Abstract
The primary objective of this study was to investigate the effects of cobalt (Co2 +), palladium (Pd2 +), platinum (Pt4 +) and vanadium (V2 +, V3 +, V4 + and V5 +) on the ability of the neutrophil chemoattractants C5a and IL-8, as well as the pneumococcal toxin, pneumolysin, to activate human neutrophils in vitro. Neutrophil activation was determined according to the magnitude of the increase in cytosolic Ca2 + concentrations using a fura-2/AM-based, spectrofluorimetric procedure, as well as by a chemotaxis assay using modified Boyden chambers. In initial screening studies, in which the metals were used at a fixed concentration of 25 μ M, the Ca2 +-mobilizing interactions of C5a, IL-8, and pneumolysin were unaffected by exposure to Co2 +, Pt4 + and V2 + − 5 +. However, exposure of C5a, IL-8, and pneumolysin to Pd2 + resulted in either partial (IL-8) or complete (C5a and pneumolysin) loss of Ca2 + -mobilizing and chemotactic activities. In dose-response experiments, these effects of Pd2 + were detectable at a threshold concentration of 6.5 μ M. These observations demonstrate that exposure to Pd2 + may compromise innate host defenses, a previously unrecognized potential health threat of environmental and/or occupational exposure to a ubiquitous heavy metal.
INTRODUCTION
Occupational and possibly environmental exposure to heavy metals is often associated with an increased frequency of respiratory symptoms, including rhinitis, wheezing, dyspnea, nasal hemorrhage, conjunctivitis, cough and sore throat, all of which are usually transient (Hughes, Citation1980; Seiler and Sigel, Citation1988; Goering, Citation1992; Ballach, Citation1997; Barceloux, Citation1999; Van Klavaren and Nemery, Citation1999; Merget and Rosner, Citation2001; Kielhorn et al., Citation2002; Brock and Stopford, Citation2003; Linna et al., Citation2003). However, persistent bronchial hyperresponsiveness, asthma, and small airways dysfunction have also been described (Swennen et al., Citation1993; Niezborala and Garnier, Citation1996; Irsigler et al., Citation1999; Mapp et al., Citation1999). These adverse effects of heavy metals on the airways result not only from immunological sensitization (Calverley et al., Citation1995; Raulf-Heimsoth et al., Citation2000), but also from irritant interactions of the metals with airway epithelium and phagocytes, resulting in the production of pro-inflammatory cytokines and reactive oxidant species (Shishodia et al., Citation1997; Wang et al., Citation2003; Ramafi et al., Citation2004; Theron et al., Citation2004; Fickl et al., Citation2006).
While their pro-allergenic and pro-irritant actions are relatively well characterized, almost nothing is known about the possible adverse effects of some heavy metals on innate host defenses operative against commonly encountered microbial pathogens. In the current study, we have investigated the effects of heavy metals of industrial and environmental significance, viz. cobalt, palladium, platinum and vanadium on the biological activities of C5a and interleukin-8 (IL-8), two key neutrophil-mobilizing chemoattractants generated by cells of the innate immune system. In addition, we have also investigated the effects of these metals on the neutrophil-activating potential of the cholesterol binding, pore-forming toxin, pneumolysin, which is produced by Streptococcus pneumoniae (Andrew et al., Citation2000) one of the major human pathogens, and one of the most common causes of community-acquired pneumonia, otitis media, sinusitis, and meningitis (Cockeran et al., Citation2003). Notwithstanding its ability to activate synthesis of pro-inflammatory cytokines via interactions with Toll-like receptor 4 on inflammatory cells (Malley et al., Citation2003), pneumolysin also initiates the generation of C5a and IL-8 as a consequence of its complement-activating and pore-forming properties, respectively (Mitchell and Andrew, Citation2000; Cockeran et al., Citation2002; Fickl et al., Citation2005; Van Rossum et al., Citation2005; Ratner et al., 2006).
MATERIALS AND METHODS
Chemicals and Reagents
Cobalt (II) chloride hexahydrate (Co2 +), platinic chloride [hydrogen hexachloroplatinate (IV) (Pt4 +)], palladium (II) chloride (Pd2 +), vanadium (II) chloride (V2 +), vanadium (III) chloride (V3 +), vanadyl sulfate hydrate (V4 +), and sodium metavanadate (V5 +) were purchased from Sigma-Aldrich (St Louis, MO). These were dissolved in distilled water to give stock concentrations of 10 mM and used in the assays described below at a maximum concentration of 25 μ M for each metal.
Recombinant human C5a and IL-8 were also purchased from Sigma-Aldrich, while recombinant pneumolysin was prepared as described previously (Saunders et al., Citation1989). C5a, IL-8, and pneumolysin were reconstituted in indicator-free Hanks' balanced salt solution (HBSS, pH 7.4, containing 1.25 mM CaCl2) to stock concentrations of 4, 2.5 and 4.2 μ g/ml, respectively.
Exposure of C5a, IL-8, and Pneumolysin to the Metals
C5a, IL-8, and pneumolysin at fixed concentrations of 2.0, 1.25, and 2.1 μ g/ml, respectively (in the Ca2 + experiments) and at concentrations of 1 μ g/ml (in the chemotaxis experiments) were co-incubated with each metal at a fixed concentration of 25 μ M for 1 min at 37°C after which the various activators were added either directly to neutrophils, or to the lower compartments of modified Boyden chambers, and evaluated for their abilities to elevate cytosolic Ca2 + or to induce a chemotactic response, respectively. In these assays, the final concentrations of C5a, IL-8, and pneumolysin were 25, 12.5, and 20 ng/ml, respectively (Ca2 + experiments) and 100 ng/ml (chemotaxis experiments), which represents a 1:100 and 1:10 dilution of each agent in the respective Ca2 + flux and chemotaxis experiments. The final concentration of each metal was 0.25 μ M, a level which was not cytotoxic according to measurement of ATP levels in neutrophils exposed to the metals for 30 min at 37°C (data not shown).
Neutrophils
This study was approved by the Faculty of Health Sciences Research Ethics Committee of the University of Pretoria (Protocol No. 43/2006). Prior informed consent was obtained from all blood donors, all of who were healthy, non-smoking, medication-free adult, human volunteers. A comprehensive health status questionnaire was used prior to venepuncture to screen for those factors that may alter the functional responses of neutrophils.
Purified neutrophils were prepared from heparinized (5 units of preservative-free heparin/ml) venous blood and separated from mononuclear leukocytes by centrifugation on Histopaque-1077 (Sigma Diagnostics) cushions at 400×g for 25 min at room temperature. The resultant pellet was suspended in phosphate-buffered saline (PBS, 0.15 M, pH 7.4) and sedimented with 3% gelatin to remove most of the erythrocytes. After centrifugation, erythrocytes were removed by selective lysis with 0.84% ammonium chloride at 4°C for 10 min. The neutrophils (which were routinely of high purity [> 95%] and viability [> 95%]) were suspended to 1 × 107/ml in PBS and held on ice until used.
Spectrofluorimetric Measurement of Cytosolic Ca2 +
Fura-2/AM was used as the fluorescent Ca2 + sensitive indicator for these experiments (Grynkiewicz et al., Citation1985). Neutrophils (1 × 107/ml) were incubated with fura-2/AM (0.5 μ M) for 25 min at 37°C in PBS, washed, and resuspended in HBSS. The fura-2-loaded cells (1 × 106/ml) were then pre-incubated for 10 min at 37°C, after which they were transferred to disposable reaction cuvettes, which were maintained at 37°C in a Perkin Elmer LS 45 luminescence spectrometer with excitation and emission wavelengths set at 340 and 500 nm, respectively. After a stable baseline was obtained (± 1 min), the neutrophils were activated by addition of the metal-treated or –untreated (control) chemoattractants or pneumolysin as described above, and alterations in cytosolic Ca2 + concentrations monitored over a 5 min time course. Peak cytosolic Ca2 + concentrations (nM) were calculated as described previously (Grynkiewicz et al., Citation1985).
To ensure that the metals at the residual concentrations of 0.25 μ M (following 1:100 dilution of the chemoattractants/pneumolysin) did not affect the reactivity of neutrophils in the Ca2 + mobilization assay, fura-2-loaded neutrophils were pre-incubated with Pd2 + at a final concentration of 0.25 μ M for 5 min at 37°C. Cytosolic Ca2 + fluxes were then monitored following addition of C5a, IL-8, or pneumolysin (25, 12.5, and 20 ng/ml, respectively) to the cells.
Assay of Neutrophil Migration
For these investigations neutrophils were suspended to a concentration of 3 × 106/ml in HBSS supplemented with 0.1% bovine serum albumin (BSA). Modified Boyden chambers in which the upper (cell) and lower (chemoattractant) chambers were separated by an 8 μ M pore-size membrane filter (Sartorius-membrane filter, Göttingen, Germany) were used to assess neutrophil migration (Anderson et al., Citation1984). Cell suspensions (200 μ l containing 6 × 105 neutrophils) were added to the upper chamber, while 1 ml of the metal-treated or -untreated chemoattractant (C5a or IL-8 at final concentrations of 100 ng/ml in BSA-supplemented HBSS) was added to the lower chamber. The chemoattractants were omitted from random migration systems, i.e., BSA-supplemented HBSS only in the lower compartment of the Boyden chamber. The fully-assembled chambers were then incubated for 45–60 min at 37°C, after which the filters were detached, fixed, stained and cleared, and the results expressed as the number of cells that had completely traversed the filter per microscope high-powered field (cells/HPF) as an average of 6 filters for each system for each experiment.
Expression and Statistical Analysis of Results
The results of each series of experiments are presented as the mean values ± standard errors of the means (SE), with the exception of some of the spectrofluorimetric determinations of cytosolic Ca2 + for which the traces are also shown. Statistical analysis of data was performed using the Mann-Whitney U-test, and ANOVA where appropriate. A computer-based software system was used for analysis and a p value of < 0.05 was taken as significant.
RESULTS
Effects of Prior Exposure to the Metals on C5a-, IL-8, and Pneumolysin-Mediated Alterations in Neutrophil Cytosolic Ca2 + Concentrations
These results are shown in , , and . In control, metal-free systems, exposure of neutrophils to C5a and IL-8 was accompanied by the typical, abruptly occurring increase in cytosolic Ca2 + which attained maximum values within 10–20 sec and declined steadily thereafter, returning to basal/or close-to-basal values after about 3 min and 2 min in the case of C5a and IL-8, respectively ( and ).
TABLE 1 Effects of pre-treatment with Co2 +, Pd2 +, Pt4 +, V2 +, V3 +, V4 +, V5 + (all at 25 μ M) on the Ca2 +-mobilizing interactions of C5a, IL-8, and pneumolysin with neutrophils
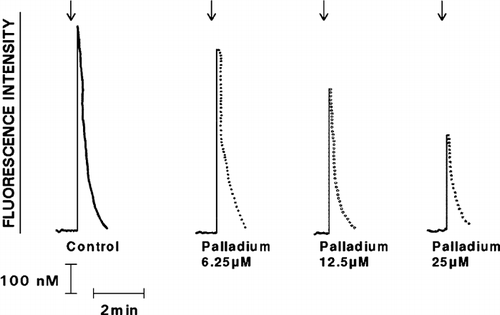
FIG. 1 Effect of exposure to Pd2 + (25 μ M) on the Ca2 +-mobilizing interactions of C5a with neutrophils shown as the fura-2 fluorescence traces of 2 representative experiments (5 in the series). The responses of cells exposed to untreated, or to Pd2 +-treated C5a (added as denoted by the arrow ↓) are shown on the left and right sides of each pair of traces, respectively.
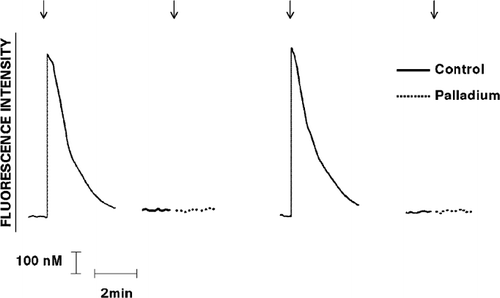
FIG. 2 Effect of exposure to Pd2 + (25 μ M) on the Ca2 +-mobilizing interactions of IL-8 with neutrophils is shown as the fura-2 fluorescence traces of 2 representative experiments (5 in the series). The responses of the cells exposed to untreated, or to Pd2 +-treated IL-8 (added as denoted by the arrow ↓) are shown on the left and right sides of each pair of traces, respectively.
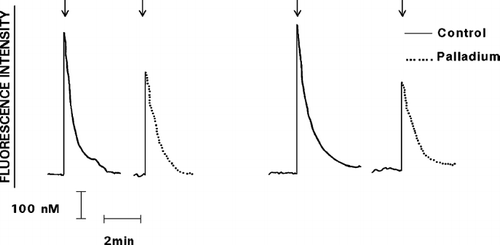
The peak response coincides with chemoattractant-mediated mobilization of Ca2 + from neutrophil intracellular stores, while the subsequent rate of decline in cytosolic Ca2 + reflects the balance between the efficacy of Ca2 + clearance systems and store-operated influx of the cation (Tintinger et al., Citation2005). As shown in , exposure of neutrophils to pneumolysin was accompanied by an increase in cytosolic Ca2 + that was evident after a lag-phase of about 1 min, rising at a slower rate than that initiated by the chemoattractants, and reaching comparable peak values that were sustained over the time course of the experiments. In this setting, increased cytosolic Ca2 + results from the pore-forming interactions of pneumolysin with neutrophils, with resultant influx of Ca2 + (Cockeran et al., Citation2002).
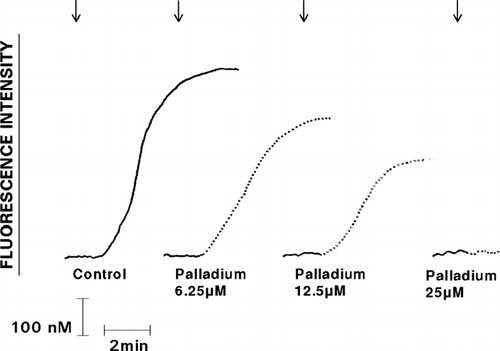
FIG. 3 Effect of exposure to Pd 2 + (25 μ M) on the Ca2 +-mobilizing interactions of pneumolysin with neutrophils is shown as the fura-2 fluorescence traces of 2 representative experiments (5 in the series). The responses of the cells exposed to untreated, or to Pd2 +-treated pneumolysin (added as denoted by the arrow ↓) are shown on the left and right sides of each pair of traces, respectively.
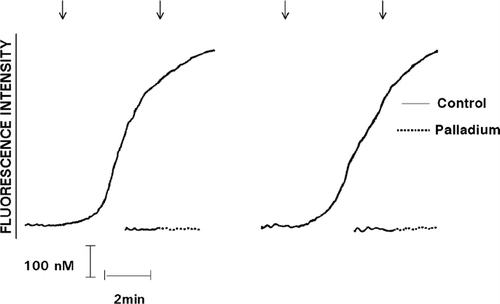
Prior exposure of C5a, IL-8, or pneumolysin to Pd2 + (25 μM), but not to any of the other metals, resulted in significant attenuation of the Ca2 +-mobilizing interactions of the chemoattractants, particularly C5a, and the toxin with the cells (, , and ).
To control for possible direct effects of Pd2 + on the responsiveness of neutrophils to C5a, IL-8, and pneumolysin, the cells were exposed to the metal at a fixed final concentration of 0.25 μM (this being the final concentration of the metal in the fura-2 fluorescence assay system) for 5 min at 37°C followed by addition of the chemoattractants or toxin. The mean peak cytosolic Ca2 + values for metal-free control cells exposed to C5a, IL-8, or pneumolysin were 577 ± 24, 449 ± 40 and 457 ± 31 nM, while the corresponding values for cells treated with 0.25 μ M Pd2 + were 577 ± 20, 456 ± 25, and 457 ± 44 nM.
The effects of exposure of IL-8 or pneumolysin to Pd2 + at concentrations ranging from 6.25–25 μ M are shown in and . The inhibitory effects of the metal on the reactivity of the chemoattractants and pneumolysin with neutrophils were detected at a concentration of 6.25 μ M.
Effects of Pd2 +on the Leukotactic Activity of C5a and IL-8
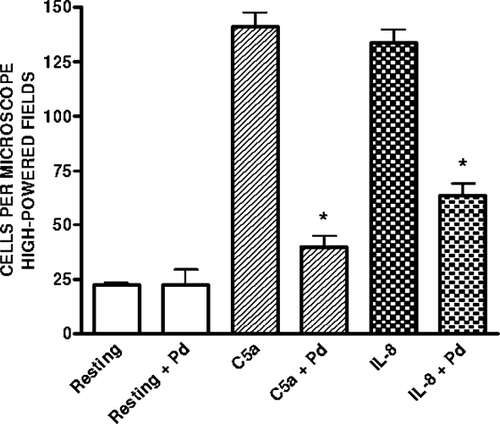
The effects of brief, prior exposure to 25 μ M Pd2 + on the chemotactic activities of C5a and IL-8 for neutrophils are shown in . Exposure of the chemoattractants to the metals was accompanied by a significant decrease, especially in the case of C5a, in chemotactic activity.
DISCUSSION
The results of the current study have demonstrated that Pd2 +, but not Co2 +, Pt4 +, or V in the various oxidation states tested, attenuate the neutrophil activating/mobilizing properties of the chemoattractants, C5a and IL-8, both of which are critical components of innate host defense. C5a is generated following activation of the alternative and mannan lectin-binding pathways of complement activation (during innate host defense) and is a potent chemoattractant for neutrophils, monocytes and macrophages (Hopken et al., Citation1996). IL-8, which possesses selective chemotactic activity for neutrophils, is produced by epithelial cells, monocytes, macrophages, and several other cell types, including neutrophils themselves, following interaction of pattern recognition molecules on these cells with microbial pathogens (Haselmayer et al., Citation2006).
In addition to inactivating the two chemoattractants, Pd2 + also neutralized the pore-forming interactions of the pneumococcal toxin pneumolysin with neutrophils, with resultant attenuation of Ca2 + influx. Pneumolysin belongs to the family of cholesterol binding, pore-forming, microbial toxins, which are produced by many different bacterial pathogens (Andrew et al., Citation2000). Depending on the local density of toxin-producing bacteria in the airways, pneumolysin, which is produced by almost all clinical isolates of the pneumococcus, may either exacerbate or prevent pneumococcal infection. In the case of the latter, exposure to small numbers of pneumococci in the airways results in the production of low, subcytolytic concentrations of pneumolysin, which induce production of IL-8 by airway epithelium by a mechanism dependent on Ca2 + influx and activation of p38 mitogen-activated protein kinase and nuclear factor-κ B (Fickl et al., Citation2005; Ratner et al., 2006); in this setting the consequent influx of neutrophils is protective, resulting in clearance of S. pneumoniae from the airways (Van Rossum et al., Citation2005; Ratner et al., 2006).
Importantly, Pd2 + at the final concentration of 0.25 μ M used in the assays of Ca2 + mobilization and chemotaxis did not affect the responsiveness of neutrophils to C5a, IL-8, or pneumolysin. These observations confirm the direct reactivity of the metal with the chemoattractants and the toxin.
These observations demonstrate that several key mediators of inflammation, of both host and bacterial origin, which act in concert to initiate a protective, neutrophil-mediated response against a commonly encountered and frequently life-threatening microbial pathogen, S. pneumoniae, are inactivated by Pd2 +. Given that C5a and IL-8 are of fundamental importance in host defense, while cholesterol-binding, pore-forming toxins are produced by many different microbial pathogens, exposure to Pd2 + may broadly favor microbial persistence in the airways.
The mechanism by which exposure to Pd2 +, but not to any of the other metals tested, attenuates the protective, biological activities of C5a, IL-8, and pneumolysin remains to be established. Interaction with protein sulfhydryls does not appear to be implicated, because all 4 test metals possess comparable activity in this respect (data not included). Interestingly, Pd2 + has been reported to acquire protease activity following binding to histidine and methionine residues, causing cleavage of the proximal upstream peptide bond (Milovic and Kostic, Citation2002a, Citation2002b, Citation2003). Although somewhat speculative, such a non-specific proteolytic mechanism would explain the susceptibility of C5a, IL-8, and pneumolysin to Pd2 +.
Notwithstanding occupational exposure to Pd2 + during extraction, concentration, refining, and separation of platinum group metals, other sources of exposure to Pd2 + include atmospheric emissions from automobile catalytic converters and corrosion of dental alloys (Drasch et al., Citation2000). In the current study, Pd2 +-mediated inactivation of C5a, IL-8, and pneumolysin was detected at concentrations of the metal as low as 6.25 μ M, which equates to 1.11 μ g/ml. This is likely to be considerably lower than concentrations of the metal which may be encountered in refineries, while a maximum load of 70.5 μ g/day has been detected in saliva of subjects with Pd2 +-containing dental restorations (Drasch et al., Citation2000). Concentrations of Pd2 + in roadside dust adjacent to motorways have been estimated at around 70 μ g/kg (Jarvis et al., Citation2001). Inhalation of Pd2 + following environmental and occupational exposure (in particular) to the metal may compromise innate host defenses in the airways, while leakage of Pd2 + from dental restorations may favor colonization of the oral cavity and throat by microbial pathogens.
In conclusion, the current study has documented interference with innate host defenses as being a possible health risk of exposure to Pd2 +. While the implications, if any, of these findings for environmental/occupational health remain to be established, the potential of heavy metals to compromise innate host defense mechanisms represents an emerging field of heavy metal toxicity (Klein-Patel et al., Citation2006).
REFERENCES
- Anderson R., Lukey P. T., Naudé S. P. E., Jooné G. Benoxaprofen: A pro-oxidant anti-inflammatory drug?. Agents Actions 1984; 14: 238–246
- Andrew P. W., Mitchell T. J., Morgan P., Gilbert R. J. C. Relationship of structure to function in pneumolysin. Streptococcus Pneumoniae: Molecular Biology and Mechanisms of Disease, A. Tomasz. Mary Ann Liebert Inc., New York 2000; 271–286
- Ballach H. J. Ozone and heavy metals from automobile catalytic converters. Environ. Sci. Pollut. Res. 1997; 4: 131–139
- Barceloux D. G. Vanadium. Clin. Toxicol. 1999; 37: 265–278
- Brock T., Stopford W. Bioaccessibility of metals in human health risk assessment: Evaluating risk from exposure to cobalt compounds. J. Environ. Monit. 2003; 5: 71N–76N
- Calverley A., Rees D., Dowdeswell R. J., Linnett P. J., Kielkowski D. Platinum salt sensitivity in refinery workers—incidence and effects of smoking exposure. Occup. Environ. Med. 1995; 52: 661–666
- Cockeran R., Anderson R., Feldman C. Pneumolysin in the immunopathogenesis and treatment of pneumococcal disease. Expert Rev. Anti-Infect. Ther. 2003; 1: 231–239
- Cockeran R., Durandt C., Feldman C., Mitchell T. J., Anderson R. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J. Infect. Dis. 2002; 186: 562–565
- Drasch G., Muss C., Roider G. Gold and palladium burden from dental restoration metals. J. Trace Elements Med. Biol. 2000; 14: 71–75
- Fickl H., Cockeran R., Steel H. C., Feldman C., Cowan G., Mitchell T. J., Anderson R. Pneumolysin-mediated activation of NF-kB in human neutrophils is antagonized by docosahexaenoic acid. Clin. Exp. Immunol. 2005; 140: 274–281
- Fickl H., Theron A. J., Grimmer H., Oommen J., Ramafi G., Steel H. C., Visser S. S., Anderson R. Vanadium promotes hydroxyl radical formation by activated human neutrophils. Free Rad. Biol. Med. 2006; 40: 146–155
- Goering P. L. Platinum and related metals: Palladium, iridium, osmium, rhodium, and ruthenium. Clinical Principles of Environmental Health, J. B. Sullivan, G. R. Krieger. Lippincott, Williams and Wilkins, Baltimore 1992; 874–881
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2 + indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985; 260: 3440–3450
- Haselmayer P., Tenzer S., Kwon B. S., Jung G., Schild H., Radsak M. P. Herpes virus entry mediator synergizes with Toll-like receptor mediated neutrophil inflammatory responses. Immunology 2006; 119: 404–411
- Hopken W. E., Lu B., Gerard W. P., Gerard C. The C5a chemoattractant receptor mediates mucosal defense to infection. Nature 1996; 383: 86–89
- Hughes E. J. Medical surveillance of platinum refinery workers. J. Soc. Occup. Med. 1980; 30: 27–30
- Irsigler G. B., Visser P. J., Spangenberg P. A. L. Asthma and chemical bronchitis in vanadium plant workers. Am. J. Ind. Med. 1999; 35: 366–374
- Jarvis K. E., Parry S. J., Piper J. M. Temporal and spectral studies of auto-catalyst-derived platinum, rhodium, and palladium and selected vehicle-derived trace elements in the environment. Environ. Sci. Technol. 2001; 35: 1031–1036
- Kielhorn J., Melber C., Keller D., Mangelsdorf I. Palladium—a review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002; 205: 417–432
- Klein-Patel M. E., Diamond G., Boniotto M., Saad S., Ryan L. K. Inhibition of β -defensin gene expression in airway epithelial cells by low doses of residual oil fly ash is mediated by vanadium. Toxicol. Sci. 2006; 92: 115–125
- Linna A., Oska P., Palmroos P., Roto P., Laippalla P., Uitti J. Respiratory health of cobalt production workers. Am. J. Int. Med. 2003; 44: 124–132
- Malley R., Henneke P., Morse S. C., Cieslewicz M. J., Lipsitch M., Thompson C. M., Kurt-Jones E., Paton S. C., Wessels M. R., Golenbock D. T. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 2003; 100: 1966–1971
- Mapp C., Boschetto P., Miotto D., DeRosa E., Fabbri L. M. Mechanism of occupational asthma. Ann. Allergy Asthma Immunol. 1999; 83: 645–664
- Merget R., Rosner G. Evaluation of the health risk of platinum group metals emitted from automotive catalytic converters. Sci. Total Environ. 2001; 270: 165–173
- Milovic N. M., Kostic N. M. Interplay of terminal amino group and coordinating side chains in directing radioselective cleavage of natural peptides and proteins with palladium(II) complexes. Inorg. Chem. 2002a; 41: 7053–7063
- Milovic N. M., Kostic N. M. Palladium(II) complexes as synthetic peptidases, regio-selectively clear the second peptide bond “upstream” from methionine and histidine side chains. J. Am. Chem. Soc. 2002b; 124: 4759–4769
- Milovic N. M., Kostic N. M. Palladium(II) complexes as a sequence-specific peptidase: Hydrolytic cleavage under mild conditions of X-Pro peptide bonds in X-Pro-Met and X-Pro-His segments. J. Am. Chem. Soc. 2003; 125: 781–788
- Mitchell T. J., Andrew P. W. Biological properties of pneumolysin. Streptococcus Pneumoniae: Molecular Biology and Mechanisms of Disease, A. Tomasz. Mary Ann Liebert Inc., New York 2000; 279–286
- Niezborala M., Garnier R. Allergy to complex platinum salts: A historical prospective cohort study. Occup. Environ. Med. 1996; 53: 252–257
- Ramafi G. J., Theron A. J., Anderson R. Pro-oxidative interactions of cobalt with human neutrophils. Inhal. Toxicol. 2004; 16: 649–655
- Ratner A. J., Hippe K. R., Aguila J. L., Bender M. H., Nelson A. L., Weiser J. N. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J. Biol. Chem. 2002; 281: 12994–12998
- Raulf-Heimsoth M., Merget R., Rihs H. P., Fohring M., Liebers V., Gellert B. T-cell receptor repertoire expression in workers with occupational asthma due to platinum salt. Eur. Respir. J. 2000; 16: 871–878
- Saunders F. K., Mitchell T. J., Walker J. A., Andrew P. W., Boulnois E. J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae does not require a thiol group for in vitro activity. Infect. Immun. 1989; 57: 2547–2552
- Handbook on Toxicity of Inorganic Compounds, H. G. Seiler, H. Sigel. Marcel Dekker, New York 1988
- Shishodia S., Shrivastava A., Sodhi A. Cisplatin-induced activation of bone marrow-derived macrophages requires protein tyrosine phosphorylation. Int. J. Immunopharmacol. 1997; 19: 683–690
- Swennen B., Buchet J. P., Stanescu D., Lison D., Lauerys R. Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. Br. J. Ind. Med. 1993; 50: 835–842
- Theron A. J., Ramafi G. J., Feldman C., Grimmer H., Visser S. S., Anderson R. Effects of platinum and palladium ions on the production and reactivity of neutrophil-derived reactive oxygen species. Free Rad. Biol. Med. 2004; 36: 1408–1417
- Tintinger G. R., Steel H. C., Anderson R. Taming the neutrophil: Calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin. Exp. Immunol. 2005; 141: 191–200
- Van Klavaren R. J., Nemery B. Role of reactive oxygen species in occupational and environmental obstructive pulmonary diseases. Curr. Opin. Pulm. Med. 1999; 5: 118–128
- Van Rossum A. M., Lysenko E. S., Weiser J. N. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 2005; 73: 7718–7726
- Wang L., Meden D., Mercer R., Overmiller D., Leonard S., Castranova V., Shi X., Ding M., Huang C., Rojanasakul Y. Vanadium-induced apoptosis and pulmonary inflammation in mice. Role of reactive oxygen species. J. Appl. Physiol. 2003; 185: 99–107