Abstract
While several skin sensitization tests have been developed and are available as regulatory toxicity tests at present, no such tests for the airway have been established. We have been developing an animal model by introducing an elicitation phase into the mouse IgE test (MIGET) for assessment of agricultural chemicals with airway sensitization potential. In the current study, trimellitic anhydride (TMA), a representative low molecular weight (LMW) airway sensitizer, was examined for its sensitization potential in our mouse model. Mice were epicutaneously sensitized to TMA on Days 0 and 7, followed by an inhalation challenge with TMA dust at high or low concentration on Day 14. Groups of different sensitization route including inhalation were established for comparison of effectiveness of immunization. Non-sensitized animals challenged with TMA dust served as controls. An ovalbumin-sensitized and -challenged animals constituted a reference group (OVA). Enhanced pause (Penh) was measured as an indicator of airflow disturbance by using a restrained flow whole body plethysmograph. The high TMA concentration group exhibited an augmented Penh, elevated IgE values, and pronounced influx of eosinophils into their BAL fluid and minor infiltration of inflammatory cells including eosinophils into the lung. The low TMA concentration group also exhibited elevated IgE values and a less frequent occurrence of minor lung inflammation, but these were not accompanied by any positive responses in Penh and BAL fluid. Almost all mice in the other immunization route groups exhibited negative responses for any parameter examined. The OVA group showed no changes in breathing pattern during the inhalation challenge despite presenting a high total serum IgE value. These results suggest that this mouse model may be useful for assessment of airway sensitization potential of agrochemicals, but by way of epicutaneous sensitization.
INTRODUCTION
In terms of adverse effects, the skin could be the main target organ for agrochemicals when these are manufactured or used in the fields. As a result, these agents are required to be tested in advance of their governmental registration. These tests include those for potential acute dermal toxicities, skin sensitization, and skin irritation, and are in compliance with the regulatory toxicity test guidelines established by the Organization for Economic Co-operation and Development (OECD), the United States Environment Protection Agency (US EPA) and the Japanese Ministry of Agriculture, Forestry, and Fisheries (JMAFF). In contrast, although the airway is also likely to be exposed to agrochemical aerosols, no regulatory tests (other than for inhalation toxicity) to assess potential toxicity to the airways are required. While the number of known respiratory chemical allergens is far fewer than that of contact sensitizers (Karol et al., Citation1996), these allergens could cause severe and fatal bronchial asthma. Thus, it is very important to examine chemicals, including agrochemicals, for their capability to elicit allergic reactions in the airway.
A number of test methods using animal models have been developed for detection of, and research on, the sensitization potential of low molecular weight (LMW) chemicals (Buehler, Citation1965; Magnusson and Kligman, Citation1969; Gad et al., Citation1986; Karol, Citation1987; Botham et al., Citation1989; Arts et al., Citation1998; Regal et al., Citation2001; Herrick et al., Citation2002; Pauluhn, Citation2003; Vanoirbeek et al., Citation2004; Matheson et al., Citation2005). Among them, widely validated tests are those for skin sensitization, and even recently, the local lymph node assay (LLNA; Kimber et al., Citation1989) has been evaluated by the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM; Sailstad et al., Citation2001) and finally adopted in the test guidelines of USEPA and OECD. On the other hand, few predictive tests have been developed for potential airway sensitizers or have undergone full interlaboratory validation studies to date (Holsapple et al., Citation2006).
Under these circumstances, since 1994, the Mouse IgE Test (MIGET; Kimber et al., Citation1996) and the Guinea Pig Test (GPT; Karol, Citation1983; Hayes et al., Citation1992; Larsen and Regal, Citation2002) have been recognized and recommended as the major airway sensitization tests by the European Center for Ecotoxicology and Toxicology of Chemicals (ECETOC; Briatico-Vangosa et al., Citation1994). The MIGET is composed of the induction phase alone in which the airway sensitization potential of a chemical is evaluated by monitoring elevations in total serum IgE antibody values in sensitized mice. The GPT is a form of definitive test (representatively reported by Obata et al., Citation1992) in which airway sensitization is evaluated by parameters such as airway responses (using whole body plethysmography), anti-antigen antibody titers, cytological observations of bronchoalveolar lavage fluid (BALF), and/or histological examinations of the lung.
The MIGET and GPT have both advantages and disadvantages. The MIGET appears to be a screening test and has advantages with respect to cost and time. Conversely, GPT is costly, time-consuming, and tends to vary in its application from researcher to researcher. Concerning the MIGET, it is uncertain how much of any elevation in total serum IgE is indicative of an actual induction of antigen-specific IgE (Arts et al., Citation1997). Another question is why under some experimental conditions, total serum IgE values are elevated in mice treated topically with 2, 4-dinitrochlorobenzene (DNCB; Potter and Wederbrand, Citation1995; Ban and Hettich, Citation2001; Guo et al., Citation2002), a potent contact sensitizer that nearly lacks any airway sensitization potential in humans and therefore has been used as a negative control. These questions may, in part, impair the reliability of MIGET; however, this is the only one test that has been widely validated (Dearman et al., Citation1998) and recognized as a robust test for detecting potential airway sensitizers (ECETOC, 1994).
We have been developing an animal model for assessing airway sensitization effects of agrochemicals. It appears that a MIGET with an elicitation phase will be suitable for such testing. That is, the airway sensitization potential of chemicals could comprehensively be assessed in an animal model by adding to the MIGET original immunological endpoint of total serum IgE values other parameters, such as changes in airway function, BALF cytology, and lung histology. Using these modified approaches, the purpose of the current study was to assess the airway sensitization potential of TMA in a new mouse model.
MATERIALS AND METHODS
Chemicals
Trimellitic anhydride (TMA; > 98.0% purity) and ovalbumin (OVA; practical grade) were purchased from Wako Ltd. (Tokyo, Japan). TMA was pulverized by an air-jet mill (Seishin Enterprise Co., Ltd., Tokyo) before commencement of the study and fine dust (mass median aerodynamic diameter [MMAD] = 5.3 μ m) was used for both sensitization and challenge.
Animals
Specific pathogen-free BALB/c female mice (8-weeks-of-age) were obtained from Charles River Japan Inc. Mice were held in an animal room (6/cage) with controlled temperature (22 ± 3°C), relative humidity (50 ± 20%), ventilation (5–10 times/hr) and illumination (12 hr on/off; lights on 7:00 AM and off 7:00 PM). They were given free access to a basal diet and sterilized well water. After acclimatization for 1 wk, they were randomly assigned to each group (group size, n = 6). All animals were handled in accordance with the guidelines for Animal Experimentation set forth by the Japanese Association for Laboratory Animal Science during the study (JALAS, Citation1987).
Groups and Sensitization Schedules
The various groups and protocols used in the study are shown in . According to the methods of MIGET, mice of the EP/EP group received: 25 μ l of TMA solution (25% [w/v] in acetone:olive oil [4:1, v/v]) on their clipped and shaved flank on Day 0; 12.5 μ l of TMA solution (12.5%) on the dorsum of each auricle on Day 7; and, inhalation challenge with a high (0.022 mg/l) or low (0.0075 mg/l) concentration (two sub-groups; EP/EPhi and EP/EPlo) of TMA on Day 14. Mice in the non-sensitized control group received the same volume of vehicle alone, followed by the inhalation challenge on the same schedule.
TABLE 1 Protocols of sensitization, challenge and measurements for each group
In order to compare the effectiveness of immunization route with the EP/EP group, ID/EP (two sub-groups; ID/EPhi and ID/EPlo), ID/ID and IH/IH groups were established. For intradermal (ID/EP or ID/ID group) sensitization at the abdomen or ears, mice were injected with 0.10 or 0.05 ml of TMA suspension (3% in corn oil). Mice in the IH/IH group were sensitized and challenged with TMA dust via the inhalation route.
In addition to the TMA groups, an OVA group was established as a reference group. Mice were immunized via intraperitoneal injection of 8 μ g OVA dissolved in aluminum hydroxide solution (6 mg/0.5 ml physiological saline) on Days 0 and 5.
Generation of TMA Aerosols and Inhalation Challenge
For the inhalation challenge, TMA dust was incorporated into a dust feeder (Sibata Scientific Technology Ltd., Tokyo) to generate the exposure chamber atmosphere. The dust on the feeder turntable was inspired by an ejector into an up-current of dried compressed air and then dispersed into the chamber equipped with nose-only plethysmographic tubes (SIS-MoR12, Sibata Scientific Technology Ltd.). The chamber concentration of TMA dust in each group was measured throughout the period of their 15-min inhalation challenge.
Before the current study was performed, a preliminary test was conducted to find a maximum TMA dust concentration that did not affect enhanced pause (Penh). Our results showed that 0.03 mg/l was the maximum TMA concentration at which the Penh of female BALB/c mice was not influenced. Frequency of breathing was also monitored; it was shown that 0.01 mg/l was already a high enough concentration to reduce the frequency. Since total IgE values have been found to peak on Day 14 after initial epicutaneous exposure to TMA in the MIGET (Kimber et al, Citation1996), inhalation challenge in the current study was set to take place at the same timepoint. In the OVA group, mice were challenged for 30 min on Day 13 with aerosols of 0.5% OVA solution (in saline) that were generated using an atomizer.
Measurement of Airway Response
Conscious mice were individually kept in the inner-holders of restrained flow whole body plethysmographs (RFWBP) without neck collars. The holder had 26 holes (4 mm in diameter) on its wall for the prevention of rise in body temperature. Each plethysmograph was connected to a nose-only exposure chamber to let the nose of mouse appear into the chamber for exposure to the test atmosphere (SIS-MoR12B, Sibata Scientific Technology Ltd., Tokyo). Each plethysmograph was equipped with a wire mesh pneumotachograph and a differential pressure transducer that converted the variable air pressure into electric signals. These signals were transmitted to an analytical recording system (Biosystem XA, Buxco, New York) wherein many breathing-related parameters could be extracted using attached software.
Prior to initiating the current study, validation of this system took place by measuring all of the proposed respiratory parameters with three different strains of mice (BALB/c, DBA/2 and C57BL/6) weekly over the period of their being 6–10 weeks-of-age, and then examining the effects of carbachol (0.05 M) for 15 min when the mice were 11 weeks-of-age. These latter studies demonstrated drastic changes in the measured parameters of the BALB/c and DBA/2 mice, but were only moderate in the C57BL/6 strain. Through these experiments, it was concluded that this system used in the current study could be applicable for examination of the respiratory responses to TMA inhalation challenge in mice. [Validation data are available upon request from the corresponding author.]
In the current study, breathing patterns of each mouse were monitored and recorded every 1 min during the periods of 5-min pre-challenge, 15-min challenge, and 40-min post-challenge. Mice in the EP/EP group were also monitored for their airway responses at 24- and 48-hr post challenge. Before each measurement, all plethysmographs were individually calibrated by infusion of 1 ml air by syringe. On monitoring the breathing patterns at TMA inhalation challenge, we selected Penh as an indicator of disturbed respiration. Penh is a dimensionless value calculated from other respiratory parameters and has been considered to be an indicator of airway resistance.
However, it has recently been recognized that Penh obtained from the unrestrained pressure whole body plethysmographs is related to ventilatory timing and unrelated to airway resistance (Lundblad et al., Citation2002; Bates et al., Citation2004). On the other hand, Pauluhn and Morh (Citation2005) have recommended a restrained volume displacement (flow type) whole plethysmograph as an ideal instrument to measure respiratory patterns and more recently Lomask (Citation2006) has demonstrated that Penh can be derived from the flow whole body plethysmograph waveform. Frequency of breathing was also monitored as another parameter of airway response to TMA inhalation challenge.
Measurement of Total Serum IgE Values
Two days after the inhalation challenge, all mice were anesthetized with intraperitoneal injection of sodium pentobarbital (70 mg/kg body weight) and blood samples withdrawn from the posterior vena cava. The blood was centrifuged at 1700 rpm for 10 min at 4°C and the recovered serum kept at −80°C until assayed. Total serum IgE values were measured with a sandwich enzyme-linked immunosorbent assay (ELISA) kit (i.e., OptEIA™ Mouse IgE Set; Pharmingen, San Diego, CA). Optical densities in these assays were read at 450 nm on a NJ-2300 microplate reader (Nalge Nunc International K.K., Tokyo).
Bronchoalveolar Lavage (BAL) Fluid
After completing the blood sampling, mice in the EP/EP, ID/EP and non-sensitized control groups were subjected to BAL fluid (BALF) analysis. Briefly, the lungs were gently lavaged twice with 0.8 ml of 5% FCS (FCS:PBS:heparin [10:189:1, v/v/v]) warmed to 37°C. The two BALF fractions were pooled and placed on ice. BALF from each animal was then centrifuged at 1700 rpm for 10 min at 4°C. The supernatant was removed and the cell pellet resuspended in 400 μ l of RPMI/5% FCS (19:1) for cell counts and differentials (200 μ l each).
Total cell numbers were counted using a Thoma's hemocytometer. Half of the re-suspension was cytocentrifuged (Cytospin, Shandon Inc., Pittsburgh, PA) at 1000 rpm for 5 min to prepare slide smears of the BAL cells. The smears were fixed and stained with Wright's stain and 200 cells in each sample examined to differentiate the numbers of macrophages, eosinophils, and neutrophils present (according to standard leukocyte typing).
Histopathology of the Lung
After the lavaging steps, the lungs of each mouse were fixed in situ with 0.8 ml of 10% neutral-buffered formalin. The tissues were then embedded in paraffin, sectioned as a whole at a thickness of 4 μ m, and stained with hematoxylin and eosin. A pathologist then examined blindly the lung preparations via light microscopy.
Statistical Analysis
The statistical significance of induced changes in Penh within each group was determined with the paired Student's t-test. One-way ANOVA, followed by Dunnett's multiple comparison test or by Dunnett type non-parametric multiple comparison test, was used to analyze the total serum IgE values and BALF data. Data were considered significant at p < 0.05.
RESULTS
Airway Responses
Remarkable changes in Penh were hardly observed during TMA challenge except for in the EP/EPhi group (). Penh was significantly increased only in the EP/EPhi group during and after the challenge period (). Nevertheless, in the other groups, one animal each in the ID/EPhi and IH/IH groups ever displayed augmented Penh. No changes were recorded in Penh in any animal in the EP/EPhi and EP/EPlo groups at 24 or 48 hr post-challenge.
FIG. 1 Penh values in pre-challenge, challenge, and post-challenge periods. Conscious mice were individually held in a restrained flow whole body plethysmograph and enhanced pause (Penh) was recorded every 1 min during 5 min pre-challenge, 15 min challenge, and 40 min post-challenge periods using an analytical recording system. Mice were challenged with fine TMA dust. Symbols represent airway responses of 6 mice/group.
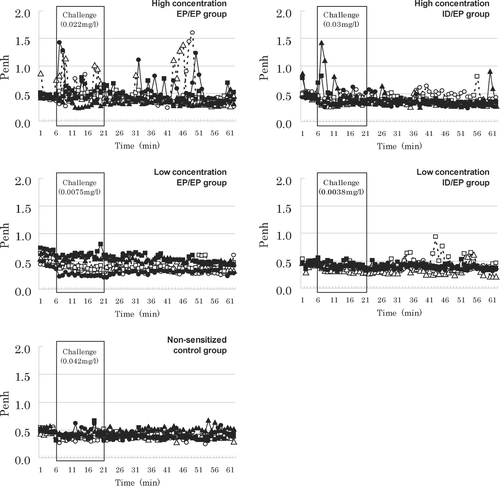
TABLE 2 Enhanced pause (Penh) before, during and after challenge
Frequency of breathing was reduced in all mice including the non-sensitized controls during the period of the 15-min inhalation challenge (data not shown). While the EP/EPhi group exhibited the greatest reduction in the ratio of frequency (i.e., During:Before challenge), magnitude of significant difference in the frequency was the same in all of the groups. In the OVA group, there were no notable changes in Penh either during or after the challenge period.
Total Serum IgE Values
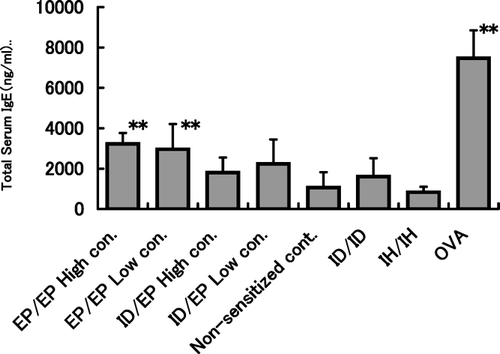
Total serum IgE values were significantly elevated in the EP/EPhi, EP/EPlo and OVA groups as compared with the non-sensitized controls (). Although total IgE values were sporadically increased in mice in the other treated groups, there were no statistically significant differences in group mean values as compared with that in the control group.
FIG. 2 Total serum IgE values in each group. Two days after inhalation challenge, 6 mice/group were anesthetized with pentobarbital and blood samples drawn from the posterior vena cava. Total serum IgE values were measured with a sandwich enzyme-linked immunosorbent assay (ELISA). ** p < 0.01 compared with non-sensitized control mice.
BALF Analysis
BALF analyses were performed on each animal in the EP/EP, ID/EP and non-sensitized control groups. There were no statistically significant differences in the total cell counts between any of the TMA groups and the control (). Regarding the differential cell counts, the percentage of eosinophils in the BAL of the EP/EPhi group was significantly increased as compared with controls (). In contrast, the percentage of macrophages in these EP/EPhi animals was significantly decreased. Other groups showed nearly the same differential cell count patterns as those in the control group.
TABLE 3 BALF analyses in the EP/EP, ID/EP and Non-sensitized control groups
Histopathology of the Lung
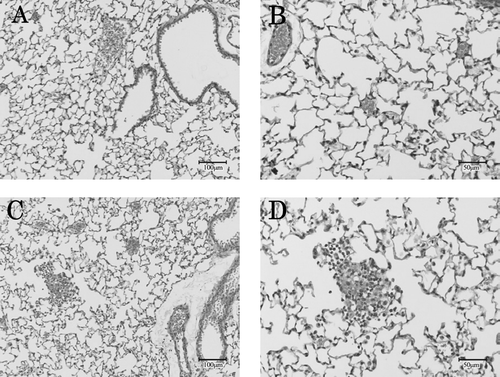
Minor cell infiltration (including eosinophils) into the airspace and perivascular portion was observed only in the EP/EP groups, with incidences of 6/6 in the EP/EPhi and 3/6 in the EP/EPlo groups ().
FIG. 3 Representative pulmonary region of lungs. Pulmonary regions from non-sensitized control group (A, B) and from EP/EPhi group (C, D) 48 hr after challenge with TMA dust. A cell infiltration (including eosinophils) into the airspace and perivascular portions was observed only in TMA-sensitized and -challenged mice.
DISCUSSION
MIGET is a widely validated rational, practical, and rapid test to predict potential LMW airway sensitizers. However, because the positive endpoint of the test is based only an elevation in total serum IgE values, it may be recognized as a screening test as compared with the GPT. While using the whole animal, MIGET needs only the serum and, as a result, may waste other useful physical materials or data. We therefore planed to develop a mouse model for LMW airway sensitizers by adding an elicitation phase to the framework of MIGET so that allergic reaction-related responses could be obtained and the sensitizing potential of chemicals comprehensively assessed.
For the above-mentioned purpose, it would be desirable if the animal model could reproduce the major characteristics of human bronchial asthma. These would include: constrictive reactions at inhalation challenge by antigen; elevated serum total or specific IgE antibody values; eosinophil influx into the airways, and lung inflammation. Although a lot of effort has been made by many researchers to develop animal models of LMW airway sensitizers, primarily using guinea pigs, rats, or mice that might manifest these desired “human” characteristics, few models have been successful in presenting all of them in a single response as yet.
In the current study, we used a restrained flow whole body plethysmograph (RFWBP) through which it was possible to monitor the respiration pattern of mice in real-time during an inhalation challenge. As compared with the well known unrestrained pressure whole body plethysmograph, RFWBP may have the following advantages; confounding effects due to habituation and grooming do not occur, the flow-derived breath structure can be better appreciated (Pauluhn and Mohr, Citation2005). To our knowledge, this is for the first time to report disturbed respiration induced in mice during and after challenge with free TMA dust. As an alternative route to inhalation exposure at challenge, intranasal or intratracheal instillation of chemicals has also been employed (Ebino et al., Citation1998; Scheerens et al., Citation1999; Regal et al., Citation2001; Herrick et al., Citation2002; Larsen and Regal, Citation2002; Sailstad et al., Citation2003; Farraj et al., Citation2004; Vanoirbeek et al., Citation2004). There are, however, some limitations to real-time monitoring of physiological respiration patterns when using these methods.
Regarding intranasal challenge, the animals need to lose the epiglottis reflex to aspirate the chemicals into the trachea and the lung. Anesthesia of the animals is inevitably required to create this condition, but the resultant unconsciousness presents a non-physiological condition.
In addition, chemicals can hardly travel beyond the epiglottis when they are dissolved in non-aqueous vehicles like oil (Ebino et al., Citation1999; Southam et al., Citation2002). Even if the chemicals are successfully aspirated as an aqueous solution under anesthesia, it would be seen that regular slow and deep anesthetic respiration is disturbed just after the aspiration and an irregular respiration would begin abruptly and last for a while. These factors make it difficult to distinguish any allergic respiratory responses from this disturbed respiration. Herrick et al. (Citation2002) were successful in demonstrating a marked lung inflammation in their mouse model of hexamethylene diisocyanate (HDI) by using an HDI-MSA conjugate in PBS for challenge. To date, this was the only successful mouse model of LMW chemical-induced asthma using intranasal instillation to demonstrate an allergic-type lung inflammation.
Intratracheal instillation is another method for challenge. Although lung inflammatory changes are readily induced via this route, non-specific acute neutrophilic inflammation generally tends to occur (Ebino et al., Citation1998; Sailstad et al., Citation2003). In any case, as with aspiration, the instillation approach has a disadvantage in that real-time monitoring of the change in respiration patterns during antigen challenge is often very hard to discern.
Among the endpoints necessary for assessment of LMW airway sensitizers, the manifestation of an allergic airway response upon antigen challenge may occupy an important part (Pauluhn, Citation2003). As has already been used and validated in a rat model for assessment of airway hypersensitivity of TMA (Pauluhn et al., Citation2002; Pauluhn, Citation2003), it was suggested in this current study that a RFWBP could also be available for assessment of disturbed respiration in the mouse.
In the EP/EPhi group, 3/6 mice showed augmented Penh shortly after the initiation of the 15 min inhalation challenge and again during the 40 min post challenge period. These three mice and one other showed elevated total serum IgE values and influx of eosinophils into the BALF at their 48 hr post-challenge examinations, exhibiting a high correlation among the endpoints. Histopathology of the lung revealed the presence of inflammatory cells in the pulmonary regions of each of the 6 mice; however, the degrees of the inflammation were minor. This may be attributable to the relatively large particle size of TMA dust (mass median aerodynamic diameter [MMAD] = 5.3 μ m).
That is, it is likely that a relatively large portion of particles may have been trapped in the nasal cavity due to impaction and that the remaining small amount of TMA that could have reached to the pulmonary region scarcely induced only minor lung inflammation. If this was the case, disturbed respiration in the upper airways may have contributed more to the induction of the augmented Penh than that in the lower airways, although it is not known how both airways shared the obstructive response to the inhaled antigens (Vanoirbeek et al., Citation2006). If finer TMA dust could be available in the future, it may be expected that our model could demonstrate major asthmatic characteristics more clearly. Thus, some of the findings of no augmented Penh, no recovery of eosinophils from the BALF, and less frequent lung inflammation despite high total serum IgE values in the EP/EPlo group could be explained using this same reason (in addition to the matter of threshold at inhalation challenge with TMA as reported by Zhang et al., Citation2004).
In the ID/EPhi group, although one animal showed an augmented Penh with a slightly higher IgE value, examination of the BALF and lung specimen failed to demonstrate positive observations. Similar observations were obtained with animals in both the ID/ID and IH/IH groups in which neither Penh nor IgE values were significantly elevated. TMA concentration used for the intradermal sensitization was 3% according to the protocol by Regal et al. (Citation2001). As compared with the IgE values in the study of Regal et al., those in the ID/EP and ID/ID groups were not too different, indicating that the weak airway and lung responses in these groups were attributable to the lower total serum IgE values than those noted in the EP/EPhi group. In the IH/IH group, as discussed above, a large portion of TMA particles may have been lost from the airways by being trapped in the nasal cavity; this would result in production of only a small amount of IgE and no induction of airway responses. Overall, these results indicate the importance of the particle size and the amount of chemicals inhaled, as well as the superiority of the epidermis as an effective route for systemic sensitization in our model (as has been reported by others, i.e., Pauluhn et al., Citation2002; Warbrick et al., Citation2002).
Frequency of breathing was reduced in both TMA sensitized and non-sensitized groups. In addition, the reduction was common to the sensitized groups regardless of Penh values, indicating that the reduction was attributable to the irritation of TMA as has been reported in the rat study (Arts et al., 2001; Pauluhn et al., Citation2002). It has also been indicated that decreases or increases in the respiratory rate are not necessarily accompanied with changes in Penh but rather are independently regulated (Hamelmann et al., Citation1997; Zhang et al., Citation2004). As the frequency of breathing was reduced in some of the TMA sensitized groups at lower concentrations than that necessary for induction of augmented Penh in the EP/EPhi group, it was likely that the changes in these two parameters of the respiratory response were induced by different mechanism(s) in our mouse model as well.
In the current study, the OVA group was established in order to clinically observe an immediate-type allergic airway response. Despite of an elevated group mean IgE value of more than 7000 ng/ml, neither an immediate-type airway constriction nor augmented Penh was observed in any animal during the half-hour challenge period. The result was in accordance with those demonstrated by Dohi et al. (Citation1999) and To et al. (Citation2001). These authors reported that a typical immediate asthmatic response was not observed in their mouse model and Penh increased gradually and reached a maximal value at 24 hr after OVA-challenge.
On the other hand, Hessel et al. (Citation1995) reported that an immediate bronchoconstrictive response was evoked during the OVA-challenge in sensitized mice. Cieslewicz et al (Citation1999) found that an airway hyperresponsiveness was induced 5–30 min after OVA-challenge in sensitized mice. It is likely that the major causes of the abovementioned differences in the airway response to OVA-challenge are attributable to the different protocols for sensitization and challenge employed in each study and our procedures were less aggressive.
Granting that the mouse is a species that does not readily respond to inhaled antigens nor develop spontaneous acute bronchoconstriction in sensitized individuals due to its poorly developed bronchial smooth muscles (Pauluhn and Morh, Citation2005; Wenzel and Holgate, Citation2006), it was of interest that TMA, a hapten, was able to induce an immediate-type airway response upon challenge that was not produced by OVA, a complete antigen used in our model. According to Regal (Citation2004), bronchoconstriction in response to antigen is the result of smooth muscle contraction and airway narrowing, and possible factors of airway narrowing are edema, increased vascular permeability and mucus production. It is still necessary for us to clarify via what mechanism(s) Penh was augmented after TMA inhalation challenge in our model. Nevertheless, basic mechanistic studies on TMA-induced immediate-type allergic symptoms or the immunopathogenesis of TMA-asthma are likely to be advanced using this mouse model.
In summary, the studies reported here indicate possibility in detection of the airway sensitization potential of TMA in a mouse model. Specifically, this model demonstrated augmented Penh upon TMA inhalation challenge, elevated total serum IgE values, pronounced influx of eosinophils into airways (and so, in their BALF), and minor infiltration of inflammatory cells (including eosinophils) into the lung, with a high correlation among all these parameters. These results permit us to suggest that the first step in the comprehensive assessment of the airway sensitization potential of agrochemicals may now be taken using this model. We also expect that our mouse model may be applicable (through some technical improvements) to the mechanical study of TMA-asthma or LMW chemical-induced airway hypersensitivity.
The authors gratefully acknowledge Koichi Hayashi for his technical assistance. This study was supported by a grant from the Japanese Ministry of Agriculture, Forestry and Fisheries.
REFERENCES
- Arts J. H., Droge S. C., Spanhaak S., Bloksma N., Penninks A. H., Kuper C. F. Local lymph node activation and IgE responses in brown Norway and Wistar rats after dermal application of sensitizing and non-sensitizing chemicals. Toxicology 1997; 117: 229–234
- Arts J. H., Kuper C. F., Spoor S. M., Bloksma N. Airway morphology and function of rats following dermal sensitization and respiratory challenge with low molecular weight chemicals. Toxicol. Appl. Pharmacol. 1998; 152: 66–76
- Ban M., Hettich D. Relationship between IgE-positive cell numbers and total serum IgE levels in mice treated with trimellitic anhydride and dinitrochlorobenzene. Toxicol. Lett. 2001; 118: 129–137
- Bates J., Irvin C., Brusasco V., Drazen J., Fredberg J., Loring S., Eidelman D., Ludwig M., Macklem P., Martin J., Milic-Emili J., Hantos Z., Hyatt R., Lai-Fook S., Leff A., Solway J., Lutchen K., Suki B., Mitzner W., Paré P., Pride N., Sly P. The use and misuse of Penh in animal models of lung disease. Am. J. Respir. Cell Mol. Biol. 2004; 31: 373–374
- Botham P. A., Rattray N. J., Woodcock D. R., Walsh S. T., Hext P. M. The induction of respiratory allergy in guinea pigs following intradermal injection of trimellitic anhydride: A comparison with the response to 2,4-dinitrochlorobenzene. Toxicol. Lett. 1989; 47: 25–39
- Briatico-Vangosa G., Braun C. L., Cookman G., Hofmann T., Kimber I., Loveless S. E., Morrow T., Pauluhn J., Sorensen T., Niessen H. J. Respiratory allergy: Hazard identification and risk assessment. Fundam. Appl. Toxicol. 1994; 23: 145–158
- Buehler E. V. Delayed contact hypersensitivity in the guinea pig. Arch. Dermatol. 1965; 91: 171–177
- Cieslewicz G., Tomkinson A., Adler A., Duez C., Schwarze J., Takeda K., Larson K. A., Lee J. J., Irvin C. G., Gelfand E. W. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Invest. 1999; 104: 301–308
- Dearman R. J., Basketter D. A., Blaikie L., Clark E. D., Hilton J., House R. V., Ladics G. S., Loveless S. E., Mattis C., Sailstad D. M., Sarlo K., Selgrade M. K., Kimber I. The mouse IgE test: Inter-laboratory evaluation and comparison of BALB/c and C57BL/6 strain mice. Toxicol. Meth. 1998; 8: 69–85
- Dohi M., Tsukamoto S., Nagahori T., Shinagawa K., Saitoh K., Tanaka Y., Kobayashi S., Tanaka R., To Y., Yamamoto K. Non-invasive system for evaluating the allergen-specific airway response in a murine model of asthma. Lab. Invest. 1999; 79: 1559–1571
- Ebino K., Kramarik J., Lemus R., Karol M. H. A mouse model for study of localized toluene diisocyanate adducts following intrabronchial administration of the chemical: Inflammation and antibody production. Inhal. Toxicol. 1998; 10: 503–529
- Ebino K., Lemus R., Karol M. H. The importance of the diluent for airway transport of toluene diisocyanate following intranasal dosing of mice. Inhal. Toxicol. 1999; 11: 171–185
- Farraj A. K., Harkema J. R., Kaminski N. E. Allergic rhinitis induced by intranasal sensitization and challenge with trimellitic anhydride but not with dinitrochlorobenzene or oxazolone in A/J mice. Toxicol. Sci. 2004; 79: 315–325
- Gad S. C., Dunn B. J., Dobbs D. W., Reilly C., Walsh R. D. Development and validation of an alternative dermal sensitization test: The mouse ear swelling test (MEST). Toxicol. Appl. Pharmacol. 1986; 84: 93–114
- Guo T. L., Zhang X. L., Leffel E. K., Peachee V. L., Karrow N. A., Germolec D. R., White K. L., Jr. Differential stimulation of IgE production, STAT activation and cytokine and CD86 expression by 2,4-dinitrochlorobenzene and trimellitic anhydride. J. Appl. Toxicol. 2002; 22: 397–403
- Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen L., Irvin C. G., Gelfand E. W. Non-invasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care. Med. 1997; 156: 766–775
- Hayes J. P., Daniel R., Tee R. D., Barnes P. J., Taylor A. J., Chung K. F. Bronchial hyperreactivity after inhalation of trimellitic anhydride dust in guinea pigs after intradermal sensitization to the free hapten. Am. Rev. Resp. Dis. 1992; 146: 1311–1314
- Herrick C. A., Xu L., Wisnewski A. V., Das J., Redlich C. A., Bottomly K. A novel mouse model of diisocyanate-induced asthma showing allergic-type inflammation in the lung after inhaled antigen challenge. J. Allergy Clin. Immunol. 2002; 109: 873–878
- Hessel E. M., Van Oosterhout A. J. M., Hofstra C. L., De Bie J. J., Garssen J., Van Loveren H., Verheyen A. K.C.P., Savelkoul H. F. J., Nijkamp F. P. Bronchoconstriction and airway hyperresponsiveness after ovalbumin inhalation in sensitized mice. Eur. J. Pharmacol. 1995; 293: 401–412
- Holsapple M. P., Jones D., Kawabata T. T., Kimber I., Salro K., Selgrade M. K., Shah J., Woolhiser M. R. Assessing the potential to induce respiratory hypersensitivity. Toxicol. Sci. 2006; 91: 4–13
- JALAS. Japanese Association for Laboratory Animal Science. Guidelines for animal experimentation. Exp. Animal 1987; 36: 285–288
- Karol M. H. Concentration-dependent immunologic response to toluene diisocyanate (TDI) following inhalation exposure. Toxicol. Appl. Pharmacol. 1983; 68: 229–241
- Karol M. H. The development of an animal model for TDI asthma. Bull. Eur. Physiopathol. Respir. 1987; 23: 571–576
- Karol M. H., Graham C., Gealy R., Macina O. T., Sussman N., Rosenkranz H. S. Structure-activity relationships and computer-assisted analysis of respiratory sensitization potential. Toxicol. Lett. 1996; 86: 187–191
- Kimber I., Hilton J., Weisenberger C. The murine local lymph node assay for identification of contact allergens: A preliminary evaluation of in situ measurement of lymphocyte proliferation. Contact Dermat. 1989; 21: 215–220
- Kimber I., Hilton J., Basketter D. A., Dearman R. J. Predictive testing for respiratory sensitization in the mouse. Toxicol. Lett. 1996; 86: 193–198
- Larsen C. P., Regal J. F. Trimellitic anhydride (TMA) dust induces airway obstruction and eosinophilia in non-sensitized guinea pigs. Toxicology 2002; 178: 89–99
- Lomask M. Further exploration of the Penh parameter. Exp. Toxicol. Pathol. 2006; 57: 13–20
- Lundblad L., Irvin C., Adler A., Bates J. A re-evaluation of the validity of unrestrained plethysmography in mice. J. Appl. Physiol. 2002; 93: 1198–1207
- Magnusson B., Kligman A. M. The identification of contact allergens by animal assay. The guinea pig maximization test. J. Invest. Dermatol. 1969; 52: 268–276
- Matheson J. M., Johnson V. J., Vallyathan V., Luster M. I. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol. Sci. 2005; 84: 88–98
- Obata H., Tao Y., Kido M., Nagata N., Tanaka I., Kuroiwa A. Guinea pig model of immunologic asthma induced by inhalation of trimellitic anhydride. Am. Rev. Respir. Dis. 1992; 146: 1553–1558
- Pauluhn J., Eidmann P., Freyberger A., Wasinska-Kempka G., Vohr H. W. Respiratory hypersensitivity to trimellitic anhydride in Brown Norway rats: A comparison of endpoints. J. Appl. Toxicol. 2002; 22: 89–97
- Pauluhn J. Respiratory hypersensitivity to trimellitic anhydride in Brown Norway rats: Analysis of dose-response following topical induction and time course following repeated inhalation challenge. Toxicology 2003; 194: 1–17
- Pauluhn J., Mohr U. Experimental approaches to evaluate respiratory allergy in animal models. Exp. Toxicol. Pathol. 2005; 56: 203–234
- Potter D. W., Wederbrand K. S. Total IgE antibody production in BALB/c mice after dermal exposure to chemicals. Fundam. Appl. Toxicol. 1995; 26: 127–135
- Regal J. F., Mohrman M. E., Sailstad D. M. Trimellitic anhydride-induced eosinophilia in a mouse model of occupational asthma. Toxicol. Appl. Pharmacol. 2001; 175: 234–242
- Regal J. F. Immunologic effector mechanisms in animal models of occupational asthma. J. Immunotoxicol. 2004; 1: 25–37
- Sailstad D. M., Hattan D., Hill R. N., Stokes W. S. ICCVAM evaluation of the murine local lymph node assay. I. The ICCVAM review process. Regul. Toxicol. Pharmacol. 2001; 34: 249–257
- Sailstad D. M., Ward M. D., Boykin E. H., Selgrade M. K. A murine model for low molecular weight chemicals: Differentiation of respiratory sensitizers (TMA) from contact sensitizers (DNFB). Toxicology 2003; 194: 147–161
- Scheerens H., Buckley T. L., Muis T. L., Garssen J., Dormans J., Nijkamp F. P., Van Loveren H. Long-term topical exposure to toluene diisocyanate in mice leads to antibody production and in vivo airway hyperresponsiveness three hours after intranasal challenge. Am. J. Respir. Crit. Care Med. 1999; 159: 1074–1080
- Southam D. S., Dolovich M., O'byrne P. M., Inman M. D. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am. J. Physiol. 2002; 282: L833–L839
- To Y., Dohi M., Tanaka R., Sato A., Nakagome K., Yamamoto K. Early interleukin 4-dependent response can induce airway hyperreactivity before development of airway inflammation in a mouse model of asthma. Lab. Invest. 2001; 81: 1385–1396
- Vanoirbeek J. A., Tarkowski M., Ceuppens J. L., Verbeken E. K., Nemery B., Hoet P. H. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxicol. Sci. 2004; 80: 310–321
- Vanoirbeek J. A., Tarkowski M., Vanhooren H. M., De Vooght V., Nemery B., Hoet P. H. Validation of a mouse model of chemical-induced asthma using trimellitic anhydride, a respiratory sensitizer, and dinitrochlorobenzene, a dermal sensitizer. J. Allergy Clin. Immunol. 2006; 117: 1090–1097
- Warbrick E. V., Dearman R. J., Kimber I. IgG and IgE antibody responses following exposure of Brown Norway rats to trimellitic anhydride: Comparison of inhalation and topical exposure. Toxicology 2002; 172: 157–168
- Wenzel S., Holgate ST. The mouse trap: It still yields few answers in asthma. Am. J. Respir. Crit. Care Med. 2006; 174: 1173–1178
- Zhang X. D., Fedan J. S., Lewis D. M., Siegel P. D. Asthmalike biphasic airway responses in Brown Norway rats sensitized by dermal exposure to dry trimellitic anhydride powder. J. Allergy Clin. Immunol. 2004; 113: 320–326
