Abstract
Trichloroethylene (TCE) is a widespread environmental toxicant known to promote CD4+ T-lymphocyte activation, IFNγ production, and autoimmunity in adult MRL+/+ mice. Because developing tissues may be more sensitive to toxicant exposure, it was hypothesized that continuous TCE exposure beginning at conception might induce even more pronounced CD4+ T-lymphocyte effects and exacerbate the development of autoimmunity in MRL+/+ mice. In the current study, MRL+/+ mice were exposed to occupationally-relevant doses of TCE from conception until adulthood (i.e., 7–8 wk-of-age). The CD4+ T-lymphocyte effects in the thymus and periphery were evaluated, as well as serum antibody levels. TCE exposure altered the number of thymocyte subsets, and reduced the capacity of the most immature CD4−/CD8− thymocytes to undergo apoptosis in vitro. In the periphery, T-lymphocyte IFNγ production was monitored in the blood prior to sacrifice by intracellular cytokine staining and flow cytometry. TCE induced a dose-dependent increase in T-lymphocyte IFNγ as early as 4–5-week-of-age. However, these effects were transient, and not observed in splenic T-lymphocytes in 7–8-week-old mice. In contrast, the serum levels of anti-histone autoantibodies and total IgG2a were significantly elevated in the TCE-exposed offspring. The data illustrated that occupationally-relevant doses of TCE administered throughout development until adulthood affected central and peripheral immune function in association with early signs of autoimmunity. Future studies will address the possibility that early-life exposure to TCE may alter some aspect of self tolerance in the thymus, leading to autoimmune disease later in life.
INTRODUCTION
The organic solvent, trichloroethylene (TCE) is commonly used as a metal degreaser in the industrial setting. Because of its increased use in the workplace. TCE has become a widespread environmental toxicant. Most of the TCE used in the United States is released into the atmosphere from vapor degreasing operations that may ultimately end up in the surface waters. In addition, TCE from contaminated soil found at disposal operations and Superfund sites also enters the groundwater that feeds 93% of the public water systems (ATSDR, Citation1997). TCE is highly lipophilic and readily absorbed into the circulation in multiple ways, including oral, dermal or inhalation routes (Bruckner et al., Citation1989). Although some individuals come into contact with TCE via environmental contamination (e.g., from use of private wells near disposal sites), a vast majority of TCE exposures occur in the workplace. It was estimated that approximately 3.5 million people are occupationally exposed to TCE in the United States every year (Wu and Schaum, Citation2000).
In an effort to better understand the risk associated with TCE exposure, several epidemiological studies have focused on the impact of TCE on the immune system and its ability to induce autoimmune disease. In an incidence of environmental contamination, populations exposed to a domestic water supply contaminated with TCE revealed an increase in lupus (Kilburn and Warshaw, Citation1992) and altered T-lymphocyte ratios (Byers et al., Citation1988). TCE was also linked with the development of systemic sclerosis (Lockey et al., Citation1987) and faciitis (Waller et al., Citation1994) in two separate case reports. More recently, human occupational exposure to TCE was shown to significantly increase the serum levels of the proinflammatory T-lymphocyte cytokines, interleukin (IL)-2 and interferon (IFN)-γ, over that of unexposed workers (Iavicoli et al., Citation2005). These epidemiological studies provided evidence that TCE promotes immune system alterations and autoimmunity. However, a direct link between TCE exposure and its effects on autoimmunity was not established until the development of a mouse model involving adult autoimmune-prone MRL+/+ mice (Gilbert et al., Citation1999).
MRL+/+ mice are considered to be autoimmune-prone because their genetic makeup contains several as yet unidentified susceptibility genes that promote a lupus-like disease late in life (50% mortality at 17 months of age). Idiopathic disease development in MRL+/+ mice progresses slowly, and is dependent upon (1) activated CD4+ T-lymphocytes that secrete increased levels of IFNγ, (2) increased serum levels of antinuclear antibodies (ANA), and (3) total serum immunoglobulin levels that precede immune complex deposition in the kidney (Theofilopoulos and Dixon, Citation1985). Instead of using a standard mouse strain to investigate toxicant-induced autoimmunity, MRL+/+ mice were chosen because a genetic predisposition was thought to be an important aspect of the multifactorial nature of autoimmune disease, including autoimmunity induced by xenobiotics (Kono et al., Citation2001; Pollard et al., Citation2001).
In our previous study, adult MRL+/+ mice were exposed short-term (4 weeks) or long-term (32 weeks) to a range of occupationally relevant concentrations of TCE in the drinking water (Griffin et al., Citation2000a, Citation2000b). Although disease pathology in the form of autoimmune hepatitis was not apparent until the 32-week timepoint a 4-week TCE exposure was sufficient to promote serological indicators of lupus (i.e., ANA levels) and effects on CD4+ T-lymphocytes. The TCE-induced CD4+ T-lymphocyte alterations could be observed at varying times depending upon the dose and the length of exposure; low to moderate doses of TCE promoted a significant increase in IFNγ from splenic CD4+ T-lymphocytes at both timepoints. In contrast, elevations in the phenotypic markers of activation in CD4+ T-lymphocytes (i.e., CD44hi, CD45RBlo) were not observed until 32 weeks of exposure. Thus, TCE-induced significant immunomodulatory effects in adult MRL+/+ mice, and provided a working model to better understand the effects of toxicants on the adult immune system. However, interpretation of these data using an adult model is somewhat limited. Such adult-only exposures did not encompass other windows of time during which the immune system is known to be vulnerable to toxicant exposure, i.e., during immune system development and maturation that primarily occurs in rodents from late gestation until approximately 40 and 60 days post-partum (Carlyle and Zuniga-Pflucker, Citation1998; Holladay and Smialowicz, Citation2000).
It is known that the fetal/neonatal immune system is quite sensitive to xenobiotics since more severe effects tend to occur at lower doses, and often persist long into adult life (Dietert and Piepenbrink, Citation2006). Examples of the more commonly studied developmental immunotoxicants that induce more severe or persistent immune effects in offspring as compared to adults include the heavy metals (e.g., mercury and lead) (Miller et al., Citation1998; Bunn et al., Citation2001), polycyclic hydrocarbons (e.g., benzo[a]pyrene) (Holladay and Smith, Citation1994), and polyhalogenated hydrocarbons (e.g., dioxin or TCDD) (Holladay et al., Citation1991; Vorderstrasse et al., Citation2006). Almost all of these studies focused on the ability of toxicants to promote thymic atrophy or T-lymphocyte hyporesponsiveness following developmental exposure. The developmental effects of toxicants like TCE that promote, rather than suppress the T-lymphocyte response is less clear. Based on previous studies using the adult MRL+/+ model, it was hypothesized that continuous TCE exposure occurring from conception until adulthood might induce an even more pronounced manifestation of CD4+ T-lymphocyte activation than observed in mice exposed only as adults, and that these manifestations may lead to an earlier appearance of autoimmunity. The present study focused on evaluating the effects of continuous exposure to TCE on the immature T-lymphocytes in the thymus and the mature T-lymphocytes in the periphery.
MATERIALS AND METHODS
Animals
Adult (6–8-week-old) female and male MRL+/+ mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in polycarbonate ventilated cages (Animal Care Systems, Littleton, CO). The animals were provided standard lab chow and drinking water ad libitum, and housed under controlled conditions of temperature and humidity with a 12-hour night/dark cycle. The mice were acclimated for 2 weeks prior to the initiation of studies. All studies were approved by Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
Experimental Design
A schematic diagram of the experimental design is represented in . TCE (purity 99+%) was purchased from Sigma (St. Louis, MO) and suspended in drinking water with 1% of the emulsifier Alkamuls (EL-620, provided by Rhodia, Cranbury, NJ). The water was changed every 2 to 3 days to ensure maintenance of the dose, as described previously (Griffin et al., Citation2000b). Female MRL+/+ mice were divided into three treatment groups (0, 0.5, and 2.5 mg/ml TCE) and housed together for 2 weeks in order to synchronize estrous cycles as much as possible. One age-matched, untreated male MRL+/+ mouse was introduced into each cage and left for a 7-day mating period. Over the next 7 days, the females were checked daily for the presence of vaginal plugs. On the morning of the appearance of vaginal plugs, defined as gestation Day 1 (GD1), the females were separated from males and housed singly, and their treatment regimen continued. The female mice in which vaginal plugs could not be positively identified remained with the males for another week. The time course of pregnancy was monitored by weighing the females at least every other day until parturition. No problems with breeding or parturition were observed, which is consistent with previous reports that oral exposure to TCE was shown not to affect mating success or hinder female fertility in rodents (Manson et al., Citation1984).
Only litters born within a 10-day span were included in the study (three litters per treatment group). From these litters, there were a total of 11 pups (0 TCE), 8 pups (0.5 mg/ml TCE) and 12 pups (2.5 mg/ml). After giving birth, the treatment regimen of the nursing dams continued until the pups were weaned at approximately 3–4 weeks of age. The offspring were separated according to their gender and housed in groups according to their exposure received in utero and via lactation. The pups were dosed via the drinking water with the same concentration used for the maternal dose for 4 weeks until sacrifice at 7–8 weeks of age. This age was chosen because it is the recommended time point for evaluating mature immune responses in rodents (Holsapple et al., Citation2005).
Intracellular IFNγ Production by Peripheral Blood CD4+ and CD8+ T-Lymphocytes
The offspring were tested for intracellular CD4+ and CD8+ T-lymphocyte IFNγ production at time intervals following weaning and ending at sacrifice. Unless otherwise specified all antibodies and reagents used for intracellular staining were purchased from BD Biosciences (San Jose, CA). Mice were anesthetized with sodium Nembutal (0.2 ml of a 5 mg/ml concentration) and blood was collected from the retro-orbital plexus into micro centrifuge tubes treated with sodium heparin (7.5 μl of 1000 U/ml stock purchased from Sigma) to prevent clotting. Whole blood was diluted 1:1 with IMDM medium (Cambrex, Baltimore, MD) and mixed. Fifty ng/ml PMA and 1 μg/ml ionomycin (both from Sigma) were added directly to the heparinized blood in the presence of 1 μg/ml protein transport inhibitor, Golgi-plug (BD Biosciences). The tubes were vortexed and aliquoted into 5 ml snap-top tubes (0.2 ml of blood per tube) and incubated for 5 hours at 37°C in 6% CO2 humidified air. After incubation, 2 ml of ammonium chloride buffer (0.16 M NH4Cl and 0.17 M Tris, pH 7.2 from Sigma) were added to lyse the erythrocytes.
The resulting lymphocytes were washed twice in staining buffer that contained 1X Dulbecco's PBS without Mg+2 or Ca+2 and 1% heat-inactivated FCS (pH 7.5). The cells were then incubated with either phycoerytherin (PE)-anti-CD4 (clone GK1.5, rat IgG2b), or PE-anti-CD8 (clone 53-6.7, rat IgG2a) for 15 minutes at room temperature. Cells were washed once with staining buffer, fixed and permeabilized by the addition of 0.5 ml of Cytofix/Cytoperm solution (BD Biosciences). The cells were vortexed and incubated at room temperature for 20 minutes. Cells were washed and resuspended in 0.05 ml Perm/Wash solution containing 10 μg/ml fluorescein isothiocyanate (FITC)-anti-IFNγ antibody. After 30 minutes, the cells were washed and resuspended in 0.5 ml PBS/2% paraformaldehyde solution and kept at 4°C in the dark until analysis by flow cytometry. The phenotypic analysis of 10,000 events per sample was carried out using a Partec Cy-Flow flow cytometer (Fort Collins, CO). The data are presented in bar graphs or as dot plots of CD4+ or CD8+ T-lymphocytes. Non-viable cells, based on low forward scatter and side scatter, were excluded in each sample. Data analysis was performed with the use of FCS Express software (Ontario, Canada). For all groups tested, staining with isotype immunoglobulin controls and unstimulated controls was also examined.
Cytokine Profile Analysis
Equal numbers of splenic lymphocytes were pooled from individual mice for an n = 3 per treatment group and enriched for CD4+ T-lymphocytes by positive selection using magnetic bead kits (Invitrogen, Carlsbad, CA). The purity of the CD4+ T-lymphocytes was determined by flow cytometry to be > 95% (data not shown). Following purification, the CD4+ T-lymphocytes (1 × 105/well) were incubated at 37°C in medium alone or in a 96-well tissue culture plate containing immobilized anti-CD3ε antibody and anti-CD28 antibody as described (Blossom et al., Citation2004). Culture supernatants were collected after 72 hours and tested for TH1/TH2 cytokines; IFNγ, IL-2, tumor necrosis factor (TNF)-α, and IL-4 using multiplex biomarker immunoassay kits (Linco Research, Inc., St. Charles, MO) and run on a Luminex 100 Bioanalyzer (Luminex Corp., Austin, TX).
Body Weight, Spleen and Thymus Cellularity and Cell Surface Staining
The offspring were weighed once weekly after weaning (3–4 weeks-of-age) and again at sacrifice. Total cellularity was determined for individual spleen and thymus with a hemacytometer using trypan blue exclusion and light microscopy. The splenic subpopulations were determined by flow cytometric analysis using antibodies purchased from BD PharMingen (La Jolla, CA). The splenocytes were triple stained with phycoerytherin (PE)-anti-CD4 (clone GK1.5, rat IgG2b), or fluorescein isothiocyanate (FITC)-anti-CD8 (clone 53-6.7, rat IgG2a), or PerCP anti-B220 (clone RA3-6B2, rat IgG2b for 30 minutes at 4°C. Relative cell number for each subpopulation was determined by multiplying the percentage by the total number of lymphocytes counted in the spleen. The percentage (%) and cellularity per thymus were determined as described above. Thymocytes were removed from individual mice at sacrifice and washed thoroughly with sterile saline to remove red cells and debris. Thymocyte cell suspensions were prepared, and 1 × 106 cells per group were 2-color-stained with FITC-anti-CD8 and PE-anti-CD4. Each monoclonal antibody was titrated prior to use to determine the optimal working concentration.
Total Immunoglobulin and Autoantibody in Serum
Blood was collected from the retro-orbital plexus from individual MRL+/+ at sacrifice. Blood was allowed to clot at room temperature for 1 hour and centrifuged at 1000 × g for 30 min. The sera were collected and stored at −20°C until assay. The sera of individual mice were tested for the presence of total immunoglobulin isotypes, IgG1 and IgG2a by ELISA. All capture and detecting monoclonal antibodies were purchased from BD-PharMingen. Capture monoclonal antibodies included purified IgG1 mAb (clone A85-3, rat IgG2a) and purified IgG2a, mAb (clone R11-89, rat IgG1). Detecting monclonal antibodies included biotinylated anti-mouse IgG1 (clone A85-1 rat IgG1) and biotinylated anti-mouse IgG2a, (clone R19-15 rat IgG2a). Relative levels of anti-single-stranded DNA (ssDNA), anti-double-stranded DNA (dsDNA), and anti-histone from the serum were determined by ELISA as described previously (Blossom et al., Citation2004) with some modifications.
Antigen preparation: Calf thymus histone, and denatured or native calf thymus DNA (1 mg/ml from Sigma) were mixed with reacti-bind DNA coating solution (Pierce Biotechnology, Rockford, IL) and incubated in a glass tube on a rotator for 10 minutes. Following an overnight incubation at 4°C, the plates were washed with a solution containing 1X PBS and 0.5% Tween-20 (Sigma) and blocked by adding 0.2 ml/well IX PBS and 10% FCS. The plates were then washed and the serum samples (1:100) were added to the wells (0.1 ml/well) overnight at 4°C. Polyclonal biotinylated goat-anti-mouse immunoglobulin (Sigma) was diluted 1:1000 and added to the plates for 1 hour at room temperature. The plates were developed with extravidin alkaline phosphatase, 0.1 ml/well and alkaline phosphatase substrate (p-nitrophenol) and measured by an ELISA reader (absorbance at 405 nm). Sera from 8–10-week-old adult MRL+/+ mice were also tested as a control for positive responses for anti-ssDNA, anti-dsDNA, and anti-histone.
Spontaneous Thymocyte Apoptosis and Activation-Induced Cell Death (AICD) in Splenic CD4+ and CD8+ T-Lymphocytes
For the thymocytes, 2 × 106 cells/ml were cultured in 24-well plates. Apoptosis was induced by culturing the cells in medium alone. After 24 hours, the cells were harvested from wells and 3-color stained with PE-anti-CD8, PerCp anti-CD4 and FITC-Annexin V, a marker of apoptosis, and examined by flow cytometry. For peripheral T-lymphocytes, AICD was induced as described previously (Blossom and Gilbert, Citation2006). Briefly, an equal number of splenic lymphocytes were pooled for an n = 3 per treatment group and activated with 5 μg/ml Conconavalin A (Con A; Type V and VI from Canavalia ensiformis; Sigma) and 5 ng/ml recombinant IL-2 (rIL-2; R&D Systems, Minneapolis, MN) for 4 days in standard RPMI medium. Viable cells were isolated by passage over Ficoll/Hypaque, counted with a hemacytometer using trypan blue exclusion and light microscopy, and then stimulated for 18 hours with a concentration of immobilized anti-CD3 antibody previously shown to induce optimal AICD (Blossom and Gilbert, Citation2006). After stimulation, the cells were stained with PE-anti-CD8, and biotinylated anti-CD4 followed by PerCP streptavidin in conjunction with FITC-labeled Annexin V and analyzed by flow cytometry as described above.
Statistical Design
Male and female offspring from three dams in each treatment group were used in this study. Since the treatment of the offspring from the exposed dams continued until adulthood, the statistical “n” was based on the individual pup instead of the entire litter (Festing, Citation2006). The cells obtained from the tissues of each offspring were pooled into 3 or 4 replicates per group, and the means of the experimental values were calculated. The values for all replicates for each exposure group were compared by one-way ANOVA. The criterion for significance was set at p < 0.05. When statistical differences were detected using ANOVA, Tukey's comparison was used to compare all the treatment groups for dose effects.
RESULTS
TCE Increased IFN-γ Production by Peripheral CD4+ and CD8+ T-Lymphocytes
The peripheral blood of individual mice was harvested at regular intervals before sacrifice in order to see whether continuous TCE exposure would accelerate T-lymphocyte production of IFNγ as tested by intracellular cytokine staining and flow cytometry. As shown in , CD4+ and CD8+ T-lymphocytes from the peripheral blood of offspring exposed to 2.5 mg/ml TCE were stimulated to produce significantly more IFNγ when compared to water-only controls in mice as early as 4–5 weeks of age, and only after 1 wk of direct TCE exposure via the drinking water (12, 15, and 22% for CD4+ T-lymphocytes, and 8, 14, and 17% for CD8+ T-lymphocytes; 0, 0.5, and 2.5 mg/ml TCE, respectively). When the offspring were 5–6 weeks of age, the TCE-induced IFNγ production was even more pronounced (34, 49, and 65% for CD4+ T-lymphocytes and 17, 33, and 45% for CD8+ T-lymphocytes; 0, 0.5 mg/ml, and 2.5 mg/ml TCE, respectively; and ), and the response was dose-dependent. Interestingly, when IFNγ secretion was tested at 7–8 weeks of age, there was very little TCE effect. Thus, the TCE-mediated increase in peripheral blood-T-lymphocyte IFNγ production was most apparent in young mice prior to adulthood.
FIG. 2 TCE increased IFNγ produced by CD4+ and CD8+ T lymphocytes. (A) Individual offspring were bled at regular intervals following weaning to test for intracellular IFNγ production by peripheral blood CD4+ and CD8+ T-lymphocytes. After stimulation of whole blood with PMA and ionomycin for 5 hours, the cells were then 2-color stained with PE-anti-CD4 or PE anti-CD8 antibody in conjunction with FITC anti-IFNγ. The data represent the mean ± SD of CD4+ or CD8+ T-lymphocytes that were also positive for IFNγ. Results statistically different from control (* p < 0.05) or statistically different from 0.5 mg/ml TCE (** p < 0.05). (B) Representative dot plots from individual mice from the data presented in (5–6-wk timepoint) of IFNγ staining on CD4+ cells from mice exposed to water or TCE. (C) Equal numbers of spleen cells from the offspring in each treatment group were pooled for an n = 3 and the CD4+ T-lymphocytes were purified. The isolated CD4+ T-lymphocytes (1 × 105 per well) were activated with plate-bound anti-CD3 antibody and soluble anti-CD28 antibody. Supernatants were collected after 72 hours incubation and analyzed for the presence of IL-2, IFNγ, IL-4, and TNFα using multiplex biomarker immunoassay kits with the Luminex 100 Bioanalyzer. The data were quantitated using standard curves and represented in the graphs as means ± SD in pg/ml.
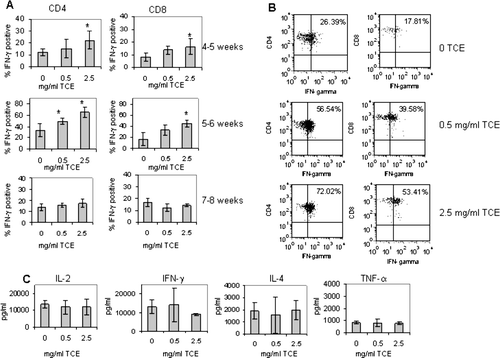
TCE-induced cytokine secretion was also measured in CD4+ T-lymphocytes isolated from the spleen at sacrifice, three days after the final interim bleed. Exposure to TCE did not significantly affect the production of splenic CD4+ T-lymphocyte IFNγ production over that of control mice (13, 293, 14, 047, 9050 pg/ml; 0, 0.5, and 2.5 mg/ml TCE, respectively) as shown in . CD4+ T-lymphocyte production of IL-2, IL-4, and TNFα was generally similar among the groups. Taken together the early increase in intracellular IFNγ observed in younger mice was transient and not reflected in the CD4+ or CD8+ T-lymphocytes from the peripheral blood or spleens of 7–8 week-old mice.
TCE Exposure Was Associated with Decreased Spleen Cellularity and Reduced Numbers of CD4+, CD8+, and B220+ Lymphocytes
In previous studies, adult MRL+/+ mice treated with TCE showed no significant changes in spleen cellularity or numbers of B- and T-lymphocyte subsets (Griffin et al., Citation2000b). In contrast, the offspring exposed to TCE in the current study demonstrated a decrease in spleen cellularity in comparison to controls, although this effect was not statistically significant (). However, the slight decrease in cellularity correlated with a significant reduction in the numbers of splenic CD4+, CD8+, and B220+ B-lymphocytes in TCE-exposed mice. Therefore the changes observed in splenic lymphocyte number appeared to be unique to early life exposure.
TABLE 1 Comparison of lymphocyte subpopulations in spleens of MRL+/+ mice exposed throughout development with TCE
Disease development in adult MRL+/+ mice was also accompanied by the expansion of memory/activated CD44hi/CD62Llo/ CD45RBlo CD4+ T-lymphocytes following 32 weeks of TCE exposure (Griffin et al., Citation2000b). However, in the present study, the majority of the splenic CD4+ and CD8+ T-lymphocyte lymphocytes isolated from the spleen from all of the groups in the present study were found to be CD44lo/CD62Lhi/CD45RBhi (data not shown), which is a T-lymphocyte phenotype typically observed in young mice (Ernst et al., Citation1993). In addition, there were no alterations in the expression of activation markers that were evaluated on the surface of B-lymphocytes (data not shown). Taken together, continuous exposure to TCE reduced B- and T-lymphocyte numbers, although this affect was apparently not associated with alterations in the activation state of the lymphocytes.
TCE Exposure Increased Levels of IgG2a and Anti-Histone Autoantibodies in the Serum
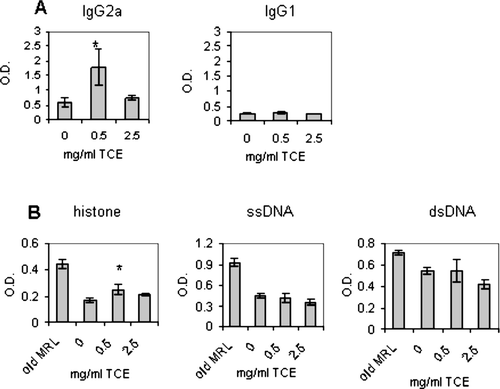
Sera were obtained from the offspring at sacrifice. It was found that the TCE-exposed offspring had significantly higher levels of IgG2a, but not IgG1 immunoglobulin, and increased levels of antibody that reacted with the nuclear antigen, histone (). In contrast, the levels of the autoantibodies, anti-ssDNA and anti-dsDNA, were not significantly different in TCE-exposed animals with respect to controls. The TCE-mediated increase in anti-histone and IgG2a antibodies was consistent with antibody responses observed in our adult model, and suggested that continuous exposure promoted early serological markers of autoimmunity. However, despite the presence of serological evidence of autoimmunity, the TCE-exposed offspring did not exhibit histopathological signs of autoimmunity upon examination of liver or kidney sections at sacrifice (data not shown).
FIG. 3 Increased IgG2a and anti-hostone levels in TCE-exposed offspring. Total IgG2a and IgG1 levels in the sera of individual mice were measured using standard ELISAs as described in Materials and Methods. Serum (diluted 1:100) was collected from the offspring at sacrifice and tested for IgG2a, IgG1, anti-histone, anti-ssDNA, or anti-dsDNA. The data are presented as the mean optical density (OD) + SD. Sera from aged adult MRL+/+ mice (8–10 months of age) were also tested as a control for positive responses for autoantibody production. *Statistically different from control (* p < 0.05).
TCE Altered Thymocyte Development in MRL+/+ Mice
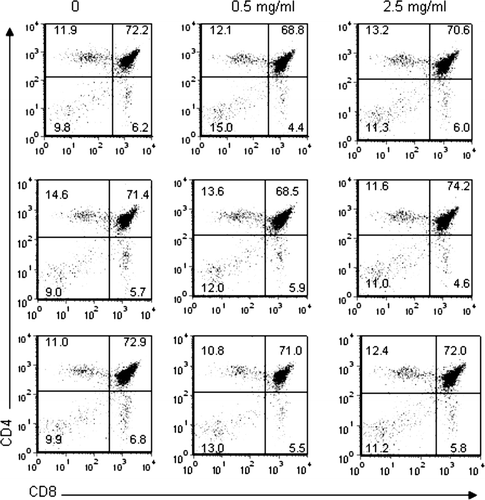
To determine whether TCE exposure affected T-lymphocyte development, thymocytes were analyzed in the TCE-exposed offspring. It was believed that looking at the relative proportion of the different thymocyte subpopulations may indicate why the TCE-exposed offspring exhibited decreased splenic lymphocyte numbers and unremarkable peripheral T-lymphocyte IFNγ responses at 7–8 weeks of age. Thymus cellularity was slightly elevated in TCE-exposed mice (). Although this increase was not statistically significant, it corresponded with a statistically significant increase in the percentage () as well as number of double negative (DN) cells (). There was also a significant increase in the number, but not the percentage, double positive (DP) cells. A quantitative increase in the number of CD4+ single positive (SP) thymocytes was also observed in mice exposed to 0.5 mg/ml TCE, although this effect was not statistically significant.
TABLE 2 Phenotypic alterations in the thymocyte profile of MRL+/+ mice exposed to TCE throughout development
FIG. 4 Percentage of thymocytes expressing CD4 and CD8. Thymocytes obtained from control and TCE-treated offspring were pooled for an n = 3 per treatment group. The cells were then subjected to staining with FITC anti-CD8 and PE anti-CD4 antibodies and examined by flow cytometry. The numbers within each dot plot represent the % of thymocytes that express CD4 and CD8.
The increase in the number of DN cells isolated from the thymus suggested that there may be a disparate proportion of the different DN subpopulations in TCE versus control thymocytes. The DN thymocytes thought to be committed to T-lymphocyte lineage are known to rearrange and eventually express TCR molecules before their maturation into DP T-lymphocyte precursors (Wilson et al., Citation1999), at which time the DN cells pass through four phenotypically distinct stages defined by their expression of CD44 and CD25 molecules (Godfrey et al., Citation1993). However, analysis of CD44 and CD25 expression on the DN thymocyte population did not reveal significant differences between TCE-exposed and control thymocytes (data not shown). Taken together, these results suggested that TCE altered thymocyte maturation by increasing the number of DN and DP thymocytes.
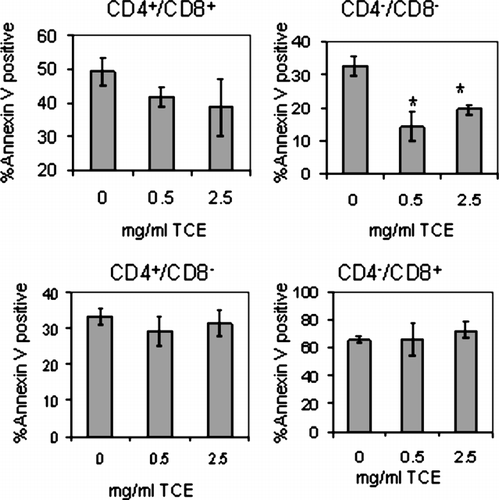
TCE Exposure-Inhibited Apoptosis in the DN Subpopulation of MRL+/+ Mice
The increase in the number of DN and DP thymocytes in TCE-exposed mice might be due to alterations in the ability of these populations to undergo apoptosis. Thymocytes were harvested from cultures after 24 hours, 3-color stained for CD4+, CD8+, and the apoptosis marker, Annexin V. For evaluation of apoptosis, the thymocytes were gated into DN, DP, CD4+ SP and CD8+ SP populations and analyzed by flow cytometry. As shown in , TCE exposure slightly decreased the apoptosis of DP thymocytes. However, TCE exposure significantly attenuated apoptosis of DN thymocytes, a population known to be generally less sensitive to apoptosis in vitro (Berki et al., Citation2002). Susceptibility to apoptosis was also conducted in the mature CD4+ and CD8+ SP populations, and there were no differences in the amount of Annexin V staining compared to control thymocytes. Thus, the ability of DN and DP thymocytes to undergo apoptosis was inhibited by TCE exposure.
FIG. 5 TCE exposure inhibited apoptosis of DN thymocytes isolated from MRL+/+ offspring. Thymocytes obtained from control and TCE-treated offspring were pooled from each treatment group for a statistical n = 4 and were cultured in medium for 24 hours (2 × 106/ml). The apoptotic cells were 3-color stained with FITC Annexin V, PE-anti-CD8 and biotinylated anti-CD4 followed by PerCP streptavidin and examined by flow cytometry. The graphs represent the mean ± SD Annexin V staining from gated SP, DN, or DP populations. *Significantly different from control (p < 0.05).
TCE Exposure Did Not Affect Peripheral CD4+ T-Lymphocyte AICD
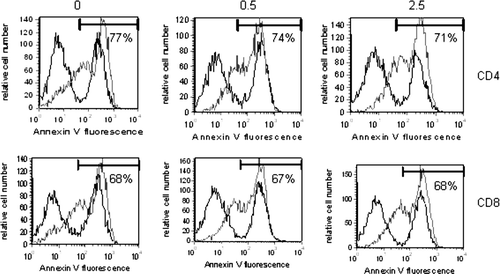
To determine whether TCE exposure similarly attenuated apoptosis in peripheral T-lymphocytes, the CD4+ and CD8+ T-lymphocytes from the MRL+/+ mice were examined for their susceptibility to AICD. The model to induce AICD, used by this group and by others, involves lymphocyte activation in the presence of IL-2 followed by CD3 receptor engagement under long-term (5-day) culture conditions (Delgado and Ganea, Citation2000; Blosom and Gilbert, Citation2006). The results revealed that the CD4+ and CD8+ T-lymphocytes from the TCE-exposed mice were similar to controls in their ability to undergo AICD upon restimulation in vitro (). Therefore, the differential sensitivity to apoptosis induced by continuous TCE exposure was observed in the thymus, but not reflected in the periphery.
FIG. 6 TCE did not alter AICD of peripheral T-lymphocytes. Spleen cells isolated form pooled samples within each treatment group (n = 3) were cultured at a concentration of 2 × 106 per ml and activated to undergo apoptosis as described in Materials and Methods. The cells were harvested and three-color stained with PE-anti-CD8, FITC Annexin V, and biotinylated anti-CD4 and PerCP streptavidin. Presented are representative FITC histograms after gating on the PE+ or PerCP+ cells. The percentage (%) within each histogram refer to the % Annexin V positive CD4+ or CD8+ T-lymphocytes treated with anti-CD3 (grey histogram). The black histograms in each plot represent Annexin V staining of CD4 or CD8 T-lymphocytes in the absence of anti-CD3 stimulation.
TCE Exposure Was Associated with Decreased Growth
The offspring and the dams were closely monitored for any gross physiological changes induced by exposure to TCE. There were no apparent effects of TCE on reproductive capacity, parturition, or the ability of the dams to mother their offspring. As shown in , the dams in each treatment group showed similar mating success, and produced litters with similar numbers of pups. However, the body weights of the offspring measured from the time of weaning until sacrifice were significantly less in TCE-exposed groups compared to control (). The significance of this finding is not clear since none of the TCE-exposed offspring demonstrated signs of overt toxicity after gross examination and necropsy following sacrifice. In addition, liver and kidney histology revealed no signs of toxicant-induced tissue damage (data not shown). The reason for the apparent TCE-induced decrease in body weight is not clear. However, this phenomenon could not be explained by dehydration in TCE-exposed mice; the offspring in all of the exposure groups consumed similar amounts of water (). In addition, the mice were exposed to similar levels of TCE in the drinking water as was shown in previous studies (Griffin et al., Citation2000b), although this evaluation does not take into account exposure levels of the offspring that were acquired during gestation and lactation.
FIG. 7 TCE exposure did not affect litter size but promoted decreased growth in the offspring. (A) Three female mice per treatment group produced litters containing similar numbers of pups. The bars in the graph represent the mean ± SD number of pups delivered per treatment group. (B) pup was weighed once weekly from the time immediately following weaning until sacrifice. The symbols in the graph represent mean ± SD of the body weights from each group. Results are significantly different from control (* p < 0.05).
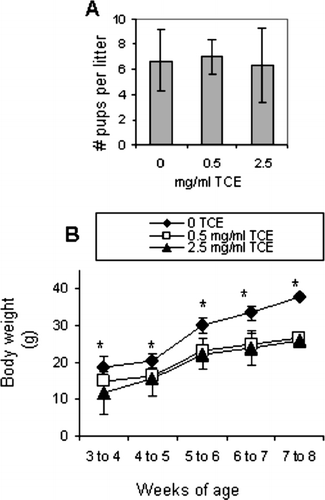
TABLE 3 Water consumption
DISCUSSION
The present study was designed to evaluate the effects of TCE on CD4+ T-lymphocyte activation and autoimmunity in MRL+/+ mice following exposure that occurred from the time of conception until the offspring reached adulthood using the same occupationally-relevant concentrations of TCE used in our adult model. Earlier work in our laboratory provided the first experimental evidence that exposure to these doses of TCE in adult mice promoted CD4+ T-lymphocyte activation and autoimmunity. TCE easily crosses the placenta (Ghantous et al., Citation1986) and enters the maternal milk (Fisher et al., Citation1990). Thus, it was hypothesized that TCE may also affect the developing immune system of the offspring.
A recent report published soon after this study was completed was the first to assess the immunotoxicological effects of TCE exposure during development (Peden-Adams et al., Citation2006). In that study, perinatal exposure to TCE in the drinking water of non-autoimmune mice significantly increased (1) T-lymphocyte mediated delayed-type hypersensitivity (DTH) responses, (2) decreased antibody-mediated plaque-forming cell (PFC) responses, and (3) enhanced thymus cellularity in 8-wk-old mice. The data presented here are compatible with that study. However, our assays designed to evaluate immune phenotype/function were quite different. The results of our previously published experiments using adult MRL+/+ mice provided the rationale to test specific immune parameters (i.e., IFNγ production) shown to be altered in these mice following adult exposure rather than evaluating the more predictive endpoints for determining immunotoxicity (Luster et al., Citation1993).
Perhaps the most striking observation in the present study was the early increase in IFNγ production by peripheral blood CD4+ and CD8+ T-lymphocytes observed in young mice (aged 4–6 weeks). The production of IFNγ appears to be am important immunological indicator of TCE-induced immunoregulation; CD4+ T-lymphocytes from adult MRL+/+ mice exposed to TCE also secreted increased levels of IFNγ at both 4 and 32 weeks of exposure. The finding that developmental and early life exposure also leads to increased IFNγ production has several important implications, since the fetal immune system in humans and in rodents, under normal circumstances, is constitutively biased towards TH2 function (Adkins and Du, Citation1998). Along this line, T-lymphocytes from neonatal and young pre-adult mice have a reduced capacity to produce IFNγ either when stimulated with mitogens (Lewis et al., Citation1991) or antigens (Adkins et al., Citation2002). Furthermore, neonatal mice that have been immunized with antigen are defective in producing TH1 subclass abs (IgG2a), but not TH2 subclass abs (IgG1) (Bowman and Holt, Citation2001). Interestingly, serum levels of IgG2a relative to IgG1 were elevated in TCE-exposed offspring in the current study, which provides further evidence of TH1 skewing.
It is interesting that, unlike what was observed with T-lymphocyte IFNγ production, there was no dose effect with antibody or autoantibody production. Although the reason remains unclear, the lack of a dose response with IgG2a or anti-histone production may be in part due to differential toxicity to the higher dose of TCE between antibody-producing B-lymphocytes and T-lymphocytes. TCE exposure was also associated with elevated serum levels of the autoantibody, anti-histone. The significance of this finding is underlined by evidence that antibodies to histone precede the development of anti-DNA autoabs in MRL mice (Laderach et al., Citation2003). Taken together, although normal individuals eventually acquire the ability to upregulate TH1 functions as they age, any event that induces an early skewing of T-lymphocyte responses toward TH1 is likely to exert deleterious effects by promoting inflammation, and may be a potential factor in the development of autoimmunity.
The phenotypic characteristics of the CD4+ and CD8+ T-lymphocytes isolated from the spleens of the offspring in all exposure groups in the current study were characteristic of a naive CD44lo, CD62Lhi, CD45RBhi phenotype that is commonly observed in young mice. In addition, spleen cellularity and total T- and B-lymphocyte numbers were also decreased in the offspring exposed to TCE. The lack of TCE-induced effects on T-lymphocyte phenotype may be due to the age of the animals in this study. For example, TCE treatment of adult MRL+/+ mice increased the number of CD4+ T-lymphocytes with a memoryactivated CD44hi/CD45RBlo phenotype after 32 wk, but not 4 wk of exposure. Thus, it is possible that a longer exposure time would be necessary to generate a significant pool of effector/memory cells. Along this line, none of the MRL+/+ mice in the present study developed significant kidney or liver pathology. However, adult MRL+/+ mice developed autoimmune liver pathology after a chronic 32-week exposure, but not at earlier time points. Consequently, it seems likely that a longer exposure to TCE would be required in order to generate tissue pathology consistent with autoimmunity. Future studies will address this possibility.
In addition to its effects on the peripheral T-lymphocytes, TCE exposure induced significant effects on developing thymocytes. The primary function of the thymus is to generate a T-lymphocyte repertoire capable of MHC-restricted antigen recognition while eliminating T-lymphocytes that are potentially autoreactive through clonal deletion (Sebzda et al., Citation1999). The maturation of DP cells that undergo positive selection to become mature SP cells is dependent upon low avidity interactions between the TCR and self MHC/peptide expressed on antigen presenting cells. In contrast, negative selection involves potent affinity interactions between the TCR and self MHC/peptide leading to deletion of autoreactive T-lymphocytes. Earlier studies found that certain environmental toxicants such as TCDD (Blaylock et al., Citation1992; Camacho et al., Citation2004) and other agents such as estrogen (Zoller and Kersh, Citation2006) induced thymic atrophy by altering thymocyte development and apoptosis. In contrast to the widely studied developmental immunotoxicant, TCDD, TCE treatment resulted in a rise in the number of DN and DP thymocytes. It is not clear why TCE exposure appeared to target these subpopulations. It is known that thymocytes undergo rapid proliferation before they emerge as DP precursors for positive and negative selection (Huesmann et al., Citation1991). Not only is the survival of DN cells dependent upon the presence of cytokines such as IL-7, it is becoming increasingly clear that costimulation and signaling events mediated by B7/CD28 molecules can potentiate the proliferation and survival of DN thymocytes (Zheng et al., Citation2004). Thus, it is possible that TCE somehow provides signaling to the DN precursors that would increase their proliferation and transition from DN to DP cells.
The increase in the number DN and DP cells was reflected by their reduced capacity to undergo apoptosis in vitro. It is not clear at this time how TCE is promoting survival of the DN population. It is possible that TCE induces a weak signal to DP thymocytes that are capable of reacting to MHC/self peptide complexes. However, neither SP population, nor the peripheral CD4+ T-lymphocytes differed with respect to controls in their ability to undergo apoptosis in vitro. Whether TCE promoted a general effect on thymopoiesis or targeted a specific event (i.e., selection or the regulation of a gene that inhibits thymocyte apoptosis) in the thymus is not known. Regardless of the mechanism, TCE appeared to target the cells in the thymus, which may have important implications regarding the role of TCE in immune function in general, as well as the development of autoimmunity. It is interesting that TCE appears to have an opposite effect on apoptosis compared to TCDD, which is known to promote thymocyte apoptosis (Camacho et al., 2005). Although no environmental chemical has ever been shown to cause autoimmunity via its effects in the thymus, it is interesting to note that the cardiac antiarrhythmic drug, procainamide, has been shown to induce autoimmunity via its effects on the selection processes in the thymus (Kretz-Rommel and Rubin, Citation2000). Whether TCE induces autoimmunity by a similar process is not currently known. Future studies will address the role of TCE in the thymus and the consequence on peripheral immune function.
Aside from the immune system effects, TCE exposure also significantly affected the body weight of the offspring. Interestingly, there was no difference in the body weights among the dams in all of the groups (data not shown). This result suggests that TCE's effect on body weight is characteristic of exposure initiated during development, but not in adults. The reason for the decreased body weight in TCE-exposed offspring is not known. The developmentaly exposed mice in the study by Peden-Adams et al. (Citation2006) also showed decreased body mass, but the weights of the offspring normalized to control weights by 8 weeks of age. In contrast, the body weights of the offspring in our study did not normalize by 7–8 weeks. This discrepancy may be explained by the fact that Peden-Adams et al. (Citation2006) administered significantly lower doses of TCE; 1.4 and 14 ppm compared to the 500 and 2500 ppm dose used in the current study. Even though the histological evaluation did not reveal any TCE-induced organ toxicity in the present study, it is possible that the doses used here were high enough to alter some aspect of development that is reflected in smaller size of the offspring.
The results of our study indicate that TCE modulates the immune system following developmental and early life exposure. Children and neonates may represent a population that is more susceptible to the effects of TCE because they may receive greater exposures per unit of body weight when compared to adults. Alternatively, the expression of CYP2E1 enzymes in the fetus or placenta could potentially result in the formation of high local concentrations of metabolites and lead to higher exposures in fetal tissues than would be expected from maternal exposure (Nakajima et al., Citation1992). Thus, it is important to consider perinatal and early life exposures in risk assessment to environmental chemicals.
Currently, the guidelines set forth by the Occupational Safety and Health Administration (OSHA) state that the 8 hr permissible exposure limits for TCE should not exceed a time-weighted average of 100 or 300 ppm for 5 minutes in any 2-hour period (ATSDR, Citation1997). These limits were extrapolated from animal studies to account for best estimates for exposure levels for the average 70 kg man. Pregnant or lactating workers were not considered in these estimates, and the effect level for their infants would presumably be lower. This study sugests a novel etiology for the development of autoimmunity and/or immune system exacerbations resulting from continuous exposure to environmental toxicants occurring from conception until adulthood. Intense focus is needed in this area to understand the mechanisms of TCE's effect on immune development and function. Similar studies in MRL+/+ mice are planned that expand the range of exposure periods to evaluate both early and later time points to determine TCE's role in immune system dysregulation and disease progression.
This work was made possible by funds from the University of Arkansas for Medical Sciences Dean's Research Development Fund (to S.J.B.).
REFERENCES
- ASTDR. Public Health Statement for Trichloroethylene. Agency for Toxic Substances and Disease Registry (ASTDR), Atlanta 1997, Available at http:/atsdr.cdc.gov
- Adkins B., Bu Y., Guevara P. Murine neonatal CD4+ lymph node cells are highly deficient in the development of antigen-specific TH1 function in adoptive adult hosts. J. Immunol. 2002; 169: 4998–5004
- Adkins B., Du R. Q. Newborn mice develop balanced TH1/TH2 primary effector responses in vivo but are biased to TH2 secondary responses. J. Immunol. 1998; 160: 4217–4224
- Berki T., Palinkas L., Boldizsar F., Nemeth P. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int. Immunol. 2002; 14: 463–469
- Blaylock B. L., Holladay S. D., Comment C. E., Heindel J. J., Luster M. I. Exposure to tetrachlorodibenzo-p-dioxin (TCDD) alters fetal thymocyte maturation. Toxicol. Appl. Pharmacol. 1992; 112: 207–213
- Blossom S. J., Gilbert K. M. Exposure to a metabolite of the environmental toxicant, trichloroethylene, attenuates CD4+ T-lymphocyte activation-induced cell death by metalloproteinase-dependent FasL shedding. Toxicol. Sci. 2006; 92: 103–414
- Blossom S. J., Pumford N. R., Gilbert K. M. Activation and attenuation of apoptosis of CD4+ T-lymphocytes following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid. J. Autoimmun. 2004; 23: 211–220
- Bowman L. M., Holt P. G. Selective enhancement of systemic TH1 immunity in immunologically immature rats with an orally-administered bacterial extract. Infect. Immun. 2001; 69: 3719–3727
- Bruckner J. V., Davis B. D., Blancato J. N. Metabolism, toxicity, and carcinogenicity of trichloroethylene. Crit. Rev. Toxicol. 1989; 20: 31–50
- Bunn T. L., Parsons P. J., Kao E., Dietert R. R. Exposure to lead during critical windows of embryonic development: Differential immunotoxic outcome based on stage of exposure and gender. Toxicol. Sci. 2001; 64: 57–66
- Byers V. S., Levin A. S., Ozonoff D. M., Baldwin R. W. Association between clinical symptoms and lymphocyte abnormalities in a population with chronic domestic exposure to industrial solvent-contaminated domestic water supply and a high incidence of leukaemia. Cancer Immunol. Immunother. 1988; 27: 77–81
- Camacho I. A., Nagarkatti M., Nagarkatti P. S. Evidence for induction of apoptosis in T-lymphocytes from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 2004; 78: 96–106
- Carlyle J. R., Zuniga-Pflucker J. C. Lineage commitment and differentiation of T- and natural killer lymphocytes in the fetal mouse. Immunol. Rev. 1998; 165: 63–74
- Delgado M., Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit antigen-induced apoptosis of mature T-lymphocytes by inhibiting Fas ligand expression. J. Immunol. 2000; 164: 1200–1210
- Dietert R. R., Piepenbrink M. S. Perinatal immunotoxicity: Why adult exposure assessment fails to predict risk. Environ. Health Perspect. 2006; 114: 477–483
- Ernst D. N., Weigle W. O., Noonan D. J., McQuitty D. N., Hobbs M. V. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T-lymphocytes correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J. Immunol. 1993; 151: 575–587
- Festing M. F. Design and statistical methods in studies using animal models of development. ILAR J. 2006; 47: 5–14
- Fisher J. W., Whittaker T. A., Taylor D. H., Clewell H. J., III, Andersen M. E. Physiologically based pharmacokinetic modeling of the lactating rat and nursing pup: A multiroute exposure model for trichloroethylene and its metabolite, trichloroacetic acid. Toxicol. Appl. Pharmacol. 1990; 102: 497–513
- Ghantous H., Danielsson B. R., Dencker L., Gorczak J., Vesterberg O. Trichloroacetic acid accumulates in murine amniotic fluid after tri- and tetrachloroethylene inhalation. Acta Pharmacol. Toxicol. (Copenhagen) 1986; 58: 105–114
- Gilbert K. M., Griffin J. M., Pumford N. R. Trichloroethylene activates CD4+ T-lymphocytes: Potential role in an autoimmune response. Drug Metab. Rev. 1999; 31: 901–916
- Godfrey D. I., Kennedy J., Suda T., Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 1993; 150: 4244–4252
- Griffin J. M., Blossom S. J., Jackson S. K., Gilbert K. M., Pumford N. R. Trichloroethylene accelerates an autoimmune response by TH1 T-lymphocyte activation in MRL+/+ mice. Immunopharmacology 2000a; 46: 123–137
- Griffin J. M., Gilbert K. M., Lamps L. W., Pumford N. R. CD4(+) T-cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol. Sci. 2000b; 57: 345–352
- Holladay S. D., Lindstrom P., Blaylock B. L., Comment C. E., Germolec D. R., Heindell J. J., Luster M. I. Perinatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p-dioxin (TCDD). Teratology 1991; 44: 385–393
- Holladay S. D., Smialowicz R. J. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000; 108(3)463–473, (Suppl)
- Holladay S. D., Smith B. J. Fetal hematopoietic alterations after maternal exposure to benzo[a]pyrene: A cytometric evaluation. J. Toxicol. Environ. Health. 1994; 42: 259–273
- Holsapple M. P., Burns-Naas L. A., Hastings K. L., Ladics G. S., Lavin A. L., Makris S. L., Yang Y., Luster M. I. A proposed testing framework for developmental immunotoxicology (DIT). Toxicol. Sci. 2005; 83: 18–24
- Huesmann M., Scott B., Kisielow P., von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T-lymphocyte receptor transgenic mice. Cell 1991; 66: 533–540
- Iavicoli I., Marinaccio A., Carelli G. Effects of occupational trichloroethylene exposure on cytokine levels in workers. J. Occup. Environ. Med. 2005; 47: 453–457
- Kilburn K. H., Warshaw R. H. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res. 1992; 57: 1–9
- Kono D. H., Park M. S., Szydlik A., Haraldsson K. M., Kuan J. D., Pearson D. L., Hultman P., Pollard K. M. Resistance to xenobiotic-induced autoimmunity maps to chromosome 1. J. Immunol. 2001; 167: 2396–2403
- Kretz-Rommel A., Rubin R. L. Disruption of positive selection of thymocytes causes autoimmunity. Nat. Med. 2000; 6: 298–305
- Laderach D., Koutouzov S., Bach J. F., Yamamoto A. M. Concomitant early appearance of anti-ribonucleoprotein and anti-nucleosome antibodies in lupus prone mice. J. Autoimmun. 2003; 20: 161–170
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin-4 and interferon-gamma production by neonatal T-lymphocytes. J. Clin. Invest 1991; 87: 194–202
- Lockey J. E., Kelly C. R., Cannon G. W., Colby T. V., Aldrich V., Livingston G. K. Progressive systemic sclerosis associated with exposure to trichloroethylene. J. Occup. Med. 1987; 29: 493–496
- Luster M. I., Portier C., Pait D. G., Rosenthal G. J., Germolec D. R., Corsini E., Blaylock B. L., Pollock P., Kouchi Y., Craig W., White K. L., Munson A. E., Comment C. E. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 1993; 21: 71–82
- Manson J. M., Murphy M., Richdale N., Smith M. K. Effects of oral exposure to trichloroethylene on female reproductive function. Toxicology 1984; 32: 229–242
- Miller T. E., Golemboski K. A., Ha R. S., Bunn T., Sanders F. S., Dietert R. R. Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicol. Sci. 1998; 42: 129–135
- Nakajima T., Wang R. S., Katakura Y., Kishi R., Elovaara E., Park S. S., Gelboin H. V., Vainio H. Sex-, age- and pregnancy-induced changes in the metabolism of toluene and trichloroethylene in rat liver in relation to the regulation of cytochrome P450IIE1 and P450IIC11 content. J. Pharmacol. Exp. Ther. 1992; 261: 869–874
- Peden-Adams M. M., Eudaly J. G., Heesemann L. M., Smythe J., Miller J., Gilkeson G. S., Keil D. E. Developmental immunotoxicity of trichloroethylene (TCE): Studies in B6C3F1 mice. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006; 41: 249–271
- Pollard K. M., Pearson D. L., Hultman P., Deane T. N., Lindh U., Kono D. H. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone bxsb mice. Environ. Health Perspect. 2001; 109: 27–33
- Sebzda E., Mariathasan S., Ohteki T., Jones R., Bachmann M. F., Ohashi P. S. Selection of the T-lymphocyte repertoire. Annu. Rev. Immunol. 1999; 17: 829–874
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985; 37: 269–390
- Vorderstrasse B. A., Cundiff J. A., Lawrence B. P. A dose-response study of the effects of prenatal and lactational exposure to TCDD on the immune response to influenza a virus. J. Toxicol. Environ. Health A 2006; 69: 445–463
- Waller P. A., Clauw D., Cupps T., Metcalf J. S., Silver R. M., Leroy E. C. Fasciitis (not scleroderma) following prolonged exposure to an organic solvent (trichloroethylene). J. Rheumatol. 1994; 21: 1567–1570
- Wilson A., Capone M., MacDonald H. R. Unexpectedly late expression of intracellular CD3ε and TCR γδ proteins during adult thymus development. Int. Immunol. 1999; 11: 164–1650
- Wu C., Schaum J. Exposure assessment of trichloroethylene. Environ. Health Perspect. 2000; 108(2)359–363, (Suppl)
- Zheng X., Gao J. X., Chang X., Wang Y., Liu Y., Wen J., Zhang H., Zhang J., Liu Y., Zheng P. B7-CD28 interaction promotes proliferation and survival but suppresses differentiation of CD4−CD8− T-lymphocytes in the thymus. J. Immunol. 2004; 173: 2253–2261
- Zoller A. L., Kersh G. J. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J. Immunol. 2006; 176: 7371–7378