Abstract
In rodents, the Plaque Assay, T-dependent antibody response to sheep erythrocytes (SRBC), has been reported to be a sensitive and predictive functional immune assay for detecting immunomodulatory compounds. However, various laboratories have chosen to use ELISA-based assays for evaluating the primary immune response in rodents. The ELISA-based assays offer several advantages over the Plaque Assay, which make them attractive for use in immunotoxicological evaluations. Among the most popular antigens used in the ELISA-based assays are SRBC and more recently KLH. While the Plaque Assay and the ELISA-based assays are both capable of evaluating the humoral immune response, they are measuring different endpoints. The Plaque Assay focuses primarily on splenic effects. ELISA-based assays, which use serum from immunized animals, are holistic in nature in that these assays measure effects of antibody production on the spleen, lymph nodes, and bone marrow. Depending on the drug or compound evaluated, different effects and degrees of sensitivity can be seen with the Plaque Assay and ELISA-based assays. One recent finding is that the sensitizing dose of KLH used in the KLH ELISA differentially affects the responses observed in rodents. Even within the same species, different strains of mice and rats produce different magnitudes of responses to the same sensitizing dose. A key component of this discussion focuses on the sensitivity of the Plaque Assay as compared to KLH ELISA-based assays. These assays were evaluated by comparing the response obtained following administration of several known immunosuppressive agents, including cyclophosphamide, azathioprine, cyclosporine A and dexamethasone. The effects on the primary IgM immune response in the B6C3F1 mice, the primary immunotoxicological rodents used by National Toxicology Program, and in the Sprague–Dawley rat, the primary rodent models used by industry are addressed.
INTRODUCTION
As discussed by the prior presentations, the antibody-forming cell response to a T-dependent antigen (TDAR) has been the primary functional assay requested by both the Food and Drug Administration (FDA, Citation2002) and the Environmental Protection Agency (EPA, Citation1998) for evaluation of potential immunomodulation. Evaluation of a TDAR is multifaceted. The assay can be evaluated using one of multiple endpoints including: the antibody-forming cell (AFC) response to sheep erythrocytes (SRBC), also referred to as the Plaque Assay, since clear areas (plaques) occur as the result of complement-mediated lysis of the SRBC which make up the red background; Enzyme-Linked Immunoabsorbency Assay (ELISA); and ELISPOT enumeration, to list a few. By far the Plaque Assay and ELISA are the most commonly used. In addition to the endpoint evaluated, multiple T-dependent antigens are available and utilized in immunotoxico-logical evaluations. Among those most often used include SRBC, keyhole limpet hemocyanin (KLH), chicken gamma globulin and tetanus toxiod (Luster et al., Citation1988; Temple et al., Citation1993; Smith et al., Citation2003; Gore et al., Citation2004). Even when the same T-dependent antigen, e.g., KLH, is utilized, the amount used to sensitize the rodent for evaluation of the primary antibody response can have marked differences on the results obtained (Peachee et al., Citation2005).
In the presentation by Bugelski and Kim, a Meta-Analysis approach was used to evaluate TDAR test results across multiple laboratories retrospectively, using positive control data from prior studies. Accordingly, the doses utilized were often not the same, nor was the route of administration, duration of exposure, or the expertise level of those conducting the studies. Collection and evaluation of the data were major undertakings with the results from multiple laboratories contributing to the data sets evaluated. In order to make the data meaningful, statistical transformations were required before the data could be evaluated in the Meta-Analysis. The Meta-Analysis studies compared the TDAR data, using multiple compounds and two T-dependent antigens, SRBC and KLH.
In the presentation by Ladics (this workshop, and Loveless et al., Citation2002, Citation2003), two test compounds were evaluated with one T-dependent antigen, SRBCs. The object of this work was to compare the sensitivity of the Plaque Assay to that of the SRBC ELISA in both ICR outbred mice and the Sprague–Dawley outbred rats. Unlike the Meta-Analysis approach, which made use of preexisting data, the study reported by Ladics was undertaken specifically to compare the 2 assays and was conducted in 4 different laboratories, each of which had extensive experience in conducting both assays. In the Meta-Analysis study, the authors concluded that there were no differences in the outcome of the SRBC and KLH studies with known immunosuppressive drugs, administered as “positive controls” in their studies.
In contrast, in the “head-to-head” comparison of the Plaque Assay and the SRBC ELISA, Ladics reported that the Plaque Assay was more sensitive than the SRBC ELISA for both the rat and mouse in evaluating the primary IgM response. These studies presented here compared the sensitivity of the Plaque Assay to the KLH ELISA in a “head-to-head” evaluation in the same laboratory and also evaluated what the role of the sensitizing dose of KLH had on the sensitivity of the primary (IgM) KLH ELISA results in mice and rats.
General Study Design
In conducting these studies, female B6C3F1 mice, the National Toxicology Program's (NTP) designated strain for immunotoxicological evaluation, were used. In the rat studies, the Sprague–Dawley outbred rat, one of the most common rats used by industry for immunotoxicological evaluations, was utilized. For each of the endpoints evaluated, i.e., Plaque Assay and KLH ELISA, the peak response day determined in our laboratory was used. In mice and rats, Day 4 (sensitized on Monday, sacrificed on Friday) was the peak response day for the SRBC Plaque Assay.
In mice, the peak IgM response to KLH occurred on Day 5 and, in rats, the peak response was observed on Day 6 in our laboratory. Similar to the approach reported by Ladics (this workshop, and Loveless et al., Citation2002, Citation2003), test compounds were administered, starting on the day of sensitization and once daily throughout the period of antibody response development. Accordingly, the animals in which the Plaque Assay was evaluated received four days of test compound exposure; mice evaluated in the KLH ELISA received five days of exposure; and rats evaluated in the KLH ELISA received six days of treatment. Thus, the mice evaluated by ELISA received one additional day of exposure and the rats two additional days as compared to the animals utilized in the Plaque Assay. The test compounds were evaluated at multiple dose levels and represented immunosuppressive compounds for which different mechanism of actions were responsible for the suppression observed.
In the Plaque Assay two endpoints are evaluated. The first is the number of antibody forming cells (AFC) per one million spleen cells. This endpoint is referred to as “Specific Activity.” The second endpoint evaluates the data expressed as the number of AFC per spleen. This endpoint represents “Total Spleen Activity.” It is critical in evaluating a compound for potential immunotoxicity to evaluate both of these endpoints. For example, if a compound produces its immunotoxic effect as a result of lysis of lymphocytes, which is observed with high doses of corticoid steroids in humans and at low dose levels in rodents, Specific Activity can be unaffected (Paul, Citation1993).
In other words, the few remaining plasma cells from treated animals can produce antibodies at the same rate as plasma cells from vehicle control animals. However, since there are less total spleen cells present and thus less plasma cells, as a result lymphocyte lysis, Total Spleen Activity will be dramatically reduced. When this occurs, one routinely sees a decrease in spleen weight and spleen cell number. Thus evaluating only Specific Activity can result in a false negative finding. In the studies presented here, similar results were observed in the compounds tested in both Specific Activity and Total Spleen Activity with the exception of mice treated with dexamethozone.
As indicated here, the potent glucocorticoid, dexametho-zone, produces immunosuppression in rodents as a result of lymphocyte lysis. While the Specific Activity results are not affected, a dose related decrease in Total Spleen Activity is observed. These results are consistent with those reported by Ladics (this workshop and Loveless et al., Citation2003).
In the KLH ELISA a single endpoint for the IgM primary antibody response is evaluated. This is the concentration of IgM anti-KLH antibody in the serum. The advantage and disadvantages of both the Plaque Assay and the KLH ELISA have been addressed previously (White, Citation2005).
Comparison of the Sensitivity of the Plaque Assay and KLH ELISA in Mice
Shown in are the results in B6C3F1 mice using four known immunosuppressive compounds in man, cyclophosphamide (CPS), azathioprine, cyclosporine A, and dexamethasone. In our initial studies with KLH, different amounts of KLH were evaluated for their ability to elicit a good response following intravenous (IV) sensitization. In these studies, concentrations of 30, 50, 100, 200, and 300 μ g per animal were evaluated to determine the optimum sensitizing concentration. It was observed that increasing amounts of KLH produced increased responses until a plateau was reached in which 100, 200, and 300 μ g per animal produced similar IgM responses (Peachee et al., Citation2005).
FIG. 1 Left panel: B6C3F1 female mice were sensitized intravenously (IV) with SRBC and evaluated on Day 4 in the Plaque Assay. Data are presented as specific activity (IgM AFC/106 spleen cells) or total spleen activity (IgM AFC/spleen). Middle panel: Animals were sensitized IV with 100 μ g/animal of KLH and serum collected on Day 5. Serum levels of IgM anti-KLH antibody were determined by ELISA. Right panel: Animals were sensitized IV with 2 mg/animal of KLH and serum collected on Day 5. Serum levels of IgM anti-KLH antibody were determined by ELISA. Results are expressed as percent of vehicle control. *p ≤ 0.05 or **p ≤ 0.01 vs. vehicle treated animals. Each group consisted of eight animals.
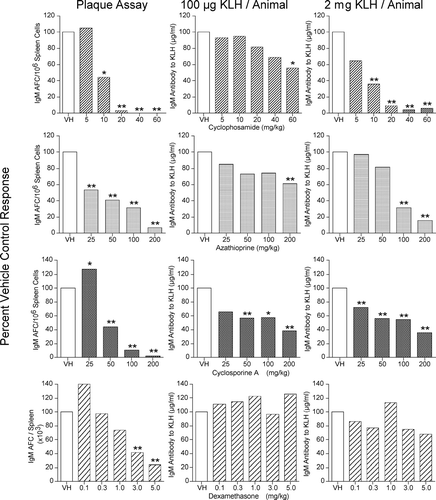
Accordingly, we utilized 100 μ g/animal as our sensitizing dose consistent with what other researchers were reporting in the peer reviewed literature (Smith et al., Citation2003; Gore et al., Citation2004). However, when we conduced additional studies in rodents with higher sensitizing amounts of KLH, similar to those used in dogs and monkeys (Finco-Kent and Kawabata Citation2005; Piccotti et al., Citation2005), even greater levels of antibody production were elicited. In addition, greater sensitivity to the immunosuppressive effects of the test compounds was observed when a 2 mg per animal amount of KLH was used as the sensitizing antigen in rodents (Peachee et al., Citation2005).
When CPS was evaluated in the Plaque Assay statistically significant immunosuppression was observed at doses of 10 mg/kg and above. In the KLH ELISA, following sensitization with 100 μ g/animal, a dose related decrease was observed, however, only the 60 mg/kg dose level reached the level of statistical significance (p ≤ 0.05). This decrease in sensitivity was observed even though the animals used in the ELISA determinations had received an additional day of drug administration. With CPS similar results were observed in the degree of immunosuppression when the Plaque Assay results were compared to the KLH ELISA, following sensitization with 2 mg/animal of KLH. Initially, based on these results, we felt that we would be able to use the KLH assay in lieu of the Plaque Assay, if we used 2 mg/animal of KLH for sensitization.
Although the KLH ELISA assay following the 2 mg/animal sensitization produced similar results as the Plaque Assay following treatment with CPS, when we evaluated the 2 assays following treatment of the mice with azathioprine, the Plaque Assay showed statistically significant suppression following 25 mg/kg in contrast to the KLH (2 mg/animal) ELISA, where statistically significant suppression was only observed at doses of 100 mg/kg and greater. When the animals were sensitized with 100 μ g/animal statically significant suppression was only observed at the highest dose, 200 mg/kg. Bear in mind that the animals in the KLH ELISA assay received an additional day of azathioprine treatment.
The Plaque Assay results with cyclosporine A in the mice were interesting in that at the lowest dose, 25 mg/kg, a statistically significant enhancement was observed, followed by a dose-response decrease at the other higher dose levels. In the KLH (100 μ g/animal) ELISA, a significant decrease was observed at dose levels at and above 50 mg/kg, however, the magnitude of the observed suppression at the higher dose levels was less than that observed in the Plaque Assay. Similarly, in the KLH (2 mg/animal) ELISA, a dose-dependent decrease was observed at all dose levels; however, the magnitude of the observed suppression was also less than that observed in the Plaque Assay. The enhanced response observed in the Plaque Assay may be due to the high sensitivity of the T-regulatory cells to immunosuppressive compounds (Lutsiak et al., Citation2005). However, additional studies would be needed to confirm this as the mechanism of the enhanced responses observed at the low dose levels.
When the glucocorticoid dexamethasone was evaluated in the Plaque Assay as Total Spleen Activity, again enhancement was observed with the lowest dose followed by a dose-dependent suppression. However, only the two highest dose levels, 3.0 and 5.0 mg/kg, reached the level of statistical significance. When dexamethasone was evaluated in the KLH ELISA, animals sensitized with 100 μ g/animal and the animals sensitized with 2 mg/animal failed to detected dexamethasone as being an immunosuppressive compound. These results are consistent with the dexamethasone SRBC ELISA data reported by Ladics (this workshop and Loveless et al., Citation2003). The failure of the ELISA to detect suppression following dexamethasone exposure with two different T-dependent antigens, contributes to the concern of using only the ELISA as the primary or only evaluation of potential immunosuppression of drugs and chemicals.
Comparison of the Sensitivity of the Plaque Assay and KLH ELISA in Rats
The results of two rat studies are presented. In our initial evaluation of KLH in the rat, we observed in the inbred Fischer 344 (F344) rat no increase in anti-KLH antibody production occurred following sensitization with 2 mg/animal as compared to 100 μ g/animal or 500 μ g/animal. In contrast, sensitization of SD rats with 2 mg/animal produced five times the amount of serum anti-KLH IgM antibody than occurred with only 100 μ g/animal. In addition, a much more robust response was observed in the outbred Sprague–Dawley (SD) rats than the F344 rats.
Shown in the top panels of are the Plaque and ELISA results, following treatment of SD rats with cylophosphamide. In the Plaque Assay, immunosuppression was observed at 5 and 10 mg/kg. In the KLH (100 μ g/animal) ELISA, an enhanced response was observed at the lower dose levels of 0.5 and 1.0 mg/kg; however, the increase did not reach the level of statistical significance. Immunosuppression was observed at the 10 mg/kg dose level in this ELISA. In animals sensitized with 2 mg/animal, dose-related enhancement was observed, reaching the level of statistical significance at the 5 mg/kg dose level of cyclophosphamide. Again, the enhancement observed may be due to the increased sensitivity of the T-regulatory to immunosuppressive compounds (Lutsiak et al., Citation2005). In the rat studies, the animals in the ELISA evaluations received six days of treatment in comparison to the four days of treatment received by the animals used in the Plaque Assay.
FIG. 2 Left panel: Sprague–Dawley (SD) female rats were sensitized with SRBC and evaluated on Day 4 in the Plaque Assay. Data are presented as specific activity (IgM AFC/106 spleen cells). Middle panel: Animals were sensitized IV with 100 μ g/animal of KLH and serum collected on Day 6. Serum levels of IgM anti-KLH antibody were determined by ELISA. Right panel: Animals were sensitized IV with 2 mg/animal of KLH and serum collected on Day 6. Serum levels of IgM anti-KLH antibody were determined by ELISA. Results are expressed as percent of vehicle control. *p ≤ 0.05 or **p ≤ 0.01 vs. vehicle-treated animals. Each group consisted of 7–8 animals.
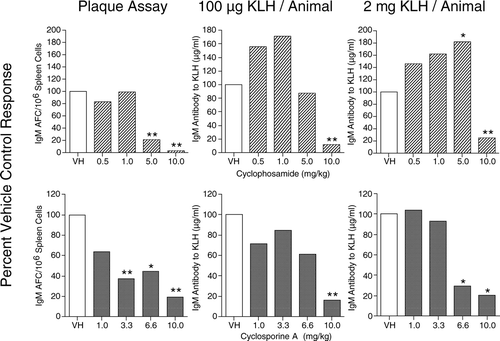
In the rat cyclosporine A study, all dose levels of cyclosporine A produced immunosup-pression, with dose levels of 3.3 mg/kg and greater being statistically significant. In the 6-day KLH (100 μ g/animal) ELISA, statistical immunosuppression was only observed at the highest dose—10 mg/kg. In the KLH (2 mg/animal) ELISA, both the 6.6 and the 10 mg/kg doses produced statistically significant suppression following six days of cyclosporine A treatment.
CONCLUSION
As was observed in the studies presented by Ladics at this workshop, as well as (Loveless et al. Citation2002, Citation2003) and Peachee et al. (Citation2005), the Plaque Assay was more sensitive than the KLH ELISA in both mice and rats. Furthermore, the use of 2 mg/animal enhanced the sensitivity of the KLH ELISA assay over that observed when 100 μ g/animal was used as the sensitizing amount of KLH. The enhanced sensitivity to immunosuppression with the higher sensitization dose of KLH in mice was not as dramatic as that observed in the rat.
Why is the SRBC plaque response more sensitive than the KLH ELISA response? Several possible explanations have been previously hypothesized (White, Citation2005). However, a fundamental concept, which may, in part, contribute to the difference in sensitivity, lies in the inherent nature of the humoral immune response. The purpose of the immune system is to maintain homeostasis and protect the host from pathogens. Since most pathogens are particulate in nature, as are sheep erythrocytes, this T-dependent antigen may have a natural advantage over a soluble protein such as KLH. Granted the body has the ability to and makes antibody to soluble proteins, e.g., tetanus toxin and neutralizing antibody to numerous soluble proteins. However, the majority of such antibodies tend to be of the IgG class, indicative of a secondary response and not IgM antibodies routinely observed in early primary antibody responses.
If the IgM KLH ELISA is utilized as a primary “screen” for potential immunomodulatory compounds or as the only functional assay used in evaluating compounds for potential immunotoxicity, the interpretations of the results need to be evaluated in the total context of “weight of evidence” for an effect on the immune system. Included in this “weight of evidence” should be, among others parameters, lymphoid organ weights and histopathology. These parameters would need to be evaluated in relation to doses that produce suppression of the antibody responses. If serum antibody levels are evaluated and lymphoid tissue or hematology are not, then immunosuppression may be missed in studies in which high doses are not used.
With low sensitivity being observed in the primary IgM anti-KLH antibody response alone, a false negative finding may results. As presented here with potent immunosuppressive drugs, the KLH ELISA may potentially identify a compound as not being immunosuppressive, when, in fact, it is.
REFERENCES
- EPA. United States Environmental Protection Agency. Health Effects Test Guidelines: Immunotoxicity. 1998, http://www.epa.gov/opptsfrs/publications OPPTS 870.7800
- FDA. United States Food and Drug Administration. Guidance for Industry: Immunotoxi-cology Evaluation of Investigational New Drugs. 2002, http//www.fda.gov/cder/guidance
- Finco-Kent D., Kawabata T. T. Development and validation of a canine T-cell-dependent antibody response model for immunotoxicity evaluation. J. Immunotoxicol. 2005; 2: 197–201
- Gore E. R., Gower J., Kurali E., Sui J. L., Bynum J., Ennulat D., Herzyk D. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology 2004; 197: 23–35
- Loveless S. E., Ladics G. S., Smith C., Holsapple M. P., Woolhiser M. R., Anderson P. K., White K. L., Jr., Musgrove D. L., Smialowicz R. J., Williams W. Inter-laboratory study of primary antibody response to sheep red blood cells in outbred rodents. The Toxicologist/Toxicol. Sci. 2002; 66(S1)238
- Loveless S. E., Ladics G. S., Smith C., Holsapple M. P., Woolhiser M. R., Anderson P. K., White K. L., Jr., Musgrove D. L., Smialowicz R. J., Williams W. Inter-laboratory study of primary antibody response to sheep red blood cells in outbred rodents following exposure to dexamethasone. The Toxicologist/Toxicol. Sci. 2003; 72(S1)104
- Luster M. I., Munson A. E., Thomas P. T., Holsapple M. P., Fenters J. D., White K. L., Jr., Lauer L. D., Germolec D. R., Rosenthal G. J., Dean J. H. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 1988; 10: 2–19
- Lutsiak C. M. E., Semnani R. T., De Pascalis R., Kashmiri S. V. S., Schlom J., Sabzevari H. Inhibition of CD4+25+ T-regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005; 105: 2862–2868
- Fundamental Immunology, 3rd Ed., W. E. Paul. Raven Press, New York 1993
- Peachee V. L., Sheth C. M., Halpen A. M., White K. L. Sensitivity of the keyhole limpet hemocyanin (KLH) ELISA model is directly related to dose used for sensitization. The Toxicologist/Toxicol. Sci. 2005; 84(S1)178
- Piccotti J. R., Alvey J. D., Reindel J. F., Guzman R. E. T-Cell-dependent antibody response: Assay development in cynomolgus monkeys. J. Immunotoxicol. 2005; 2: 191–196
- Smith H. W., Winstead C. J., Stank K. K., Halstead B. W., Wierda D. A predictive F344 rat immunotoxicology model: Cellular parameters combined with humoral response to NP-Cγ G and KLH. Science Direct Toxicology 2003; 194: 129–145
- Temple L., Kawabata T. T., Munson A. E., White K. L., Jr. Comparison of ELISA and plaque-forming cell assay for measuring the humoral immune response to SRBC in animals treated with benzo(a)pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993; 21: 412–419
- White K. L., Jr. Plaque versus ELISA assays. Evaluation of humoral immune responses to T-dependent antigens. Encyclopedic Reference of Immunotoxicology, H. W. Vohr. Springer, New York 2005; 505–508
