Abstract
Dichloroacetic acid (DCA) is a by-product of chlorination that occurs in drinking water disinfected with chlorine. Metabolism of trichloroethene (TCE) also generates DCA. TCE exposure is associated with the development of autoimmune diseases, which may be induced by TCE metabolites, such as DCA. Thus, it is important to understand immunotoxic responses to DCA. We chose 2 murine models, autoimmune-prone MRL+/+ and normal B6C3F1 mice. Both strains of mice were exposed to DCA for 12 weeks. Following DCA treatment, liver weights and liver-to-body weight ratios were significantly increased in both strains of mice when compared to their respective controls. The serum activity of alanine and aspartate aminotransferases was not significantly altered in either strain. In MRL+/+ mice, the serum concentrations of IgG and IgM were significantly increased, whereas in B6C3F1 mice, only serum IgG3 was increased. DCA treatment did not change the levels of inflammatory cytokines in the serum. However, independent of treatment, the concentrations of G-CSF in the serum were lower in MRL+/+ mice than in B6C3F1 mice, whereas IL-12 serum levels were higher in MRL+/+ mice. DCA treatment decreased IL-10 and KC chemokine concentrations in the livers of MRL+/+ mice, whereas T-helper cell cytokines (IL-4, IL-5, IL-10, IFNγ, and GM-CSF), pro-inflammatory cytokines (IL-6, IL-12, and G-CSF), and KC chemokine were increased in the livers of DCA-treated B6C3F1 mice. Stimulation of splenic T-lymphocytes with antibodies against CD3 and CD28 resulted in a marked difference in the secreted cytokines between the two strains of mice. T-lymphocytes from MRL+/+ mice secreted more IL-2, IL-4 and IL-10, but less IFNγ and GM-CSF, than did T-lymphocytes from B6C3F1 mice. Thus, the cytokine levels in serum and liver, and the cytokine secretion patterns from stimulated splenic T-lymphocytes suggested a higher propensity of inflammatory responses in B6C3F1 than in MRL+/+ mice. Treatment with DCA also affected lipid accumulation in the liver more severely in B6C3F1 than in MRL+/+ mice. Thus, these results indicate that DCA induced stronger inflammatory responses leading to more severe hepatotoxicity in B6C3F1 mice than in MRL+/+ mice, and more pronounced immune responses in the latter.
INTRODUCTION
Dichloroacetic acid (DCA) is formed as a by-product of water disinfection by chlorine (Uden and Miller, Citation1983; Chen and Weisel, Citation1998; Boorman, Citation1999). Chlorination is the most common method for disinfection of drinking water. Analysis of 35 water treatment facilities across the United States revealed that haloacetic acids are the second most abundant water disinfection by-products. An estimated 150 million people in the United States ingest 2 L/day of chlorinated drinking water (Christman et al., Citation1983) derived from surface or ground water containing DCA (Krasner et al., Citation1989). Therefore, a sizable population is continuously exposed to DCA (Kim et al., Citation1999; Levin et al., Citation2002). A concentration range of 0.38–160 μ g/L of DCA has been reported in U.S. drinking water supplies (Uden and Miller, Citation1983; Chen and Weisel, Citation1998; Kim et al., Citation1999). Oral ingestion via drinking water and, skin absorption and inhalation during showering (Uden and Miller, Citation1983; Kim and Weisel, Citation1998) contribute equally to DCA absorption (Jo et al., Citation1990). The daily intake of DCA could be significant under high exposure conditions where constant exposure to haloacetic acids via drinking waters might be deleterious to human health.
Metabolism of trichloroethene (TCE) also generates DCA (Bruckner et al., Citation1989; IARC, Citation1995). Exposure to TCE can occur through the air via evaporation from metal-degreasing operations or from waste disposal sites and contaminated ground water. TCE has also been found as an indoor contaminant following its use in adhesives, spot removers and carpet cleaning fluids, paint removers, and strippers. It has been found at more than 60% of the 1,428 hazardous waste dump sites proposed for inclusion in the national priority list for remediation (ATSDR, Citation1997). Up to 34% of the United States drinking water supply is contaminated with TCE (ATSDR, Citation1997).
Inhalation or oral exposure to TCE causes damage to the liver, kidney, lungs, and the immune system, and is potentially carcinogenic. DCA itself induces hepatocellular carcinoma (Herren-Freund et al., Citation1987; Bull et al., Citation1990; Pereira, Citation1996; Boorman, Citation1999). Hypomethylation of DNA may represent an epigenetic mechanism in hepatocellular carcinogenesis following DCA exposure (Tao et al., 1998, 2000, Citation2004). DCA has also been used as an investigational drug for metabolic and cardiovascular disorders (Moore et al., Citation1979; Stacpoole and Green, Citation1992; Stacpoole et al., Citation1992; Shangraw et al., Citation1994). However, due to adverse effects in laboratory animals (Stacpoole et al., Citation1979; DeAngelo et al., Citation1991, Citation1996), therapeutic use of DCA has been limited. The toxicity of DCA has been studied in animals, but its immunotoxicity is not known.
We have previously demonstrated that TCE exacerbate autoimmunity in MRL+/+ mice, possibly resulting from its metabolites (Khan et al., Citation1995). Dichloroacetyl chloride, which is immunogenic (Khan et al., Citation1995, Citation1997; Cai et al., Citation2006), is one of the reactive intermediates of TCE metabolism, yielding DCA as a stable metabolite upon hydrolysis. We hypothesized that immunotoxic effects of TCE may be mediated by DCA. Therefore, we evaluated the immunotoxic effects of DCA in autoimmune-prone MRL+/+ mice and compared with normal B6C3F1 mice.
MATERIAL AND METHODS
Animal Treatment
Five-week-old female MRL+/+ and B6C3F1 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were acclimatized for one week in a humidity- and temperature-controlled animal care facility at UTMB which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Lab chow and drinking water were provided ad libitum. DCA (purity 99%) was purchased from Sigma-Aldrich (St. Louis, MO). Mice from each strain were randomly divided into control and treatment groups. Animals were given DCA in the drinking water at 0.5 mg/ml (pH 6.5–7.0, neutralized with 4.0 N NaOH) ad libitum for 12 wk. Control animals received tap water. Water bottles were changed twice weekly, and the water intake was measured.
The average water intake by DCA-treated MRL+/+ mice was 220 μ l/g/d (corresponding to 110 μ g DCA/g/d) compared to 241 μ l/g/d by the control animals. The average water intake by DCA-treated B6C3F1 mice was 184 μ l/g/d (92 μ g DCA/g/d) compared to 195 μ l/g/d by the control mice. Mice were weighed biweekly. At the termination of the experiment, mice were euthanized and blood was withdrawn from the heart. The liver was removed, weighed, and a portion of the liver was immediately stored at −80°C. Serum was obtained and stored in small aliquots at −80°C for further analysis. All experiments were performed in accordance to the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch.
Serum Alanine and Aspartate Aminotransferases
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using kits from Biotron Diagnostics (Hemet, CA). The protocol was modified slightly to adapt it for small amounts of serum (12.5 μ l). Optical density (OD) values were read using a microplate reader (Bio-Rad, Hercules, CA).
Serum Immunoglobulins
Immunoglobulin isotypes (IgG and IgM) and IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3) were quantified using mouse radial immunodiffusion kits (The Binding Site, Birmingham, England). IgE was quantified with an ELISA kit from Bethyl (Montgomery, TX) using a 1:10 diluted serum. Due to the limited availability of the serum, only a single dilution was used. The absorbance was read at 450 nm using a microplate reader (Bio-Rad).
Serum Cytokines
Cytokines [G-CSF, GM-CSF, IL-1α, IL-5, IL-6, IL-10, IL-12 (p40/p70), IFNγ, TNFα, and KC] were measured using multiplex antibody bead kits following the manufacturer's instructions (Biosource, Camarillo, CA). The fluorescence was measured using a Luminex 100 apparatus (Bio-Rad).
Liver Cytokines
A portion of the liver was homogenized in 0.5 mM PBS, pH 7.4 (20 ml/g of liver tissue), and centrifuged at 10,000 × g for 10 min. The supernatant was used for measuring cytokines. Twelve cytokines [G-CSF, GM-CSF, IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p40/p70), IFNγ, TNFα, and KC] were measured using multiplex antibody bead kits (Biosource).
Cytokines Secreted by Activated Splenocytes
Splenocytes were isolated as described earlier (Cai et al., Citation2006). Splenocytes were cultured at 4 × 105 cell/well in flat-bottom 96-well plates, and stimulated with antibodies against CD3 and CD28 as described (Cai et al., Citation2006). The following cytokines were measured in 48-hr culture supernatants using multiplex cytokine analysis: IL-2, IL-4, IL-5, IL-10, IL-12 (p70), GM-CSF, IFNγ, and TNFα
Histopathology and Detection of Proliferating Cell Nuclear Antigen (PCNA)
Livers were fixed in 10% neutral-buffered formalin and stained with hematoxylin and eosin (H & E) for morphological evaluation. The liver tissue sections were also stained with Oil Red O stain for lipid deposition. Digital color images of liver sections of Oil Red O stained area were semi-quantified using a Leica DC Viewer (version 3.0.2.0) image analysis system (Leica Microsystems Inc., Bannickburn, IL). Proliferating cell nuclear antigen (PCNA) staining of the liver sections was performed following the manufacturer's instructions. A biotinylated mouse anti-PCNA primary antibody was used (ZYMED Laboratories Inc., San Francisco, CA).
Statistical Analysis
The data are presented as mean ± standard error of the mean (SEM). For statistical significance, the data were subjected to a two-sided student t-test, and p values < 0.05 were considered statistically significant. For cytokine analyses from serum and stimulated splenocyte cultures, the data were subjected to analysis of variance (ANOVA) followed by a post-hoc test (Least Significant Difference test with Bonferroni correction).
RESULTS
Water Intake, Body and Liver Weight
To monitor the general health status of the mice, water intake and body weight were measured over the course of the experiment. Mice were exposed to DCA for 12 wk, and liver weights were then measured. Water intake decreased over time in both strains of mice, independent of treatment with DCA. In general, MRL+/+ mice consumed ∼ 20% more water than did B6C3F1 mice (not shown). No significant treatment-related body weight changes were observed in either mouse strain (). However, the liver weight and the liver:body weight ratios were significantly increased in both treatment groups ().
TABLE 1 Body weight and liver/body weight ratios of MRL+/+ and B6C3F1 mice treated with DCA
Serum ALT and AST
To detect whether exposure to DCA induced overt liver injury, we measured serum levels of ALT and AST. Enzyme activities of ALT and ASP did not differ significantly between control and treatment groups (data not shown).
Serum Immunoglobulins
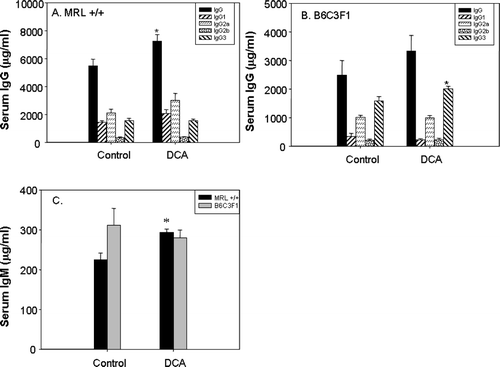
Exposure to TCE metabolites can result in increases in total serum immunoglobulin, indicating activation of the adaptive immune system (Cai et al., Citation2006). Therefore, it was important to determine whether DCA could induce similar changes in immune parameters. In MRL+/+ mice, treatment with DCA resulted in statistically significant increases in total serum immunoglobulins IgG (32% increase over control; ) and IgM (30% increase over control; ). In B6C3F1 mice, only IgG3 was significantly increased by DCA treatment (27% over control; ). Total IgG was elevated, but the difference compared to that in untreated B6C3F1 mice did not reach statistical significance.
Serum Cytokines
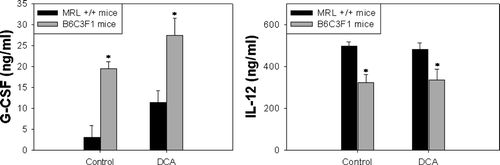
Cytokines are predominantly acting in an autocrine and paracrine manner at the site of production (Feghali and Wright, Citation1997). Furthermore, cytokines exert their effects at specific times during an infection or inflammatory response. Thus, potentially elevated serum levels of inflammatory cytokines may have subsided at later stages in an inflammation. For our study, however, it was also important to understand the differences in inflammatory potential between the two mouse strains used. Treatment with DCA did not significantly alter the concentration of cytokines in the serum from either MRL+/+ or B6C3F1 mice. The concentration of G-CSF was increased in both strains by DCA treatment, however statistical significance was not achieved (). Irrespective of DCA treatment, G-CSF levels were significantly higher in the serum from B6C3F1 mice than in the serum from MRL+/+ mice. On the other hand, the concentration of IL-12 was lower in the serum from B6C3F1 than in the serum from MRL+/+ mice ().
Liver Cytokines
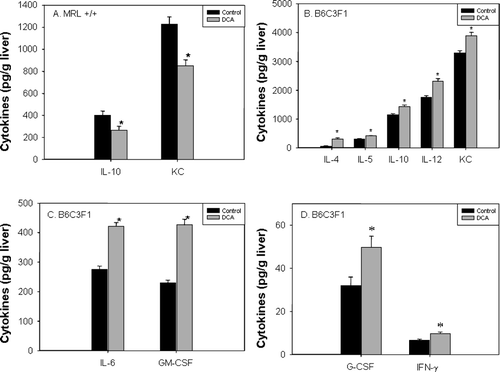
The increase in liver weight in DCA-treated mice indicated potential inflammatory responses. Therefore, we measured the concentration of cytokines in the liver. In MRL+/+ mice, DCA treatment caused a statistically significant decrease in IL-10 (-34%) and KC chemokine (−31%) in the liver (). Other cytokines were not detectable. In the livers of DCA-treated B6C3F1 mice, cytokines characteristic of T-helper (TH) lymphocytes, namely IL-4 (+400%), IL-5 (+33%), IL-10 (+25%), IFNγ (+45%), and GM-CSF (+42%) were significantly increased compared to levels seen in the untreated B6C3F1 mice (–). Also, the inflammatory cytokines IL-6 (+53%), IL-12 (+32%), and G-CSF (+56%), and the KC chemokine (+18%) were significantly increased in the livers of the DCA-treated B6C3F1 mice.
Cytokine Secretion by Activated Splenocytes
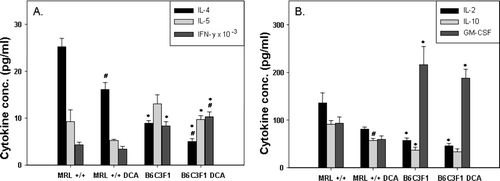
To determine whether DCA treatment preferentially induced the differentiation of specific T-lymphocyte subsets (e.g., TH1 cells, which secrete predominantly IFNγ and IL-2 or TH2 cells, which secrete mainly IL-4, IL-5, and IL-10), we stimulated isolated splenocytes in culture with antibodies against the T-cell receptor component CD3 and against the co-stimulatory receptor CD28 (Cai et al., Citation2006). Antibody binding to CD3 induces T-cell receptor signaling, and antibody binding to CD28 induces a costimulatory signal required for the activation of resting T-lymphocytes (Nishijama et al., Citation2005). We measured cytokines secreted into the culture medium by activated lymphocytes after 48 hr of incubation. Treatment with DCA statistically significantly decreased IL-4 secretion by lymphocytes of both MRL+/+ and B6C3F1 mice (). In cultures from DCA-treated MRL+/+ mice, IL-10 secretion was significantly decreased compared to cultures from untreated MRL+/+ mice (), whereas in cultures from DCA-treated B6C3F1 mice, IFNγ secretion was significantly increased, (). Secretion of several cytokines differed between the two mouse strains, independent of DCA treatment. Secretion of IL-2, IL-4, and IL-10 was significantly lower in cultures from B6C3F1 mice than in cultures from MRL+/+ mice. On the other hand, the pro-inflammatory, lymphocyte-secreted cytokines GM-CSF and IFNγ were significantly increased in cultures from B6C3F1 mice as compared to cultures from MRL+/+ mice. Thus, cytokines secreted by activated T-lymphocytes from MRL+/+ and B6C3F1 mice differed in their inflammatory potential, with MRL+/+-derived lymphocytes secreting predominantly anti-inflammatory factors, and B6C3F1-derived lymphocytes secreting predominantly pro-inflammatory factors.
FIG. 4 Cytokines secreted by splenic T-lymphocytes from MRL+/+ and B6C3F1 mice exposed to vehicle or DCA for 12 wk and activated in culture with antibodies against CD3 and CD8 for 48 hr. The values represent the mean (n = 6); *p < 0.05 for comparisons between mouse strain; #p < 0.05 for comparisons between control and DCA treatment within a strain.
Morphological Changes in the Liver
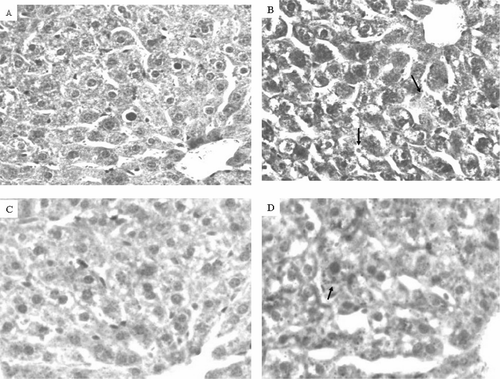
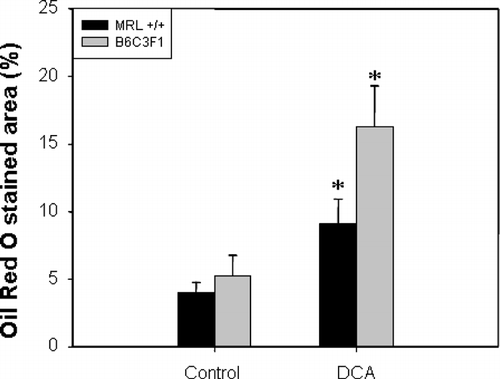
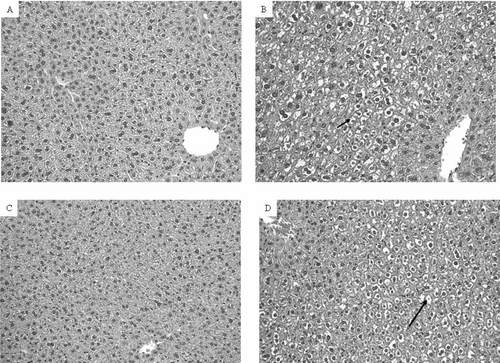
Although overt liver damage as measured by serum concentrations of ALT and AST was not detectable, the increase in liver weights following exposure to DCA suggested that histological changes might have occurred. Such changes could be early indicators of hepatotoxicity. Histological evaluation of the livers showed lipid accumulation within the hepatocytes, compressing and displacing the nucleus to the periphery of the hepatocyte as shown in H&E-stained sections (). Lipid accumulation was especially pronounced in B6C3F1 mice confirmed by Oil Red O staining (). The stained area was 1.3 times as high in DCA-treated MRL+/+ mice and 2.1 times as high in DCA-treated B6C3F1 mice than in their respective controls (). PCNA is a marker of S-phase, and thus can serve as measure for hepatocellular proliferation (Brown et al., Citation2006). PCNA staining was negative in the liver sections (data not shown).
FIG. 5 Liver histopathology of MRL+/+ (A and B) and B6C3F1 mice (C and D) exposed to vehicle (A and C) or DCA (B and D) for 12 wk. Liver sections were stained with H & E. Arrows indicate lipid accumulation within hepatocytes, compressing and displacing the nucleus to the periphery (magnification 200×).
DISCUSSION
Earlier studies from this laboratory showed that TCE and its reactive intermediate DCAC exacerbate autoimmune responses in autoimmune-prone MRL+/+ mice (Khan et al., Citation1995, Citation1997; Cai et al., Citation2006). The metabolism of TCE progresses through hydrolysis of its reactive intermediate DCAC to the stable metabolite DCA (Lash et al., Citation2000). While several studies have been conducted to determine the effects of TCE (Khan et al., Citation1995; Griffin et al., Citation2000a and Citationb; Gilbert et al., Citation2004) or DCAC (Khan et al., Citation1997; Cai et al., Citation2006) on autoimmune responses in susceptible MRL+/+ mice, animal studies with DCA have focused on hepatocarcinogenicity (Bull et al., Citation1990, Citation2002, Citation2004). Here, we evaluated the potential for immunotoxicity and hepatotoxicity of DCA in MRL+/+ mice and in the normal strain B6C3F1. The latter strain was chosen for comparison, because it shares some genetic elements with MRL+/+ mice, which were derived by a series of crosses between C57BL/6J, C3H/HeDi, AKR/J, and LG/J mice.
Conventional toxicity parameters such as liver weight/body weight ratio, and histopathological alterations indicated higher hepatotoxicity of DCA in B6C3F1 mice than in MRL+/+ mice. The immunoglobulins IgG and IgM were increased in MRL+/+ mice following DCA treatment, suggesting immune activation in this strain. Only IgG3 levels were increased significantly in B6C3F1 mice, which is consistent with the higher levels of inflammatory cytokines observed in B6C3F1 mice, because class switching to IgG3 is induced by IFNγ (Snapper et al., Citation1992). Thus, antibody responses in both strains were enhanced by DCA treatment, but IgG subtype distribution suggested differences in cytokine-induced class switching.
The general increase in levels of TH1/TH2 and inflammatory cytokines in liver extracts from B6C3F1 mice following exposure to DCA suggests that inflammation occurred in these mice. Our findings are also consistent with previous reports on the induction (by DCA and trichloroacetic acid) of in vivo hepatocyte replication, which is transient (Stauber and Bull, Citation1997) and occurs in the absence of detectable mitogenic activity in vitro (Walgren et al., Citation2005). Thus, after 12 wk of exposure to DCA, we measured increased liver weights in treated mice, although PCNA staining was negative. Therefore, hepatocyte proliferation may be caused by DCA-induced inflammatory responses in B6C3F1 mice. Compensatory hepatocyte proliferation in response to chronic inflammation is an important step in the development of hepatocellular carcinoma (Maeda et al., Citation2005).
On the other hand, responses to DCA in MRL+/+ mice were not inflammatory in nature, possibly reflecting the higher humoral autoimmune potential of this mouse strain. In a previous investigation, we determined autoimmune responses to the reactive intermediate of TCE, DCAC, and the related acylating agent dichloroacetic anhydride (DCAA) in MRL+/+ mice (Cai et al., Citation2006). IgM levels are decreased in MRL+/+ mice following treatment with DCAC, whereas we found increases in IgM after DCA treatment. In addition, T-lymphocytes from DCAC- or DCAA-treated MRL+/+ mice that are activated in vitro with antibodies against CD3 and CD28 secrete substantially increased levels of inflammatory cytokines, including IFNγ (Cai et al., Citation2006), whereas DCA treatment did not increase the secretion of cytokines by antibody-activated lymphocytes with the exception of IFNγ, which was increased in cultures from DCA treated B6C3F1 mice. These observations suggest that immune responses to DCA were lower compared with responses to DCAC or DCAA. Importantly, we found significant differences between the two strains, MRL+/+ and B6C3F1, in this study. Lymphocytes from the latter strain secreted more IFNγ and GM-CSF, but less IL-2, IL-4, and IL-10, than did lymphocytes from MRL+/+ mice, independent of DCA treatment. Thus, antibody-stimulated lymphocytes from MRL+/+ mice secreted predominantly anti-inflammatory TH2-type cytokines, whereas stimulated lymphocytes from B6C3F1 mice secreted higher levels of pro-inflammatory cytokines.
In conclusion, we found that DCA treatment of MRL+/+ mice enhanced TH2 responses, but induced very little hepatotoxicity, whereas in B6C3F1 mice, hepatotoxicity was a major sequelae of DCA exposure, with no obvious TH2 responses. These findings are important for understanding differential effects of exposure to TCE and its metabolites on human populations. Ongoing experiments evaluate the effects of DCA on hepatic inflammation and cancer in the two mouse strains.
This publication was made possible by grant ES11584 from National Institute of Environment Health Sciences (NIEHS) and its content are solely the responsibility of the authors and do not necessarily represent the view of the NIH or NIEHS. Helpful discussions with Biotransformation and Oxidative Stress Research Core members of UTMB supported by NIHES center grant (P30ES06676), is gratefully acknowledged.
REFERENCES
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Trichloroethylene (Update). United States Department of Health and Human Services, Atlanta, GA 1997; 191
- Boorman G. A. Drinking water disinfection by-products: Review and approach to toxicity evaluation. Environ. Health Perspect. 1999; 107(S1)207–217
- Brown K. E., Mathahs M. M., Broadhurst K. A., Weydert J. Chronic iron overload stimulates hepatocyte proliferation and cyclin D1 expression in rodent liver. Transl. Res. 2006; 148: 55–62
- Bruckner J. V., Davis B. D., Blancato J. N. Metabolism, toxicity, and carcinogenicity of trichloroethylene. CRC Crit. Rev. Toxicol. 1989; 20: 31–50
- Bull R. J., Orner G. A., Cheng R. S., Stillwell L., Stauber A. J., Sasser L. B., Lingohr M. K., Thrall B. D. Contribution of dichloroacetate and trichloroacetate to liver tumor induction in mice by trichloroethylene. Toxicol. Appl. Pharmacol. 2002; 182: 55–65
- Bull R. J., Sanchez I. M., Nelson M. A., Larson J. L., Lansing A. J. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology 1990; 63: 341–359
- Bull R. J., Sasser L. B., Lei X. C. Interactions in the tumor-promoting activity of carbon tetrachloride, trichloroacetate, and dichloroacetate in the liver of male B6C3F1 mice. Toxicology 2004; 199: 169–183
- Cai P., König R., Khan M. F., Qiu S., Kaphalia B. S., Ansari G. A. S. Autoimmune response in MRL+/+ mice following treatment with dichloroacetyl chloride or dichloroacetic anhydride. Toxicol. Appl. Pharmacol. 2006; 21: 248–255
- Chen W. J., Weisel C. P. Halogenated DBP concentrations in a distribution system. J. Am. Water Works Assoc. 1998; 90: 151–163
- Christman C. F., Norwood D. L., Millington D. S., Johnson J. D. Identity and yield of halogenated products of aquatic fulvic acid chlorination. Environ. Sci. Technol. 1983; 17: 625–628
- DeAngelo A. B., Daniel F. B., Most M. M., Olsen G. R. The carcinogenicity of the dichloroacetic acid in the male F344 rat. Toxicology 1996; 114: 207–221
- DeAngelo A. B., Daniel F. B., Stober A. J., Olson G. R. The carcinogenicity of dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1991; 16: 337–347
- Feghali C. A., Wright T. M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997; 2: d12–26
- Gilbert K. M., Whitlow A. B., Pumford N. R. Environmental contaminant and disinfection by-product trichloroacetaldehyde stimulates T-cells in vitro. Int. Immunopharmacol. 2004; 4: 25–36
- Griffin J. M., Blossom S. J., Jackson S. K., Gilbert K. M., Pumford N. R. Trichloroethylene accelerates an autoimmune response by TH1 T-cell activation in MRL+/+ mice. Immunopharmacology 2000b; 46: 123–137
- Griffin J. M., Gilbert K. M., Lamps L. W., Pumford N. R. CD4+ T-cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol. Sci. 2000a; 57: 345–352
- Herren-Freund S. L., Pereira M. A., Khoury M. D., Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol. Appl. Pharmacol. 1987; 90: 183–189
- IARC (International Agency of Research on Cancer). Trichloroethylene. IARC Monographs on the Evaluation of Carcinogenic Ricks to Humans. World Health Organization, LyonFrance 1995; Vol. 63: 75–158
- Jo W. K., Weisel C. P., Lioy P. J. Chloroform exposure and health risk associated with multiple uses of chlorinated water. Risk Anal. 1990; 10: 581–585
- Khan M. F., Kaphalia B. S., Ansari G. A. S. Time-dependent autoimmune response of dichloroacetyl chloride in female MRL+/+ mice. Immunopharmacol. Immunotoxicol. 1997; 19: 265–277
- Khan M. F., Kaphalia B. S., Prabhakar B. S., Kanz M. F., Ansari G. A. S. Trichloroethene-induced autoimmune response in female MRL+/+ mice. Toxicol. Appl. Pharmacol. 1995; 134: 155–160
- Kim H., Haltmeier P., Klotz J. B., Weisel C. P. Evaluation of biomarkers of environmental exposures: Urinary haloacetic acids associated with ingestion of chlorinated drinking water. Environ. Res. Sec. A 1999; 80: 187–195
- Kim H., Weisel C. P. Dermal absorption of dichloro- and trichloroacetic acids from chlorinated water. J. Expos. Anal. Environ. Epidemiol. 1998; 8: 555–575
- Krasner S. W., McGuire M. J., Jacangelo J. G., Patania N. L., Reagan K. M., Aieta E. M. The occurrence of disinfection by-products in U.S. drinking water. J. Am. Water Works Assoc. 1989; 81: 41–53
- Lash L. H., Fisher J. W., Lipscomb J. C., Parker J. C. Metabolism of trichloroethylene. Environ. Health Perspect. 2000; 108(S2)177–200
- Levin R. B., Epstein P. R., Ford T. E., Harrington W., Olson E., Reichard E. G. U.S. drinking water challenges in the twenty-first century. Environ. Health Perspect. 2002; 110(S1)43–52
- Maeda S., Kamata H., Luo J. L., Leffert H., Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121: 977–990
- Moore G. W., Swift L. L., Rabinowitz D., Crofford O. B., Oates J. A., Stacpoole P. W. Reduction of serum cholesterol in two patients with homozygous familial hypercholesterolemia by dichloroacetate. Atherosclerosis 1979; 33: 285–293
- Nishijima K., Ando M., Sano S., Hayashi-Ozawa A., Kinoshita Y., Iijima S. Costimulation of T-cell proliferation by anti-L-selectin antibody is associated with the reduction of a cdk inhibitor p27. Immunology 2005; 116: 347–353
- Pereira M. A. Carcinogenic activity of dichloroacetic acid and trichloroacetic acid in the liver of female B6C3F1 mice. Fundam. Appl. Toxicol. 1996; 31: 192–199
- Shangraw R. E., Winter R., Hromco J., Robinson S. T., Gallaher E. J. Amelioration of lactic acidosis with dichloroacetate during liver transplantation in humans. Anesthesiology 1994; 81: 1127–1138
- Snapper C. M., McIntyre T. M., Mandler R., Pecanha L. M., Finkelman F. D., Lees A., Mond J. J. Induction of IgG3 secretion by interferon gamma: A model for T-cell-independent class switching in response to T-cell-independent Type 2 antigens. J. Exp. Med. 1992; 175: 1367–1371
- Stacpoole P. W., Moore G. W., Kornhauser D. M. Toxicity of chronic dichloroacetate. New Engl. J. Med. 1979; 300: 372
- Stacpoole P. W., Wright E. C., Boumgartner T. G., Bersin R. M., Buchalter S., Curry S. H., Duncan C. A., Harman E. M., Henderson G. H., Jenkinson S., Lachin J. M., Lorenz A., Schneider S. H., Siegel J. H., Summer W. R., Thompson D., Wolfe C. L., Zorovich B., the Dichloroacetate-Lactic Acidosis Study Group. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. New Engl. J. Med. 1992; 26: 1564–1569
- Stauber A. J., Bull R. J. Differences in phenotype and cell replicative behavior of hepatic tumors induced by dichloroacetate (DCA) and trichloroacetate (TCA). Toxicol. Appl. Pharmacol. 1997; 144: 235–246
- Tao L., Yang S., Xie M., Kramer P. M., Pereira M. A. Effect of trichloroethylene and its metabolites, dichloroacetic acid and trichloroacetic acid, on the methylation and expression of c-jun c-myc protooncogenes in mouse liver: prevention by methionine. Toxicol. Sci. 2000a; 54: 399–407
- Tao L., Yang S., Xie M., Kramer P. M., Pereira M. A. Hypomethylation and overexpression of c-jun c-myc protooncogenes and increased DNA mehtyltransferase activity in dichloroacetic and trichloroacetic acid-promoted mouse liver tumors. Cancer Lett. 2000b; 158: 185–193
- Tao L., Li Y., Kramer P. M., Wang W., Pereira M. A. Hypomethylation of DNA and the insulin like growth factor-II gene in dichloroacetic and trichloroacetic acid-promoted mouse liver tumors. Toxicology 2004; 196: 127–136
- Uden P. C., Miller J. W. Chlorinated acids and chloral in drinking water. J. Am. Water Works Assoc. 1983; 75: 524–527
- Walgren J. L., Kurtz D. T., McMillan J. M. Lack of direct mitogenic activity of dichloroacetate and trichloroacetate in cultured rat hepatocytes. Toxicology 2005; 211: 220–230