Abstract
EPA guidelines provide a choice in evaluating humoral immune system function in rats and mice immunized with sheep red blood cells (sRBC): an antibody-forming cell (AFC) assay or a sRBC-specific serum IgM enzyme-linked immunosorbent assay (ELISA). Four different laboratories used both methods to detect suppression of the antibody response by cyclophosphamide (CP) or dexamethasone (DEX). Attempts were made to minimize interlaboratory variability through the use of common reagents and vendors; each laboratory used the same source for rodents, immunosuppressive agents, and one sheep for sRBCs, and determined optimal sRBC concentration for immunization and peak day of antibody response in female CD rats and CD1 mice. The CP dose at which statistical significance was first observed in each species was quite similar within each lab using either assay. For DEX, the AFC assay detected significant and greater suppression at lower concentrations compared to the ELISA in both rats and mice. All labs detected DEX suppression using an AFC assay, whereas only one lab detected significant suppression in both species using an ELISA. For both compounds the magnitude of suppression was greater using the AFC assay, and resulted in ID50 values which were lower in the AFC assay when compared to the ELISA. In addition, cross-species comparisons of ID50 values suggested rats were more sensitive than mice. These initial experiments with two chemicals indicated that the AFC assay is consistently better at identifying suppression of a T-dependent antibody response across laboratories following xenobiotic exposures in outbred rats and mice. Additional compounds will need to be evaluated before concluding that one method is superior or more sensitive to the other in detecting suppression of the antibody response.
INTRODUCTION
Humoral immunity involves the production of specific antibodies by plasma cells and requires the cooperation and interaction of antigen presenting cells, T-lymphocytes and B-lymphocytes, all of which represent potential targets for xenobiotics. Luster et al. (Citation1988) demonstrated the importance of assessing humoral immune function in evaluating immunotoxicity and reported that the quantification of a specific T-dependent antibody response was one of the best predictors of immunotoxicity in mice. In 1998 EPA issued Health Effects Test Guidelines for Immunotoxicity (OPPTS 870.7800), that:
At the end of the exposure period, either the plaque forming cell (PFC) assay or an enzyme linked immunosorbent assay (ELISA) must be performed to determine the effects of the test substance on the splenic anti-SRBC (IgM) response or serum anti-SRBC IgM levels, respectively. (EPA, 1998, p. 2)
These guidelines were subsequently codified in 2005. While the antibody or plaque-forming cell (AFC or PFC) assay has been extensively evaluated and demonstrates good sensitivity and specificity regarding the detection of immune suppression, it is reported to be a more labor intensive approach to measuring antibody production than an ELISA The AFC assay, however, is able to evaluate the different cells required for antibody production compared to the ELISA and can be modified (e.g., cell purification) to readily identify the cellular mode of action by which a xenobiotic is acting.
Compared to the AFC assay the ELISA allows the investigator more flexibility in the timing of assessment since serum samples can be frozen until analysis. Furthermore, since removal of the spleen is not required, the ELISA allows for multiple serum samples from the same animal. Thus, a time course may be performed or, upon re-challenge with the same antigen, the secondary IgG-mediated immune response may be determined. Also, as reported by Ladics et al. (1995, 1998), it appears that the ELISA may be used to measure humoral immunity in rats on standard toxicology studies without altering standard toxicological endpoints. As a result, a separate satellite group may not be needed to assess humoral immune function, thereby reducing animal usage and cost. The measurement of an antigen-specific antibody in the serum integrates the total antibody production from multiple sites of antibody formation (i.e., spleen, lymph nodes, bone marrow).
However, the sRBC ELISA is not without drawbacks, including consistent preparation of the sRBC antigen used to coat the wells of the ELISA plates, and standardization of data analysis. Both the sRBC ELISA and AFC assay require the identification of a sheep whose erythrocytes produce a robust response. Currently no sRBC antibody standard is commercially available, which necessitates analysis using midpoint, endpoint titer, or another analysis procedure. Furthermore, special equipment such as a plate reader or spectrophotometer and the appropriate validated software are needed that will meet GLP requirements for studies submitted to regulatory agencies.
Although both approaches are accepted as a means to evaluate the integrity of the antibody response following xenobiotic exposure, the relative sensitivity of each assay remains contested. Therefore, the objective of this study was to test the hypothesis that the ELISA is equivalent in sensitivity to the AFC assay. Each assay was evaluated in four different laboratories using two known immunosuppressive agents cyclophosphamide (CP) and dexamethasome (DEX) in an outbred strain of rat and mouse. Where possible, attempts were made to minimize interlaboratory variability through the use of common reagents and vendors (e.g., immunosuppressive agents, same sheep as a source of sRBCs, rodents obtained from the same supplier).
MATERIALS AND METHODS
Reagents
Cyclophosphamide (CP) was obtained from Sigma Chemical (St. Louis, MO) and stored desiccated at approximately 4°C. CP was prepared in sterile saline. Dexamethasone (DEX) was received in a 4.75% ethanol solution at 2 mg/ml and was stored at room temperature (Phoenix Pharmaceuticals, St. Joseph, MO).
All laboratories utilized sRBC from the same sheep (Covance Research Products, Denver, PA). Preparation and handling of the sRBC are described in detail in Holsapple (Citation1995).
Animals
All laboratories obtained female Crl:CD BR rats and CD-1 mice from Charles River Breeding Laboratories (Raleigh, NC). Animals were quarantined for 1 week prior to exposure. Rodents were housed individually in stainless steel wire-mesh cages or group housed in accordance with the individual laboratory IACUC requirements and provided food and tap water ad libitum. All laboratories used rodents between 8–12 weeks of age.
Immunization
Rodents were injected intravenously into a lateral tail vein with sRBC (test Day 0) using concentrations determined by each laboratory to give optimal antibody responses. The number of sRBCs injected per mouse in 0.2 ml ranged between 4 × 107 and 2 × 108 and per rat in 0.5 ml ranged between 108 and 3 × 108. The peak antibody response for all labs was determined to be Day 4 for the AFC assay in either mice or rats, whereas Day 5 was the peak day for the ELISA in mice and Day 6 for rats.
Chemical Exposure
Mice immunized with sRBC were then injected intraperitoneally (IP) with 0, 5, 10, 20, 40 or 60 mg/kg/day CP on Days 0–3 (AFC) or 0–4 (ELISA). Rats immunized with sRBC were then injected IP with 0, 0.3, 1, 3, 10 or 30 mg/kg/day CP on Days 0–3 (AFC) or 0–5 (ELISA).
DEX dosing solutions were prepared and further diluted using 4.75% ethanol in saline and were administered IP with 0, 0.1, 0.3, 1, 3 or 5 mg/kg to mice on Days 0–7 (AFC) or 0–8 (ELISA), and to rats on Days 0–7 (AFC) or 0–9 (ELISA). All animals were immunized intravenously (IV) with sRBC on Day 4 of the DEX treatment.
ELISA for Measuring the Primary IgM Response to sRBC
Animals were sacrificed via CO2 asphyxiation followed by cardiac puncture, and serum was prepared. Sera were stored at −20°C until analysis. Serum samples were analyzed for sRBC-specific IgM antibody as described by Temple et al. (Citation1993).
AFC Assay
Spleens were weighed and placed in RPMI containing 5% fetal bovine serum FBS or Earls Balanced Salts Solution (EBSS) containing HEPES, based on each laboratory's experience. A single-cell suspension was prepared from each spleen and spleen cell numbers were determined using automated counters. Cell suspensions were evaluated for sRBC-specific IgM antibody forming cells using the AFC assay as described (Holsapple, Citation1995). Numbers of antibody-forming cells were subsequently expressed as AFC/106 splenocytes (specific activity) and as AFC/spleen (total response per animal).
Data Analysis
Data were collated by each laboratory and sent to DuPont Haskell Laboratory for statistical analysis. For AFC/106splenocytes, AFC/spleen, and ELISA, the lowest dose at which statistical significance was first observed was determined using the Jonckheere–Terpstra trend test (alpha = 5%) (Gross and Clark, Citation1975).
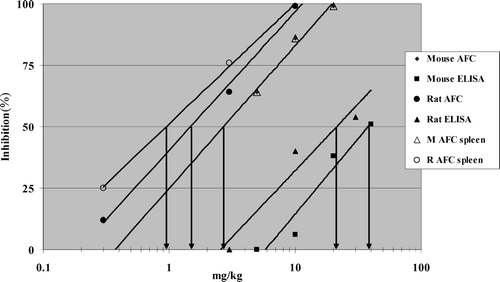
Dose-response curves for percent inhibition were plotted in Microsoft Excel, a best-fitting trend line drawn, and the dose of each compound to inhibit the response (AFC/106splenocytes; AFC/spleen; ELISA) by 50% (ID50) was visually determined An example illustrating one laboratory's results using cyclophosphamide is shown in .
FIG. 1 Cyclophosphamide Inhibition of the Antibody Response to sRBC from a Representative Laboratory. Dose response curves for percent inhibition were plotted in Microsoft Excel, a best-fitting trendline drawn, and the dose of each compound to inhibit the response (AFC: Antibody Forming Cell/106 splenocytes; AFC spleen: AFC/spleen; ELISA) by 50% (ID50) in rats (R) or mice (M) was visually determined.
RESULTS
Cyclophosphamide
In the rat, the lowest dose at which statistically significant inhibition of the antibody response was observed was 10 mg/kg in 3 of 4 labs using either the AFC assay (AFC/106splenocytes) or ELISA (). Comparison of the assays revealed equal or greater inhibition of the antibody response with the AFC assay compared to the ELISA. This pattern was also seen with mice, where the lowest statistically significant dose was either identical or within one concentration for either the AFC assay or ELISA. As was seen with the rat, equal or greater inhibition of the antibody response in mice was observed in the AFC assay in 3 of the 4 labs. Overall, either assay detected statistically significant suppression in rats or mice at identical or similar doses in 7 of 8 evaluations. For inhibition of the antibody response to be statistically significant, greater suppression was generally necessary for the AFC assay vs. the ELISA in both species.
TABLE 1 Lowest dose of cyclophosphamide at which statistical significance was observed and percent inhibition
ID50 values were determined for each experiment and compared for each endpoint (AFC/106 splenocytes; AFC/spleen; ELISA). Expressing the results of the AFC assay as either AFC/106 splenocytes or AFC/spleen resulted in very similar ID50values within each laboratory for both rats and mice (). In both species, the AFC assay detected a 50% inhibition of the antibody response to sRBC at about an order of magnitude lower dose of cyclophosphamide compared to the ELISA in 7 of 8 evaluations. In addition, ID50 values were also lower in rats when compared to mice in 7 of 8 evaluations.
TABLE 2 Cyclophosphamide ID50 values from all four laboratories using the AFC and ELISA assays to evaluate immunosuppression
Dexamethasone
In rats, the AFC assay detected statistically significant suppression at lower doses than the ELISA assay (). In addition, the percent inhibition of the antibody response was greater with the AFC assay and quite consistent across all four laboratories (Range: 47–80%). In Lab #1, the ELISA assay failed to detect a statistically significant inhibition at any dose of dexamethasone. In mice, 3 of 4 labs failed to detect statistically significant inhibition at any dose of dexamethasone using the ELISA assay. Two of these labs also did not detect a statistically significant inhibition at any dose of dexamethasone when measuring specific activity in the AFC assay (AFC/106 splenocytes), but did detect significant inhibition when expressing the data in total activity (AFC/spleen). As with cyclophosphamide, the rat was slightly more sensitive than the mouse to dexamethasone–induced suppression of the sRBC response.
TABLE 3 Lowest dose of dexamethasone at which statistical significance was observed and percent inhibition
When both assays were compared with respect to ID50values, the AFC assay in the rat across the four laboratories detected a 50% inhibition of the antibody response to sRBC at DEX concentrations nearly 2 orders of magnitude lower than that of the ELISA (). Expressing AFC results as AFC/spleen yielded lower ID50 values, compared to AFC/106 splenocytes, especially in the mouse. Because very little to no inhibition of the antibody response was observed in mice as measured by the ELISA, extrapolation to a 50% inhibition and an ID50 determination was not meaningful. A 50% inhibition of the antibody response was detected at lower concentrations in rats compared to mice.
TABLE 4 Dexamethasone ID50 values from all four laboratories using the AFC and ELISA assays to evaluate immunosuppression
DISCUSSION
Since many elements of the immune system are utilized in the primary antibody response to a foreign antigen, especially a T-dependent antigen, measurement of this response following xenobiotic exposure has become the preferred evaluation for detecting immunosuppression (Luster et al., Citation1992). In comparing the results from the AFC response and the ELISA one needs first to understand what is actually being evaluated. In the AFC assay, the number of antigen-specific antibody forming cells (i.e., AFCs or plasma cells) in the spleen is being enumerated as plaques. All plaques are not identical sizes, suggesting that each plasma cell may be releasing somewhat different amounts of specific antibody. In the ELISA, one is measuring the combined antibody levels in the serum, which reflects the contribution not only from the spleen, but also from lymph nodes, the bone marrow, and perhaps other organs (e.g., liver). It is conceivable, therefore, that the immunosuppressive effect of one dose of a chemical may differ depending upon what specific endpoint is being measured.
In fact, it has been the experience of some of the coauthors that when both the AFC assay and ELISA were conducted with the same compound, different sensitivities were obtained from the two assays (Temple et al., Citation1993; Johnson et al., Citation2000). In some cases, the ELISA response was more affected than the AFC assay; however, in general, the AFC assay appeared to be more sensitive than the ELISA, namely, that greater inhibition was detected at the same dose. The ELISA appears to be more sensitive than the AFC assay, however, with compounds that have a pronounced adverse effect on the bone marrow (Karrow et al., Citation2000).
While the ELISA does offer several advantages discussed here, major national and international validation studies have not been conducted. The AFC assay, on the other hand, has undergone numerous national and international ring studies (Luster et al., Citation1988; White, Citation1992; White et al., Citation1994; Dayan et al., Citation1998).
A popular misconception is that the AFC assay is more difficult to conduct and more labor intensive and resource demanding compared to the ELISA. In reality, in order to meet GLP regulations, significantly more documentation and personnel time are needed with the ELISA than the AFC assay. If one calculates the total cost of conducting the ELISA, including preparation of membrane antigens, purchase of ELISA plates, detector antibodies and substrate, and additional documentation and subsequent quality assurance review time, the ELISA is typically about 30% more expensive compared to the AFC assay.
Another misconception is that the AFC determinations must be conducted on the day of sacrifice, in contrast to the ELISA where the serum can be frozen and analyzed on a later date. While not as flexible as frozen sera, spleens may be aseptically removed, placed in media and kept on ice overnight and AFCs determined the following day with no difference in detecting immunosuppression (Burns-Naas et al., Citation1998; Jovanovic et al., Citation1999; Woolhiser and McCay, Citation1999).
In conducting the ELISA, a single endpoint is obtained, either a serum titer, or some type of concentration of antibody level in the serum. In the AFC assay; two endpoints are obtained: AFC/106 spleen cells, which represents specific activity of the response and AFC/spleen, which represents total animal activity. With some compounds, e.g., steroids, specific activity is oftentimes not affected but total spleen activity is dramatically decreased. For compounds that result in cell death, expressing the data as specific activity can be misleading. Although the number of cells in the spleen is dramatically decreased, those that remain can function properly indicating that specific activity would not necessarily be decreased. However, since the number of cells in the spleen has been decreased, the animal can be considered immunocompromised. This condition is illustrated with the DEX mouse data where ELISA and AFC/106 spleen cells were found not to be significantly affected by 2 of the 4 labs, but all labs observed immunosuppression when the data was presented as AFC/spleen. Therefore, we recommend expressing the results of the AFC assay as both AFC/106 spleen cells and AFC/spleen.
Due to the many pros and cons of the sRBC AFC assay and ELISA, both of which require by definition access to a reliable source of immunogenic sheep erythrocytes, an attempt has been made to find an alternative standardized antigen, one which could be commercially produced under GMP conditions. Keyhole limpet hemocyanin (KLH) has been proposed and evaluated as an alternative to the sRBC as the preferred antigen for assessing immunotoxicity (Gore et al., Citation2004). It is a respiratory protein found in shellfish and is quite immunogenic, partially due to the phylogenic distance between shellfish and mammals. Initial preparations of KLH resulted in an insoluble protein, but more recent attempts have yielded a soluble protein that has been purified under GMP conditions.
Both the sRBC AFC assay and the KLH ELISA have been evaluated following exposure to model immunosuppressive agents, with the AFC assay being more sensitive than the KLH ELISA (Peachee et al., Citation2005). Despite a concerted effort, a validated protocol does not appear to have been established yet and the large variability in the antibody response to KLH in vehicle-treated outbred animals is problematic for using KLH to detect unknown immunosuppressive chemicals (Piccotti et al., Citation2006).
In general, the AFC assay appears to be more sensitive than the ELISA, but the sensitivity of each assay no doubt varies by compound. Although the T-cell-dependent antibody response is a key element in determining the immunosuppressive potential of xenobiotics, it is important to emphasize that a weight of evidence approach is critical for hazard identification purposes, and that either or both assays may be appropriate for that purpose.
Present address for Michael P. Holsapple is: The Weinberg Group, 1220 19th Street NW, Suite 300, Washington, DC 20036-2400
REFERENCES
- Burns-Naas L. A., Mast R. W., Klykken P. C., McCay J. A., White K. L., Jr., Mann P. C., Naas D. J. Toxicology and humoral immunity assessment of decamethylcyclo-pentasiloxane (D5) following a 1-month whole body inhalation exposure in Fischer 344 rats. Toxicol. Sci. 1998; 43: 28–38
- Dayan A. D., Kuper F., Madsen C., Smialowicz R. J., Smith E., Van Loveren H., Vos J. G., White K. L., Jr. Report of validation study of assessment of direct immunotoxicity in the rat. Toxicology 1998; 125: 183–201
- Gore E. R., Gower J., Kurali E., Sui J., Bynum J., Ennulat D., Herzyk D. J. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immuotoxicity evaluation. Toxicology 2004; 197: 23–35
- Jonckheere's test. Non-Parametric Statistical Methods, A. J. Gross, V. A. Clark, 1975; 120–133
- Holsapple M. P. The plaque-forming cell (PFC) response in immunotoxicology: An approach to monitoring the primary effector function of B-lymphocytes. Methods in Immunotoxicology, Vol. 1, G. R. Burleson, J. H. Dean, A. E. Munson. Wiley-Liss, Inc., New York 1995; 71–108
- Johnson C. W., Williams W. C., Copeland C. B., DeVito M. J., Smialowicz R. J. Sensitivity of the SRBC PFC assay versus ELISA for detection of immunosuppression by TCDD and TCDD-like congeners. Toxicology 2000; 156: 1–11
- Jovanovic M. L., Seaton T. D., Gallavan R. H., Jr., Burns-Naas L. A. Immuno-toxicology sample longevity; Phenotypic analysis and the NK assay. Toxicol. Meth. 1999; 9: 11–23
- Karrow N. A., McCay J. A., Brown R. D., Musgrove D. L., Pettit D. A., Germolec D. R., Munson A. E., White K. L., Jr. Thalidomide stimulates splenic IgM antibody response and cytotoxic T-lymphocyte activity, and alters leukocyte sub-populations in female B6C3F1 mice. Toxicol. Appl. Pharmacol. 2000; 165: 237–244
- Ladics G. S., Smith C., Elliot G. S., Slone T. W., Loveless S. E. Further evaluation of the incorporation of an immunotoxicological functional assay for assessing humoral immunity for hazard identification purposes in rats on standard toxicology study. Toxicology 1998; 126: 137–152
- Ladics G. S., Smith C., Heaps K., Elliot G. S., Slone T. W., Loveless S. E. Possible incorporation of an immunotoxicological functional assay for assessing humoral immunity for hazard identification purposes in rats on a standard toxicology study. Toxicology 1995; 96: 225–238
- Luster M. I., Munson A. E., Thomas P. T., Holsapple M. P., Fenters J., White K., Lauer L. D., Dean J. H. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 1988; 10: 2–19
- Luster M. I., Portier C., Pait D. G., White K. L., Jr., Gennings C., Munson A. E., Rosenthal G. J. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol 1992; 18: 200–210
- Peachee V. L., Sheth C. M., Halpen A. M., White K. L. Sensitivity of the keyhole limpet hemocyanin (KLH) ELISA model is directly related to dose used for sensitization. The Toxicologist 2005; 38, (Abstract #178)
- Piccotti R. R., Alvery J. D., Slim R. M., Morris D. L., Kawabata T. T. Evaluation of inter-animal variability in the T-cell-dependent antibody response in rats. The Toxicologist 2006, (Abstract #1737)
- Temple L., Kawabata T. T., Munson A. E., White K. L., Jr. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol 1993; 21: 412–419
- White K. L., Jr. Validation: State of the art–United States National Toxicology Program and Fischer 344 rat cyclosporine-A study. J. Toxicol. Clin. Exp. 1992; 12: 429–447
- White K. L., Jr., Gennings C., Murray M. J., Dean J. H. Summary of an international methods validation study, carried out in nine laboratories, on the immunological assessment of cyclosporine A in the Fischer 344 rat. Toxicol. In Vitro 1994; 8: 957–961
- Woolhiser M. R., McCay J. A. Delayed assessment of immunological function allowing for transportation of lymphoid tissues to distant laboratory sites. Toxicol. Meth. 1999; 9: 165–171