Abstract
Cytokines play pivotal roles in regulation of immune responses. Signaling proteins involved in cytokine signal transduction pathways can be potential targets of toxins causing aberrant immune responses. Binding of cytokines to their specific receptors induces activation of signal transduction pathways. In this review, an overview of the cytokine/cytokine receptor system, signaling pathways activated by cytokine receptors, their regulation mechanisms, pathological conditions caused by aberrant cytokine signaling, and issues to be elucidated in the near future is provided.
INTRODUCTION
In this review, a short overview of signal transduction from Type I and Type II cytokine receptors and several recent important findings in this field is presented. For further information, other detailed general information and an additional complete set of literature reported is in recent comprehensive reviews (Levy and Darnell, Citation2002; Leonard, Citation2003). New findings in the past years after the publication of these reviews are discussed in this review. Unsolved issues in the cytokine research field are also discussed.
TYPE I AND TYPE II CYTOKINES AND THEIR RECEPTORS
Type I Cytokine Receptors
Cytokines are proteins secreted by cells and act on the cytokine-producing cells (autocrine) or on other cells (paracrine). Cytokines are classified according to their structure and the structure of their receptors. Type I cytokines are those that share a four α -helical bundle structure, and their receptors share similar structural features (Leonard, Citation2003). Type I cytokines are further classified according to the usage of receptor subunit and include:
users of the common cytokine receptor γ chain (γ c) and related cytokines: interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-13, IL-15, IL-21, and thymic stromal lymphopoietin (TSLP);
common β chain (β c) users: IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF);
gp130 users: IL-6, IL-11, oncostatin M, leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNF), and novel neurotrophin-1 (NNT-1)/B-lymphocyte-stimulating factor-3 (BSF-3); and,
users of homodimeric receptors including growth hormone (GH), prolactin, erythropoietin (EPO), thrombopoietin (TPO), leptin, and granulocyte colony-stimulating factor (G-CSF);
users of tyrosine kinase receptors, such as macrophage colony-stimulating factor (M-CSF) and stem cell factor (SCF).
FIG. 1 Schematic structure of Type I and Type II cytokine receptors. Ig-like domain: immunoglobulin-like domain; Cys: cysteine; WS motif: WSXWS sequence (W: tryptophane; S: serine; X: non-conserved amino acid).
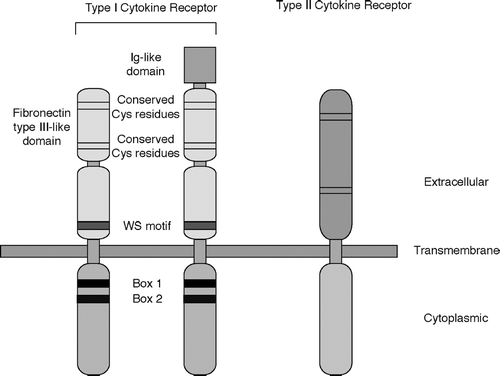
Type I cytokine receptors are homodimers, heterodimers, or oligomers. One of the important features of cytokine receptors is that one or two subunits of receptors can be shared by several different cytokines as described above. This is one of the molecular bases by which different cytokines show similar biological effects (redundancy in action). One of the important implications of this fact is that loss-of-function of a shared receptor subunit causes loss or defect of action of all the cytokines that share the subunit. A striking example is X-linked severe combined immunodeficiency (X-SCID) resulting from a mutation in γ c (Noguchi et al., Citation1993). Mutations in γ c lead to defective signal transduction of cytokines that use γ c. Among others, defects in IL-7 and IL-15 signaling cause defective development of T-lymphocytes and natural killer (NK) cells observed in X-SCID patients.
Type II Cytokine Receptors
Type II cytokines are the interferons (IFNs) and related cytokines. They include (i) Type I IFNs: IFN-α, IFN-β, IFN-ω, IFN-τ; (ii) Type II IFN: IFNγ; and, (iii) IL-10 family cytokines: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29 (Leonard, Citation2003). Receptors of Type II cytokines share structural similarities and are called Type II cytokine receptors (). Receptors of Type II cytokines also consist of two or more receptor subunits.
Three-Dimensional Structure of Cytokine Receptors
Three-dimensional structures of GH/GH receptor (GHR) (de Vos et al., Citation1992) and IFNγ /IFNGR1 (Walter et al., Citation1995) have been available. Recently, a three-dimensional structure of IL-2 associated with its α, β, and γ c receptor subunits was revealed (Wang et al., Citation2005). This is the first example of elucidation of a three-dimensional structure of a cytokine associated with its hetero-oligomeric receptor. The bases of the IL-2Rβ and γ c converge to form a Y shape, and IL-2 binds in the fork region. The binding of IL-2Rα, which is not the Type I cytokine receptor, to IL-2 stabilizes the binding site of IL-2 formed by the IL-2Rβ and γ c. Since Type I cytokine receptors share similar structural features (), other Type I cytokine receptors are supposed to bind its ligand in a similar manner as IL-2R interacts with IL-2.
So far, there is no information on three-dimensional structures of cytoplasmic domains of cytokine receptors. Elucidation of structures of cytoplasmic domains of cytokine receptors associated with signaling molecules will provide us with insight into the molecular basis of signal transduction from cytokine receptors.
SIGNALING PATHWAYS ACTIVATED BY CYTOKINE-CYTOKINE RECEPTOR INTERACTION
Binding of cytokines to their specific receptors induces activation of multiple signaling pathways, including the Jak-Stat pathway, Ras-mitogen-activated protein kinase (MAPK) pathway, Src/ZAP70, phosphatidylinositol 3-kinase (PI3K), insulin receptor substrates 1 and 2 (IRS-1, 2), phosphatases, and others (Taniguchi, Citation1995; Leonard, Citation2003).
Jak-Stat Pathway
Among other pathways, the Jak-Stat pathway has been one of the most extensively studied (Levy and Darnell, Citation2002). The pathway was independently identified by two research groups. One group discovered this pathway by establishing IFN-unresponsive mutants and genetic complementation of these mutants (Stark et al., Citation1998). The other performed promoter analysis of IFN-inducible genes (Levy and Darnell, Citation2002).
There are four Jak kinases in mammals: Jak1, Jak2, Jak3, and Tyk2. Jak kinases have seven conserved domains (Jak homology region: JH1 to JH7), among which the JH1 domain is the tyrosine kinase domain and the JH2 domain is the pseudokinase domain (). N-terminal domains are required for interaction with the cytoplasmic domains of cytokine receptors. Specificity of Jak kinase activation by cytokines is determined by specific interaction of Jak kinases with the cytoplasmic domains of cytokine receptors. For example, Jak3 specifically binds to the cytoplasmic domain of γ c (Boussiotis et al., Citation1994; Miyazaki et al., Citation1994; Russell et al., Citation1994). This is the molecular basis by which binding of γ c user cytokines leads to activation of Jak3. Jak kinases are constitutively associated with the cytoplasmic domains of cytokine receptors.
FIG. 2 Schematic structure of Jak kinases (A) and Stat proteins (B). Jak kinases have seven regions with sequence similarity. JH: Jak homology. Stat proteins have six domains with sequence similarity. SH2: Src homology 2 domain.
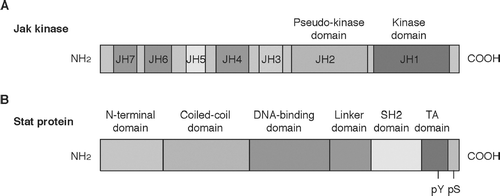
There are seven Stat proteins in mammals: Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6 (Ihle, Citation1996). Each Stat protein consists of amino-terminal domain, coiled-coil domain, DNA-binding domain, linker domain, Src homology 2 (SH2) domain, and the transactivation domain ().
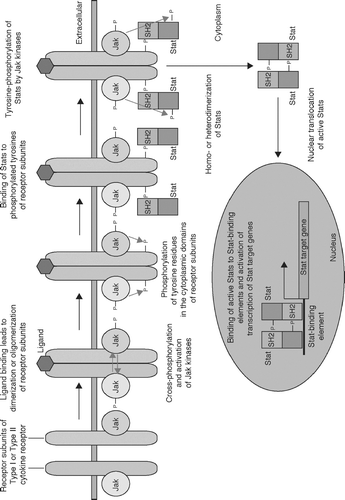
Binding of cytokines to their specific receptors leads to dimerization or oligomerization of cytokine receptor subunits, making receptor-associated Jak kinases close to each other (). This induces cross-phosphorylation of Jak kinases, leading to their maximal activation. Activated Jak kinases, in turn, phosphorylate tyrosine residues of the cytoplasmic domains of cytokine receptors. These phosphorylated tyrosines are recognized by the SH2 domains of Stat proteins, and Stat proteins bind to the cytoplasmic domains of the cytokine receptors. Then, receptor-bound Stat proteins are phosphorylated by Jak kinases at their carboxyl-termini. Phosphorylated Stat proteins are released from the cytoplasmic domains of cytokine receptors and form homodimers, heterodimers, or heterotrimers via the interaction of their SH2 domains and phosphorylated tyrosines. These complexes are transported into the nucleus by importins. The Stat complexes bind to their specific recognition sequences of their target genes and regulate their expression. As this activation mechanism suggests, specificity of Stats is determined by the specific recognition of the critical tyrosine residues in the cytoplasmic domains of receptor subunits by the SH2 domains of Stats.
FIG. 3 The Jak-Stat pathway. Binding of cytokines to their specific receptors leads to dimerization or oligomerization of cytokine receptor subunits, making receptor-associated Jak kinases close to each other. This induces cross-phosphorylation of Jak kinases, leading to their maximal activation. Activated Jak kinases in turn phosphorylate tyrosine residues of the cytoplamic domains of cytokine receptors. These phosphorylated tyrosines are recognized by the SH2 domains of Stat proteins, and Stat proteins bind to the cytoplasmic domains of the cytokine receptors. Then, receptor-bound Stat proteins are phosphorylated by Jak kinases at their carboxyl-termini. Phosphorylated Stat proteins are released from the cytoplasmic domains of cytokine receptors and form homodimers, heterodimers, or heterotrimers via the interaction of their SH2 domains and phosphorylated tyrosines. These complexes are transported into the nucleus by importins. The Stat complexes bind to their specific recognition sequences of their target genes and regulate their expression.
Regulation Mechanisms of Jak-Stat Pathway
Regulation mechanisms, especially negative regulation mechanisms, of Jak-Stat pathway have been extensively studied ().
Cytoplasmic tyrosine phosphatases: Considering the importance of tyrosine phosphorylation in the activation of the Jak-Stat pathway, it is not surprising that tyrosine phosphatases play an important role in its negative regulation. It has been shown that cytoplasmic tyrosine phosphatases SHP1, SHP2, and PTP1B are involved in this process (Kile et al., Citation2001; Qu, Citation2002; Bourdeau et al., Citation2005).
SOCS Proteins: SOCS (suppressor of cytokine signaling) proteins are cytokine-induced proteins that bind to the cytoplasmic domains of cytokine receptors and/or Jak kinases to inhibit activation of Stat proteins (Alexander and Hilton, Citation2004; Kishimoto, Citation2005; Yoshimura, Citation2005). SOCS proteins have the conserved SH2 domain and a region called the SOCS box. There are a total of eight SOCS family members.
Nuclear Phosphatases: It has been shown that Stat proteins are dephosphorylated in the nucleus (Haspel and Darnell, Citation1999), indicating that nuclear phosphatases are involved in negative regulation of Stat proteins. The nuclear phosphatases involved in this process are yet to be identified.
PIAS Proteins: PIAS (proteins that inhibit activated Stats) proteins are a group of negative regulators of Stat proteins that interact with tyrosine-phosphorylated Stat proteins (Shuai and Liu, Citation2003).
In vitro studies showed that transfection and overexpression of the PIAS genes block activation of Stat proteins. Recent study using pias1-deficient mice showed that PIAS1 protein is involved in fine-tuning of Stat1 signaling by blocking binding of Stat1 to its weak recognition sequences (Liu et al., Citation2004).
Truncated Forms of Stats: It has been suggested that naturally occurring truncated forms of Stat proteins function as dominant-negatives (O'Shea, Citation1997; Stark et al., Citation1998). However, this conclusion has been drawn from in vitro over-expression studies. In vivo studies showed that Stat3β, a truncated form of Stat3, does not exert the dominant-negative effect, but Stat3α and β have unique and specific functions (Maritano et al., Citation2004). In addition, Stat4β, a truncated form of Stat4, has been shown to activate transcription (Hoey et al., Citation2003). Therefore, it is likely that naturally occurring truncated forms of mammalian Stat proteins are not dominant-negative forms but play distinct roles in vivo. In contrast, in Drosophila, a naturally occurring truncated from of Stat92E, which lacks the first 130 amino acid residues, acts as a negative regulator of the long form (Hou et al., Citation1996; Yan et al., Citation1996).
SLIM Proteins: Stat1 is regulated by the ubiquitin-proteasome pathway (Kim and Maniatis, Citation1996). It has not been shown which E3 ligase is used. In this regard, recently, a novel nuclear E3 ligase that negatively regulates Stat signaling was revealed (Tanaka et al., Citation2005). This protein, SLIM (Stat-interacting LIM protein), contains PDZ and LIM domains and interacts with activated Stat4. SLIM acts on Stat proteins to cause their proteasome-mediated degradation. Database searches revealed that there are at least five SLIM family members (unpublished results). It is of great interest whether each of the SLIM family proteins interacts with specific Stats to cause their degradation.
TABLE 1 Negative regulators of Stat proteins
Other Signaling Molecules Involved in Cytokine Signaling
Although the Jak-Stat pathway plays a critical role in cytokine signaling, other signaling pathways also play important roles (Taniguchi, Citation1995; Leonard, Citation2003).
PTKs Other Than Jak Kinases: It has been shown that tyrosine kinases other than Jak kinases are associated with cytoplasmic domains of cytokine receptors. They include Src-family PTKs, Syk/ZAP-70, Tec-family PTKs, and Fes PTK (see Taniguchi, Citation1995 for review). The physiological importance of these PTKs in cytokine signaling is yet to be clarified.
IRS proteins/PI3K/Akt: IRS-1 and IRS-2 proteins have been shown to be tyrosine-phosphorylated upon cytokine signaling. The function of IRS proteins in cytokine signaling remains unclear. It is suggested that they serve as docking sites of signaling molecules. One of those IRS-associated signaling molecules is PI3K. PI3K is a lipid kinase that consists of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit (Fruman et al., Citation1998). Activated PI3K generates phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] from phosphatidylinositol 4,5-bisphosphate (PIP2). PI(3,4,5)P3 activates signaling molecules that contain the plekstrin homology (PH) domain. One of these PH domain-containing proteins is the protein serine kinase, Akt. It has been shown that Akt plays a critical role in inhibiting apoptosis by regulating the mitochondrial pathway involving Bcl-2 family proteins.
Ras-MAPK Pathway: It has been shown that many cytokine signaling use this pathway. It is postulated that Shc adaptor molecule binds to a phosphorylated tyrosine(s) in the cytoplasmic domain of cytokine receptors upon cytokine binding to its receptor. Shc mediates recruitment of Grb2 and Sos, leading to activation of Ras. Ras in turn initiates the well-established pathway involving activation of MAPK.
PATHOLOGICAL CONDITIONS CAUSED BY ABERRANT CYTOKINE SIGNALING
Mutations in genes involved in cytokine signaling cause pathological conditions including immunodeficiency, autoimmunity, cancer, developmental defects, and others (Levy and Darnell, Citation2002; Leonard, Citation2003). For example, aberrant IL-2 signaling leads to autoimmunity. Classically, promotion of lymphocyte proliferation has been thought to be the main function of IL-2. In fact, IL-2 was discovered in an assay system to identify T-lymphocyte growth factors (Smith, Citation1988). Therefore, it was a surprise that the lack of IL-2 causes autoimmunity including inflammatory bowel diseases in mice (Horak et al., Citation1995; Kramer et al., Citation1995). Further analyses showed that deficiency in IL-2Rα or IL-2Rβ also produces fatal lymphoproliferative inflammatory diseases (Suzuki et al., Citation1995; Willerford et al., Citation1995). We showed that the cytoplasmic subregions of IL-2Rβ that mediate activation of Stat5/Stat3 or Lck/SHP2/Ras are dispensable for the suppressive function of IL-2Rβ for autoimmunity (Fujii et al., Citation1998). Although γ c-deficient mice have severe defects in thymocyte development, activated T-lymphocytes accumulate in the periphery as they age (Cao et al., Citation1995), suggesting defective immunological tolerance in γ c-deficient mice.
Recent studies showed that IL-2Rα and IL-2Rβ are necessary for expansion of regulatory T-lymphocytes (T-regs) (D'Cruz and Klein, Citation2005; Fontenot et al., Citation2005), whereas γ c is essential for generation of T-regs (Fontenot et al., Citation2005). T-regs were originally identified as CD4(+) CD25(+) T-lymphocytes, depletion of which causes multiple autoimmune diseases in mice. Foxp3, a member of forkhead-winged-helix family transcription factors, has been shown to be essential for generation of T-regs (Fontenot et al., Citation2003; Hori et al., Citation2003; Khattri et al., Citation2003). It is an interesting future issue how IL-2 and other related cytokines regulate development and expansion of T-regs. Expression regulation of Foxp3 by these cytokines is currently under extensive investigation.
UNSOLVED ISSUES IN CYTOKINE SIGNALING
Relative Importance of the Jak-Stat Pathway in Cytokine Signaling
Accumulating evidence indicates the critical importance of the Jak-Stat pathway in cytokine signaling. For example, IL-4 specifically activates Stat6, and Stat6-deficient mice have severe defects in IL-4 signaling (Kaplan et al., Citation1996; Shimoda et al., Citation1996; Takeda et al., Citation1996). However, since Stat proteins are involved in up-regulation of cytokine receptor subunits, it is not surprising that cells show severely impaired responses to cytokines whose receptor subunits are up-regulated by the Jak-Stat pathway. Therefore, it should be necessary to clarify whether Stat proteins play other roles in addition to receptor up-regulation. Evaluation of cellular responses in Stat-deficient cells over-expressing cytokine receptor subunits regulated by the Stat protein would give us a clearer answer. The popular belief that the Jak-Stat pathway can explain all the aspects of cytokine signaling might be wrong. In this regard, we have recently established a novel reporter gene-based system to identify nuclear translocating signaling molecules (Hoshino et al., Citation2004). Novel non-Jak/Stat signaling pathways will be identified with this system. In addition, this system successfully identified a novel mutant of Stat1 with impaired protein stability (Hoshino et al., Citation2006). Thus, this system is also useful for analyzing regulation mechanisms of the Jak/Stat pathway.
Specific Regulation of Cellular Responses by Cytokines Using the Same Stat Proteins
As mentioned previously, the same Stat proteins are activated by several different cytokines. However, different cytokines lead to specific outcomes. For example, it has been shown that Stat5 activation is important for EPO-induced globin gene expression (Chretien et al., Citation1996; Iwatsuki et al., Citation1997; Wakao et al., Citation1997; Gregory et al., Citation1998; Chida et al., Citation1999; Socolovsky et al., Citation1999). Although Stat5 is activated by a variety of cytokines including EPO, IL-2, IL-3, IL-5, and others, only EPO and a small number of other cytokines induce erythrocyte differentiation in vivo and in in vitro model systems (Constantinescu et al., Citation1999; Ihle, Citation2001). This problem is underscored when different cytokine receptors that induce Stat5 activation are expressed on a single cell and still exert specific biological outcomes. Expression of globin genes is one of examples of the differential expression regulation by cytokines. It is not well understood how the specificity of Stat5-mediated globin gene expression by EPO is ensured. Another example of cytokine-specific expression of Stat target genes is IL-2Rα. Expression of IL-2Rα is up-regulated by IL-2 in a Stat5-dependent manner (Nakajima et al., Citation1997; Fujii et al., Citation1998). However, IL-3, which also activates Stat5, cannot induce IL-2Rα up-regulation (Ascherman et al., Citation1997). Analysis of the molecular mechanisms of cytokine-specific expression of Stat target genes will be an interesting future research area.
ACKNOWLEDGMENT
The author wishes to thank S. Saint Fleur for the critical reading of this manuscript. This work is supported by NIH Grant R01 AI059315.
REFERENCES
- Alexander W. S., Hilton D. J. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Ann. Rev. Immunol. 2004; 22: 503–529
- Ascherman D. P., Migone T. S., Friedmann M. C., Leonard W. J. Interleukin-2 (IL-2)-mediated induction of the IL-2 receptor α chain gene. J. Biol. Chem. 1997; 272: 8704–8709
- Bourdeau A., Dube N., Tremblay M. L. Cytoplasmic protein tyrosine phosphatases, regulation and function: The roles of PTP1B and TC-PTP. Curr. Opin. Cell Biol. 2005; 17: 203–209
- Boussiotis V. A., Barber D. L., Nakarai T., Freeman G. J., Gribben J. G., Bernstein G. M., D'Andrea A. D., Ritz J., Nadler L. M. Prevention of T-cell anergy by signaling through the γ c chain of the IL-2 receptor. Science 1994; 266: 1039–1042
- Cao X., Shores E. W., Hu-Li J., Anver M. R., Kelsall B. L., Russell S. M., Drago J., Noguchi M., Grinberg A., Bloom E. T., Paul W. E., Katz S. I., Love P. E., Leonard W. J. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 1995; 2: 223–238
- Chida D., Miura O., Yoshimura A., Miyajima A. Role of cytokine signaling molecules in erythroid differentiation of mouse fetal liver hematopoietic cells: Functional analysis of signaling molecules by retrovirus-mediated expression. Blood 1999; 93: 1567–1578
- Chretien S., Varlet P., Verdier F., Gobert S., Cartron J. P., Gisselbrecht S., Mayeux P., Lacombe C. Erythropoietin-induced erythroid differentiation of the human erythroleukemia cell line TF-1 correlates with impaired STAT5 activation. EMBO J. 1996; 15: 4174–4181
- Constantinescu S. N., Ghaffari S., Lodish H. F. The erythropoietin receptor: Structure, activation and intracellular signal transduction. Trends Endocrinol. Metab. 1999; 10: 18–23
- D'Cruz L. M., Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T-cells in the absence of interleukin-2 signaling. Nat. Immunol. 2005; 6: 1152–1159
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor. Science 1992; 255: 306–312
- Fontenot J. D., Gavin M. A., Rudensky A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T-cells. Nat. Immunol. 2003; 4: 330–336
- Fontenot J. D., Rasmussen J. P., Gavin M. A., Rudensky A. Y. A function for interleukin-2 in Foxp3-expressing regulatory T-cells. Nat. Immunol. 2005; 6: 1142–1151
- Fruman D. A., Meyers R. E., Cantley L. C. Phosphoinositide kinases. Ann. Rev. Biochem. 1998; 67: 481–507
- Fujii H., Ogasawara K., Otsuka H., Suzuki M., Yamamura K., Yokochi T., Miyazaki T., Suzuki H., Mak T. W., Taki S., Taniguchi T. Functional dissection of the cytoplasmic subregions of the IL-2 receptor β c chain in primary lymphocyte populations. EMBO J. 1998; 17: 6551–6557
- Gregory R. C., Jiang N., Todokoro K., Crouse J., Pacifici R. E., Wojchowski D. M. Erythropoietin receptor and STAT5-specific pathways promote SKT6 cell hemoglobinization. Blood 1998; 92: 1104–1118
- Haspel R. L., Darnell J. E., Jr. A nuclear protein tyrosine phosphatase is required for the inactivation of Stat1. Proc. Natl. Acad. Sci. USA 1999; 96: 10188–10193
- Hoey T., Zhang S., Schmidt N., Yu Q., Ramachandani S., Xu X., Naeger L. K., Sun Y. L., Kaplan D. H. Distinct requirements for the naturally occurring splice form Stat4α and Stat4β in IL-12 responses. EMBO J. 2003; 22: 4237–4248
- Horak I., Lohler J., Ma A., Smith K. A. Interleukin-2 deficient mice: A new model to study autoimmunity and self-tolerance. Immunol. Rev. 1995; 148: 35–44
- Hori S., Nomura T., Sakaguchi S. Control of regulatory T-cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061
- Hoshino A., Matsumura S., Kondo K., Hirst J. A., Fujii H. Inducible translocation trap: A system for detecting inducible nuclear translocation. Mol. Cell 2004; 15: 153–159
- Hoshino A., Saint Fleur S., Fujii H. Regulation of Stat1 protein expression by phenylalanine 172 in the coiled-coil domain. Biochem. Biophys. Res. Commun. 2006; 346: 1062–1066
- Hou X. S., Melnick M. B., Perrimon N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell 1996; 84: 411–419
- Ihle J. N. STATs: Signal transducers and activators of transcription. Cell 1996; 84: 331–334
- Ihle J. N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 2001; 13: 211–217
- Iwatsuki K., Endo T., Misawa H., Yokouchi M., Matsumoto A., Ohtsubo M., Mori K. J., Yoshimura A. STAT5 activation correlates with erythropoietin receptor-mediated erythroid differentiation of an erythroleukemia cell line. J. Biol. Chem. 1997; 272: 8149–8152
- Kaplan M. H., Schindler U., Smiley S. T., Grusby M. J. Stat6 is required for mediating responses to IL-4 and for development of TH2 cells. Immunity 1996; 4: 313–319
- Khattri R., Cox T., Yasayko S. A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T-regulatory cells. Nat. Immunol. 2003; 4: 337–342
- Kile B. T., Nicola N. A., Alexander W. S. Negative regulators of cytokine signaling. Int. J. Hematol. 2001; 73: 292–298
- Kim T. K., Maniatis T. Regulation of interferon-γ-activated STAT1 by the ubiquitin-proteasome pathway. Science 1996; 273: 1717–1719
- Kishimoto T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005; 23: 1–21
- Kramer S., Schimpl A., Hunig T. Immunopathology of interleukin (IL) 2-deficient mice: Thymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. J. Exp. Med. 1995; 182: 1769–1776
- Leonard W. J. Type I cytokines and interferons and their receptors. Fundamental Immunology, W. E. Paul. Lippincott Williams & Wilkins, Philadelphia, PA 2003; 701–747
- Levy D. E., Darnell J. E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002; 3: 651–662
- Liu B., Mink S., Wong K. A., Stein N., Getman C., Dempsey P. W., Wu H., Shuai K. PIAS1 selectively inhibits interferon-inducible genes and is imporatnt in innate immunity. Nat. Immunol. 2004; 5: 891–898
- Maritano D., Sugrue M. L., Tininini S., Dewilde S., Strobl B., Fu X., Murray-Tait V., Chiarle R., Poli V. The STAT3 isoforms α and β have unique and specific functions. Nat. Immunol. 2004; 5: 401–409
- Miyazaki T., Kawahara A., Fujii H., Nakagawa Y., Minami Y., Liu Z. J., Oishi I., Silvennoinen O., Witthuhn B. A., Ihle J. N., Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 1994; 266: 1045–1047
- Nakajima H., Liu X. W., Wynshaw-Boris A., Rosenthal L. A., Imada K., Finbloom D. S., Hennighausen L., Leonard W. J. An indirect effect of Stat5a in IL-2-induced proliferation: A critical role for Stat5a in IL-2-mediated IL-2 receptor α chain induction. Immunity 1997; 7: 691–701
- Noguchi M., Yi H., Rosenblatt H. M., Filipovich A. H., Adelstein S., Modi A. S., McBride O. W., Leonard W. J. Interleukin-2 receptor γ chain mutation results in X-linked severe combined Immunodeficiency in human. Cell 1993; 73: 147–157
- O'Shea J. J. Jaks, STATs, cytokine signal transduction, and immunoregulation: Are we there yet?. Immunity 1997; 7: 1–11
- Qu C. K. Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim. Biophys. Acta 2002; 1592: 297–301
- Russell S. M., Johnston J. A., Noguchi M., Kawamura M., Bacon C. M., Friedmann M., Berg M., McVicar D. W., Witthuhn B. A., Silvennoinen O., Goldman A. S., Schmalstieg F. C., Ihle J. N., O'Shea J. J., Leonard W. J. Interaction of IL-2Rβ and γ c chains with Jak1 and Jak3: Implications for XSCID and XCID. Science 1994; 266: 1042–1045
- Shimoda K., van Deursen J., Sangster M. Y., Sarawar S. R., Carson R. T., Tripp R. A., Chu C., Quelle F. W., Nosaka T., Vignali D. A., Doherty P. C., Grosveld G., Paul W. E., Ihle J. N. Lack of IL-4-induced TH2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 1996; 380: 630–633
- Shuai K., Liu B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003; 3: 900–911
- Smith K. A. Interleukin 2: Inception, impact and implications. Science 1988; 240: 1169–1176
- Socolovsky M., Faloon A. E. J., Wang S., Brugnara C., Lodish H. F. Fetal anemia and apoptosis of red cell projenitors in Stat5a−/−5b−/− mice: A direct role for Stat5 in Bcl-XL induction. Cell 1999; 98: 181–191
- Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. How cells respond to interferons. Annu. Rev. Biochem. 1998; 67: 227–264
- Suzuki H., Kündig T. M., Furlonger C., Wakeman A., Timms E., Matsuyama T., Schmits R., Simard J. J., Ohashi P. S., Griesser H., Taniguchi T., Paige C. J., Mak T. W. Deregulated T-cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 1995; 268: 1472–1476
- Takeda K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., Akira S. Essential role of Stat6 in IL-4 signalling. Nature 1996; 380: 627–630
- Tanaka T., Soriano M. A., Grusby M. J. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity 2005; 22: 729–736
- Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science 1995; 268: 251–255
- Wakao H., Chida D., Damen J. E., Krystal G., Miyajima A. A possible involvement of Stat5 in erythropoietin-induced hemoglobin synthesis. Biochem. Biophys. Res. Commun. 1997; 234: 198–205
- Walter M. R., Windsor W. T., Nagabhushan T. L., Lundell D. J., Lunn C. A., Zauodny P. J., Narula S. K. Crystal structure of a complex between interferon-γ and its soluble high-affinity receptor. Nature 1995; 376: 230–235
- Wang X., Rickert M., Garcia K. C. Structure of the quaternary complex of interleukin-2 with its α, βâ and γc receptors. Science 2005; 310: 1159–1163
- Willerford D. M., Chen J., Ferry J. A., Davidson L., Ma A., Alt F. W. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 1995; 3: 521–530
- Yan R., Small S., Desplan C., Dearolf C. R., Darnell J. E., Jr. Identification of a Stat gene that functions in Drosophila development. Cell 1996; 84: 421–430
- Yoshimura A. Negative regulation of cytokine signaling. Clin. Rev. Allergy Immunol. 2005; 28: 205–220
