Abstract
The current study characterizes the mechanism by which the seed extract of Moringa oleifera Lam (Moringaceae) decreases the mast cell-mediated immediate type hypersensitivity reaction. The immediate type hypersensitivity reaction is involved in many allergic diseases such as asthma and allergic rhinitis. Moringa oleifera, a shrub widely used in the traditional medicine in India, has been reported to possess anti-cancer, hypotensive, anti-arthritic, and anti-inflammatory activities. In the present study, the effects of the ethanolic extract of seeds of Moringa oleifera (MOEE-herbal remedy) on systemic and local anaphylaxis were investigated. The potential anti-anaphylactic effect of MOEE was studied in a mouse model of Compound 48/80-induced systemic anaphylactic shock. Passive cutaneous anaphylaxis activated by anti IgE-antibody was also used to assess the effect of MOEE. In addition, rat peritoneal mast cells (RPMC) were used to investigate the effect of MOEE on histamine release induced by compound 48/80. When administered 1 hr before 48/80 injection, MOEE at doses of 0.001–1.000 g/kg completely inhibited the inducible induced anaphylactic shock. MOEE significantly inhibited passive cutaneous anaphylaxis activated by anti-IgE antibody at a dose of 1 g/kg. When MOEE extract was given as pretreatment at concentrations ranging 0.1–100 mg/ml, the histamine release from the mast cells that was induced by the 48/80 was reduced in a dose-dependent manner. These results suggest a potential role for MOEE as a source of anti-anaphylactic agents for use in allergic disorders.
INTRODUCTION
Moringa oleifera Lam (synonym: Moringa pterygosperma), a small tree (7–12 m high) with thick grey bark, fragrant white flowers, and long green pods, is commonly referred to as the ‘drumstick tree’ or ‘horseradish tree’ (Makkar and Becker, Citation1997). It is a member of the Moringaceae family that grows throughout most of the tropics, including Pakistan, Bangladesh, Afghanistan, and northwest India (Makkar and Becker, Citation1996). As a traditionally important food commodity, M. oleifera has received attention as “natural nutritient of the tropics”; the leaves, flowers, fruits, and roots of this multipurpose tree are locally esteemed as a vegetable (Anwar and Bhanger, Citation2003).
In the Indian traditional system of folk medicine and in Ayurveda, various plant based preparations, like that of the M. oleifera, have been and still are given to patients without clinical trials, based upon the suggestive potential or possible efficacy of these extracts. With time, it has come to be shown that some of these potential or possible efficacies were, in fact, supported by animal data using experimental models to help discern particular pharmacological activities and mechanisms. Based on these types of observations, the plant has become well positioned in Ayurvedic, Unani, and even allopathic systems of medicine (Mughal et al., Citation1999). The findings from both the folk medicine/non-clinical and laboratory settings (i.e., in the animal models) have allowed the conclusion to be reached that M. oleifera has surprising medicinal attributes and is useful in the treatment of ascites, rheumatism, venomous bites, and as a cardiac and circulatory stimulant (Anwar et al., Citation2005).
The leaves exhibit strong hypotensive (Faizi et al., Citation1995), anti-diabetic (Marles and Farnsworth, Citation1995), anti-microbial, spasmolytic, and diuretic activity (Udupa et al., Citation1994), and have been seen to be useful against inflammation in anaphylaxis (Mahajan et al., Citation2006) helminthesis, and scurvy (Caceres et al., Citation1992). The plants seeds have been shown to possess anti-microbial (Caceres, Citation1991) and anti-fungal (Chuang et al., Citation2007) activity, and have also been shown to display anti-fertility (Prakash et al., Citation1988), anti-hepatotoxic (Ruckmani et al., Citation1998), analgesic (Rao et al., Citation2003), anti-arthritic (Mahajan et al., Citation2007), anti-tumour (Guevara et al., Citation1999), anti-pyretic, and anti-inflammatory effects (Varier, Citation1997). Guevara et al. (Citation1999) has also reported anti-genotoxic activity within the ethanolic extract of these seeds.
Beyond those myriad benefits, consumption of the plant/its derivatives has also been reported to elicit potential curative responses in children suffering from upper respiratory tract infections. It has also been reported that an alkaloid from the plant closely resembles ephedrine in its action and potential use in treating asthma, and that it likely acts through the induction of bronchiole relaxation (Kirtikar and Basu, Citation1975). Recent studies from this laboratory have also clearly shown the potential anti-asthmatic activities from treatment with extracts of this plant (Mahajan et al., Citation2007). Ayurvedic practitioners have administered the seed extract nasally in diseases like rhinitis and have also successfully used the dried seeds as an “anti-allergic” agent (Chopra et al., Citation1938). With respect to this latter finding, it has not been clearly shown how this material prevents allergic diseases and related disorders in experimental models. So far, no systematic study has closely examined the anti-anaphylactic properties of the extract of M. oleifera seeds. The present study sought to establish the scientific validity for the anti-anaphylactic property of M. oleifera seed extract during antigen-mediated anaphylaxis.
In general, immunoglobulin E (IgE)-mediated anaphylactic reactions that are involved in urticaria, allergic rhinitis, and asthma are mediated by various chemical mediators released from mast cells (Wasserman and Marquardt, Citation1988; Miescher and Vogel, Citation2002). Among the substances released on degranulation of mast cells, histamine is a well-characterized potent vasoactive mediator implicated in the acute phase of immediate-type hypersensitivity reactions (Peterson et al., 1996; Moon et al., Citation2003). Activated mast cells can also produce a wide variety of other inflammatory mediators and several pro-inflammatory and chemotactic cytokines, e.g., tumor necrosis factor-α (TNFα), and interleukins (IL)-1, -4, -6, -8, and -13 (Metcalfe et al., Citation1981; Royer et al., Citation2001; Stassen et al., Citation2001; Na et al., Citation2002).
Mast cell degranulation can be elicited by the synthetic compound 48/80. As a well-known histamine releaser, it has been used as a direct and convenient reagent to study the mechanism of anaphylaxis (Ennis et al., Citation1980; Kim et al., Citation2003). Similarly, the secretory responses of mast cells can be induced by corresponding antigen-induced aggregation of cell surface-specific receptors for IgE (Metzger et al., Citation1986; Hong et al., Citation2003) or by bridging with anti-IgE antibodies. With respect to the latter, it has been established that the anti-IgE antibody can induce a passive cutaneous anaphylaxis (PCA) reaction. As typical models for immediate-type hypersensitivity and anaphylactic reactions, both rats and guinea pigs are useful for studying PCA (Saito and Nomurda, Citation1989). The present study evaluated the effect of MOEE on the compound 48/80-induced systemic anaphylactic shock, anti-IgE-induced passive cutaneous anaphylaxis reactions, and inhibition of histamine release from rat peritoneal mast cells (RPMC).
MATERIALS AND METHODS
Reagents
Compound 48/80 (condensation product of N-methylp-methoxyphenylethylamine with formaldehyde), o-phthaldialdehyde (OPA), metrizamide, gelatin, and egg albumin (EA) were purchased from Sigma (St Louis, MO). All analytical solvents, Evans blue, toluidine blue, and trypan blue dyes were of analytical grade and purchased from S.D. Fine Chemicals Pvt. Ltd. (Mumbai, India).
Animals
Wistar rats (200–250 g) and ICR mice (20–30 g) were procured from Zydus Cadila Limited (Ahmedabad, India) and were maintained in approved housing facilities at the L.M. College of Pharmacy, Ahmedabad, India. The animals were housed six per cage in rooms maintained at ambient temperature (22 ± 1°C), relative humidity (55 ± 5%), and with a 12 hr light-dark cycle. Animals had free access to standard pellet diet (certified Amrut brand rodent feed, Pranav Agro Industries, Pune, India) and filtered tap water was given ad libitum. The animal experiments were conducted as per protocol approved by Institutional Animal Ethics Committee (IAEC) and as per Indian norms laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi. Throughout the entire study period, the rats were monitored for growth, health status, and food intake capacity to be certain that they were healthy. Freshly-prepared solutions of drugs or chemicals were used throughout the study.
Procurement of Plant Material and Extraction Procedure
Seed kernels of M. oleifera were obtained from a commercial supplier of Ahmedabad and were identified and authenticated by Department of Pharmacognosy, L. M. College of Pharmacy, Ahmedabad, India. Voucher specimens were deposited at the Department of Pharmacognosy, Ahmedabad.
The coarse powder (500 g) of the dried seed kernels of M. oleifera was defatted using petroleum ether and then it was exhaustively extracted using 95% ethanol (2L) in a soxhlet extractor at 55°C for 6 hr. The extract was concentrated under reduced pressure to yield a syrupy mass. The extract was stored in airtight container in cool place and used throughout the study.
Phytochemical Analysis
Preliminary phytochemical studies of the extract were performed for alkaloids, flavanoids, saponins, glycosides, phenols, steroids, tannins and terpenoids according to published standard methods (Peach and Tracey, Citation1955).
Compound 48/80-Induced Systemic Anaphylaxis in Mice
Compound 48/80-induced systemic anaphylactic reactions were examined using a slight modification of a protocol previously described by Kim et al. (Citation2005). Briefly, mice (n = 10 per regimen) were given an intraperitoneal (IP) injection of 0.008 g compound 48/80/kg body weight (BW) or vehicle only (control). Mortality was then monitored for 1 hr after induction of anaphylactic shock. To determine the effects of the extract, MOEE was dissolved in distilled water and administered orally at a doses ranging from 0, 0.001, 0.01, 0.1, of 1 g/kg 1 hr before the 48/80 injection.
Passive Cutaneous Anaphylaxis (PCA) Reaction in Rats
The passive cutaneous anaphylaxis (PCA) reaction was studied here using the modified method of Gupta et al. (Citation1995). Rats were injected subcutaneously with 100 μ g egg albumin (EA, mixed with aluminium hydroxide) on Days 1, 3, and 5 of the treatment regimen in order to induce both sensitization and homologous antiserum. On Day 10, blood was collected under light anaesthesia from the retro-orbital vein and the isolated serum containing IgE antibodies was separated by centrifugation (5000 × g, 30 min, 4°C).
An IgE-dependent cutaneous reaction was generated by sensitizing skin with an intradermal injection of anti-IgE antibody. Here, naive rats were sensitized with an intradermal injection of 0.1 ml of rat homologous antiserum (containing IgE antibodies) into each of four dorsal skin sites that had been shaved (and outlined with a water-insoluble red marker). After 48 hr, MOEE (0.001–1 g/kg per os) or saline (control) was then provided to respective groups of four rats each. One hour after this treatment, each rat received an intravenous injection (into tail vein) of 0.5 ml 1% EA in phosphate-buffered saline containing 0.5% (v/v) Evans blue dye. One hour after the challenge, the rats were sacrificed and the dorsal skin at the four sites removed for measurement of their respective pigment area.
The amount of the Evan's blue dye at each site was determined colorimetrically after extraction with 1 ml 1.0 M KOH and 9 ml of a mixture of acetone and phosphoric acid (5:13) (Katayama et al., Citation1978). The intensity of absorbance was then measured at 620 nm in a spectrophotometer (UV 1601, Shimadzu (Asia Pacific) Pte Ltd., Sydney, Australia). Using the maximum color intensity of dye (absorbance by spectrophotometer) observed in the saline-treated control samples as the 100% level associable with the inducible PCA response in these studies, the percent inhibition of PCA induction in each MOEE-treated group was calculated accordingly.
Preparation of Rat Peritoneal Mast Cells (RPMC)
RPMC were isolated as described by Kim et al. (Citation1998a, Citation1998b). In brief, rats were anesthetized by ether and injected with 20 ml Tyrode buffer B (150 mM NaCl, 0.1% glucose, 3 mM NaHCO3, 3.7 mM KCl, 3.5 mM NaH2PO4, and 0.1% gelatin) into the peritoneal cavity. After the abdomen was gently massaged, the peritoneal cavity was carefully opened and the fluid containing peritoneal cells recovered. The peritoneal cells present in the fluid were sedimented at 150 × g (10 min, room temperature) and resuspended in Tyrode buffer B. Mast cells were separated from the major components of the peritoneal cells, i.e., macrophages and small lymphocytes, according to the method of Yurt et al. (Citation1977). Peritoneal cells suspended in 1 ml Tyrode buffer B were layered on 2 ml metrizamide (22.5% [w/v], density = 1.120 g/ml) and centrifuged at 400 × g (15 min, room temperature). The cells remaining at the buffer-metrizamide interface were aspirated and discarded; the cells in the pellet were washed and resuspended in 1 ml Tyrode buffer A containing calcium. Mast cell preparations isolated by this method were ≈ 95% pure, as assessed by toluidine blue staining. More than 97% of the cells isolated using these methods were viable, as judged by trypan blue uptake.
Histamine Assay
Mast cell suspensions (2 × 105 cells/ml in Tyrode buffer A containing calcium) were pre-incubated at 37°C for 10 min before addition of 0.5 ml Compound 48/80 (10 μ g/ml). Extract-treated cells were pre-incubated at 37°C with MOEE (0.1–100 mg/ml) and then incubated for 10 min with compound 48/80. Each reaction was stopped by cooling the tubes in ice. The cells were then separated from their released histamine by centrifugation at 400 × g (5 min, 4°C). Residual histamine in the cells was released by disrupting the cells with perchloric acid and centrifugation at 400 × g (5 min, 4°C). The histamine content was then measured by the o-phthaldialdehyde spectrofluorimetric procedure of Shore et al. (Citation1959). The fluorescence intensity was measured in a spectrofluorometer (Shimadzu, RF-5301 PC) at an emission of 438 nm and an excitation of 353 nm. The inhibition percentage of histamine release was then calculated using the following:
where A is the level of histamine release in the absence of MOEE treatment and B, the release when it was present.
Statistical Analysis
All data are reported as the mean ± the standard error of the mean (SEM) in each of the different experiments. The Dunnett's test was used to make a statistical comparison between the various treatment groups. Results with p values < 0.05 were considered statistically significant. All statistical analyses were performed using Graph Pad software (San Diego, CA).
RESULTS
Phytochemical Study
The preliminary phytochemical screening of the ethanolic extract of M. oleifera showed the presence of alkaloids, flavanoids, glycosides, tannins and terpenoids ( and ).
TABLE 1 Preliminary phytochemical study on M.oleifera seed extract
TABLE 2 List of major phytochemical constituents of the seeds of M. oleifera
Effect of MOEE on Compound 48/80-Induced Systemic Anaphylaxis in Mice
To assess any contribution of MOEE against an anaphylactic reaction, an in vivo model of systemic anaphylaxis was first examined. Here, Compound 48/80 (8 mg/kg BW) was used as the inducer of a fatal systemic allergic reaction. After the IP injection of this agent, the mice were monitored for 1 hr and the mortality rate determined. As shown in , oral administration of saline prior to injection of 48/80 resulted in an induced fatal shock in 100% of the mice in that cohort. When MOEE was administered orally at concentrations ranging from 0.001 to 1 g/kg BW for 1 hr prior to exposure to 48/80, mortality was dose-dependently reduced. Of note, MOEE completely inhibited anaphylactic reaction at a dose of 1 g/kg. Treatment with the MOEE itself at this level induced no outward physiological effects in the mice.
TABLE 3 Effect of MOEE on compound 48/80-induced systemic reaction in mice
Effect of MOEE on Anti-IgE Antibody-Induced Passive Cutaneous Anaphylaxis in Rat
PCA is one of the most important in vivo models of anaphylaxis in local allergic reactions (Wershil et al., Citation1987). As described above, local extravasation was induced by a local injection of anti-IgE antibody followed by an intravenous antigenic challenge. The cutaneous anaphylactic reaction was then visualized by extravasation of a dye around the injection site. When administered orally 1 hr prior to the challenge with antigen, MOEE dose-dependently inhibited PCA reactions. Specifically, MOEE significantly inhibited PCA at a dose of 0.1 g/kg (i.e., to 47.6%); at 1.0 g/kg, the degree of inhibition of PCA was 61.2% ().
TABLE 4 Effect of MOEE on the passive cutaneous anaphylaxis reaction in rats
Effect of MOEE on Histamine Release from RPMC
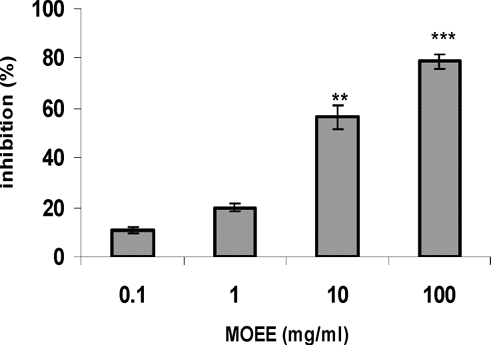
The inhibitory effect of MOEE on Compound 48/80-induced histamine release from RPMCs is shown in . MOEE dose-dependently inhibited the induced histamine release at concentrations of 0.1–100 mg/ml. In particular, MOEE extract significantly inhibited the 48/80-induced release beginning at a concentration of 10 mg/ml (inhibition of ≈ 60% vs. level of release in control stimulated cells).
FIG. 1 Effect of MOEE compound 48/80 induced histamine release from RPMC. RPMC (2 × 105 cells/ml) were pre-incubated with the drug at 37°C for 10 min prior to incubation with compound 48/80 (10 μ g/ml) for 10 min. Each bar represents the mean ± SEM of four independent experiments. **p < 0.01; ***p < 0.001; value significantly different from that in the saline treatment group.
DISCUSSION
Immediate-type allergic reactions (anaphylactic allergic reaction) are a life-threatening syndrome induced by the sudden systemic release of inflammatory mediators, such as histamine, heparin, and various cytokines from mast cells. The mast cells are thought to play a major role in the development of many physiological changes during allergic responses (Shin et al., Citation2005). These cells are located through out the human body, and upon allergen exposure, they are stimulated via the IgE-receptors (Kemp and Lockey, Citation2002). The results of this study demonstrated that MOEE has anti-allergic properties. Specifically, MOEE treatment greatly affected compound 48/80-induced systemic anaphylaxis, IgE-induced PCA reactions, and also attenuated compound 48/80-induced histamine release from RPMC.
Numerous reports established that stimulation of mast cells with compound 48/80 initiates the activation of a signal transduction pathway that leads to histamine release. Several studies have shown that compound 48/80 and other polybasic compounds are able, apparently directly, to activate G proteins (Mousli et al., Citation1990a, Citation1990b). The evidence indicates that the protein is Gi-like and that the activation is inhibited by benzalkonium chloride (Bueb et al., Citation1990). Compound 48/80 increases the permeability of the lipid bilayer membrane of cell by causing a perturbation in the membrane. This result indicates that the increase in membrane permeability may be an essential trigger for the release of the mediator from mast cells. In this sense, anti-allergic agents having a membrane stabilizing action may be desirable. The results of the studies here showed that MOEE treatment profoundly affected Compound 48/80-induced systemic anaphylaxis and histamine release. Thus, it is possible to hypothesize that MOEE might stabilize the lipid bilayer membrane, thereby preventing the perturbation being induced by Compound 48/80, and regulating the degranulation of mast cells in rat skin by stabilizing membrane fluidity.
In spite of increasing evidence of the role of several other mediators (Rafferty and Holgate, Citation1989; Rimmers and Church, Citation1990), histamine is still regarded to be the principal mediator of antigen-induced skin reactions. In addition, intradermal and intranasal application of chemical mediators and chemical mediator releasers increase vascular permeability in a manner similar to that of allergic models (Inagaki et al., Citation1989, Citation1990). PCA is very effective way to test skin allergic reactions and has been successfully applied in murine models to assess the effects of oriental medicines (Lee et al., Citation1997; Kim and Lee, Citation1999; Kim et al., Citation2000). The present study also utilized PCA for testing protective effect from IgE-mediated local allergic reaction. The mechanism of the protection against anti-IgE is suggested only in some particular conditions so far. It is conceivable that MOEE extract inhibits the initial phase of immediate type allergic reactions, probably through interference with the degranulation system. MOEE administered rats were protected from IgE mediated local anaphylaxis (). This finding suggests that MOEE may be applicable to the treatment of allergic skin reactions.
Various plants and plant-based preparations have been used in traditional medicine for centuries to treat allergic diseases in the Indian subcontinent. Crude extracts of Syzygium aromaticum (Kim et al., Citation1998), Vitex rotundifolia (Shin et al., Citation2000), Crinum glaucum (Okpo and Adeyemi, Citation2002), Striga orobanchioides (Harish et al., Citation2001) have been studied for effects upon mast cell-mediated anaphylactic reactions. Recently activity of the extracts of L. lucidus against mast cell-mediated allergic reactions has been reported by Yun et al. (Citation2003). Among the various components from L.lucidus, betulinic acid, a pentacyclic triterpene modulated the production of TNFα and IL-6 by monocytes and macrophages during immune responses. An anti-allergic activity of caffeic acid (Hossen et al., Citation2006), oligopeptide (Singh et al., Citation1998), and eugenol (Kim et al., Citation1997) has also been demonstrated in murine models of IgE-mediated reactions. These compounds have mast cell stabilizing activity and thus are able to inhibit the release of histamine.
In conclusion, the results obtained in the present study provide evidence that MOEE might contain compounds with actions that inhibit mast cell-mediated anaphylactic reactions. Further investigation is necessary to clarify unknown anti-anaphylactic constituents that may be more active than the MOEE extract itself. Because we used the whole crude extract of seeds of M. oleifera not a purified component, the active components that are responsible for biological effect are not clear this time. However qualitative phytochemical investigation of MOEE here and by other investigators has shown the presence of various active components (see and ). In addition, we have also succeeded in isolating benzylisothiocynate from the extract in our laboratory. The active principle(s) listed in the table might be responsible for one or more mechanisms for such activities. The effort to identify active components from MOEE in the immediate-type allergic reaction is ongoing in our laboratory. Recently, the protective effect of MOEE against immune-mediated inflammatory responses and autoimmune disorders has been reported (Mahajan et al., Citation2007). Building upon the results reported here, future work should address the possibility that MOEE may be active in the inhibition of mast cell degranulation and therefore of some potential use in the treatment of anaphylactic responses.
The authors acknowledge the financial support given by Department of Science and Technology (DST), New Delhi, India.
REFERENCES
- Anwar F., Bhanger M. I. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem 2003; 51: 6558–6563
- Anwar F., Ashraf M., Bhanger M. I. Interprovenance variation in the composition of Moringa oleifera oil seeds from Pakistan. J. Am. Oil Chem. Soc. 2005; 82: 45–51
- Bueb J. L., Mousli M. C., Bronner C., Rouot B., Landry Y. Activation of Gi-like proteins, a receptor-independent effect of kinins in mast cells. Mol. Pharmacol. 1990; 38: 816–822
- Caceres A., Cebreva O., Morales O., Miollined P., Mendia P. Pharmacological properties of M. oleifera 1. Preliminary screening for antimicrobial activity. J. Ethnopharmacol. 1991; 33: 213–216
- Caceres A., Saravia A., Rizzo S., Zabala L., De-Leon E., Nave F. Pharmacological properties of M. oleifera 2. Screening for anti-spasmodic, anti-inflammatory and diuretic activity. J. Ethnopharmacol. 1992; 36: 233–237
- Chopra's Indigenous Drugs of India, R. N. Chopra, I. C. Chopra, K. Handa, D. Kapur. Dhur & Sons, Ltd., Calcutta 1938
- Chuang P. H., Lee C. W., Chou J. Y., Murugan M., Shieh B. J., Chen H. M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresource Technol 2007; 98: 232–236
- Dahot M. U., Memon A. R. Nutritive significance of oil extracted from Moringa oleifera seeds. J. Pharm. Univ. Karachi 1985; 3: 75–80
- Das B. R., Kurup P. A., Narasimha Rao P. L. Antibiotic principle from Moringa Pterosperma Part IX. Inhibition of transaminase by isocyanates. Ind. J. Med. Res 1958; 46: 75–77
- Dayrit F. M., Alcantara A. D., Villasenor I. M. The antibiotic compound and its deactivation in aqueous solution. Phil. J. Sci 1990; 119: 23–26
- Ennis M., Pearce F. L., Weston P. M. Some studies on the release of histamine from mast cells stimulated with polysine. Br. J. Pharmacol 1980; 70: 329–334
- Faizi S., Siddiqui B. S., Saleem R., Siddiqui S., Aftab K., Gilani A. H. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry (Oxford) 1995; 38: 957–963
- Guevara A. P., Vargas C., Sakurai H., Fujiwara Y., Hashimoto K., Maoka T., Kozuka M., Ito Y., Tokuda H., Nishino H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res 1999; 440: 181–188
- Gupta P. P., Srimal R. C., Srivastava M., Singh K. L., Tondon J. S. Anti-allergic activity of Arbortritosides from Nyctanthus arbortrists. Int. J. Pharmacog. 1995; 33: 70–72
- Harish M. S., Mallikarjun N., Badami S. Antihistaminic and mast cell-stabilizing activity of Striga orobanchioides. J. Ethnopharmacol. 2001; 76: 197–200
- Hong S. H., Jeong H. J., Kim H. M. Inhibitory effects of Xanthii fructus extract on mast cell-mediated allergic reaction in murine model. J. Ethnopharmacol 2003; 88: 229–234
- Hossen M. A., Inoue T., Shinmel Y., Minami K., Fujil Y., Chiaki K. Caffeic acid inhibits Compound 48/80-induced allergic symptoms in mice. Biol. Pharm. Bull. 2006; 29: 64–66
- Inagaki N., Miura T., Daikoku M., Nagai H., Koda A. Inhibitory effects of β -adrenergic stimulants on increased vascular permeability caused by passive cutaneous anaphylaxis, allergic mediators, and mediator releasers in rats. Pharmacology 1989; 39: 19–27
- Inagaki N., Miura T., Ohira K., Nagai H., Xu Q., Koda A. Effect of CV-3988, a specific antagonist against platelet activation factor, on homologous passive cutaneous anaphylaxis in the mouse ear. J. Pharmacobiodynam. 1990; 13: 272–277
- Katayama S., Shionoya H., Ohtake S. A new method for extraction of extravasated dye in the skin and the influence of fasting stress on passive cutaneous anaphylaxis in guinea pigs and rats. Microbiol. Immunol. 1978; 22: 89–101
- Kemp S. F., Lockey R. F. Anaphylaxis: A review of causes and mechanisms. J. Allergy Clin. Immunol 2002; 110: 341–348
- Kim H. M., Lee Y. M. Role of TGF-β 1 on the IgE-dependent anaphylaxis reaction. J. Immunol. 1999; 162: 4960–4965
- Kim H. M., Lee E. H., Cho H. H., Moon Y. H. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by spirulina. Biochem. Pharmacol. 1998a; 55: 1071–1076
- Kim H. M., Lee E. H., Hong S. H., Song H. J., Shin M. K., Kim S. H., Shin T. Y. Effect of Syzygium aromaticum extract on immediate hypersensitivity in rats. J. Ethnopharmacol 1998b; 60: 125–131
- Kim H. M., Lee E. H., Kim C. Y., Chung J. G., Kim S. H., Lim J. P., Shin T. Y. Anti-anaphylactic properties of eugenol. Pharmacol. Res. 1997; 36: 475–480
- Kim M. S., Na H. J., Han S. W., Jin J. S., Song U. Y., Lee E. J., Song B. K, Hong S. H., Kim H. M. Forsythia fructus inhibits the mast-cell-mediated allergic inflammatory reactions. Inflammation 2003; 27: 129–135
- Kim S. H., Choi C. H., Kim S. Y., Eun J. S., Shin T. Y. Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allergy model. Exp. Biol. Med 2005; 230: 82–88
- Kim S. H., Choi Y. K., Jeong H. J., Kang H. U., Moon G., Shin T. Y., Kim H. M. Suppression of immunoglobulin E-mediated anaphylactic reaction by Alpinia oxyphylla in rats. Immunopharmacol. Immunotoxicol. 2000; 22: 267–277
- Kirtikar K. R., Basu B. D. Indian Medicinal Plants, 2nd Edition, D. Dun, B. Singh, M. P. Singh. M/s Bishen Singh Mahendra Pal Singh, New Cannaught Place, Dehradun 1975; Vol. 1: 676–683
- Laandrault N., Pouchert P., Ravel P., Gase F., Cros G., Teissedro P. L. Antioxidant activities phenolic level of French wines from different varieties and vintages. J. Agric Food Chem 2001; 49: 3341–3343
- Lako J., Trenerry V. C., Wahlqvist M., Wattanapenpaiboon N., Sotheeswaran S. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007; 101: 1727–1741
- Lee Y. M., Kim C. Y., Kim Y. C., Kim H. M. Effects of Poncirus trifoliata on type I hypersensitivity reaction. Am. J. Chin. Med. 1997; 25: 51–56
- Mahajan S. G., Mali R. G., Mehta A. A. Anti-anaphylactic and anti-inflammatory activity of hydroalcoholic extract of leaves of Moringa oleifera. Planta Indica 2006; 2: 9–13
- Mahajan S. G., Mali R. G., Mehta A. A. Effect of Moringa oleifera Lam. seed extract on toluene diisocyanate-induced immune-medicated inflammatory responses in rats. J. Immunotoxicol 2007
- Mahajan S. G., Mali R. G., Mehta A. A. Protective effect of ethanolic Extract of seeds of Moringa oleifera Lam. against inflammation associated with development of arthritis in rats. J. Immunotoxicol. 2007; 4: 39–47
- Makkar H. P., Becker K. Nutritional value and anti-nutritional components of whole and extracted Moringa oleifera leaves. Anim. Feed Sci. Technol 1996; 63: 211–228
- Makkar H. P., Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. 1997; 128: 311–322
- Marles R. J., Farnsworth N. R. Anti-diabetic plants and their active constituents. Phytomedicine 1995; 2: 137–189
- Memon G. M., Khatri L. M. Isolation and spectroscopic studies of mono-palmitic, di-oleic triglyceride from seeds of Moringa oleifera Lam. Pak. J. Sci. Ind. Res 1987; 30: 393–395
- Metcalfe D. D., Kaliner M, Donlon M. A. The mast cell. Crit. Rev. Immunol. 1981; 3: 23–74
- Metzger H., Alcaraz G., Gogman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Ann. Rev. Immunol. 1986; 4: 419–470
- Miescher S. M., Vogel M. Molecular aspects of allergy. Mol. Aspects Med. 2002; 23: 413–462
- Moon P. D., Na H. J., Kim H. M. Action of enzyme food, Green Life Enzyme, on systemic and local anaphylaxis. Orient. Pharm. Exp. Med. 2003; 3: 46–50
- Mousli M. C., Bronner C., Bockaert J., Rouot B., Landry Y. Interaction of substance P, compound 48/80, and mastoparan with α subunit C-terminal of G protein. Immunol. Lett. 1990a; 25: 355–358
- Mousli M. C., Bronner C., Landry Y., Bockaert J., Rouot B. Direct activation of GTP-binding regulatory proteins (G proteins) by Substance P and Compound 48/80. FEBS Lett. 1990b; 259: 260–262
- Mughal M. H., Ali G., Srivastava P. S., Iqbal M. Improvement of drumstick (Moringa pterygosperms Gaertn.) a unique source of food and medicine through tissue culture. Hamdard Med 1999; 42: 37–42
- Na H. J., Jeong H. J., Bae H., Kim Y. B., Park S. T., Yun Y. G., Kim H. M. Tongkyutang inhibits mast cell-dependent allergic reactions and inflammatory cytokine secretion. Clin. Chim. Acta 2002; 319: 35–41
- Njoku O. U., Adikwu M. U. Investigation on some physicochemical antioxidant and toxicological properties of Moringa oleifera seed oil. Acta. Pharma. Zagr 1997; 47: 287–290
- Okpo S. O., Adeyemi O. O. The antianaphylactic effects of Crinum glaucum aqueous extract. J. Ethnopharmacol. 2002; 81: 187–190
- Modern Methods of Plant Analysis, D. Paech, M. V. Tracey. Springer- Verlag, Berlin 1955; Vol. IV: 373–374
- Petersen L. J., Mosbech H., Skov P. Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: Characterization of factors influencing histamine release ability. J. Allergy Clin. Immunol 1996; 97: 672–679
- Prakash A. O., Pathak S., Shukla S., Mathur R. Pre- and post-implantation changes in the uterus of rats: Response to Moringa oleifera Lam. extract. Ancient Sci. Life 1988; 8: 49–54
- Rafferty P., Holgate S. T. Histamine and its antagonists in asthma. J. Allergy Clin. Immunol. 1989; 84: 144–151
- Rao C. V., Ojha S. K., Mehrotra S. Analgesic effect of Moringa oleifera leaf extract on rats. Proceedings of the Second World Congress on Biotechnological Developments of Herbal Medicine, LucknowIndia, 2003; 42
- Rimmers S. J., Church M. K. The pharmacology and mechanisms of action of histamine H1-antagonist. Clin. Exp. Allergy 1990; 20: 3–17
- Royer B., Varadaradjalou S., Saas P., Gabiot A. C., Kantelip B., Feger F., Guillosson J. J., Kantelip J. P., Arock M. Autocrine regulation of cord blood derived human mast cell activation by IL-10. J. Allergy Clin. Immunol. 2001; 108: 80–86
- Ruckmani K., Kavimani S., Anandan R., Jaykar B. Effect of Moringa oleifera Lam. on paracetamol induced hepatotoxicity. Ind. J. Pharm. Sci 1998; 60: 33–35
- Saito H., Nomura Y. Screening methods for drug evaluation-3. Pharmaceutical Research and Development, L. Suzuki, H. Tanaka, H. Yajima, H. Fukuda, H. Sezaki, K. Koga, M. Hirobe, T. Nakajime. Hirokawa, Tokyo 1989; 22
- Shin T. Y., Kim S. H., Lim J. P., Suh E. S., Jeong H. J., Kim B. D., Park E. J., Hwang W. J., Rye D. G., Baek S. H., An N. H., Kim H. M. Effect of Vitex rotundifolia on immediate-type allergic reaction. J. Ethnopharmacol. 2000; 72: 443–450
- Shin T. Y., Kim S. H., Suk K., Ha J. H., Kim I. K., Lee M. G., Jun C. D., Kim S. Y., Lim J. P., Eun J. S., Shin H. Y., Kim H. M. Anti-allergic effects of Lycopus lucidus on mast cell-mediated allergy model. Toxicol. Appl. Pharmacol 2005; 209: 255–262
- Shore P. A., Burkhalter A., Cohn V. H. A method for fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959; 127: 182–186
- Singh R., Nath A., Gupta P. P., Shukla M., Khare S. K., Kundu B. Anti-allergic/anti-asthmatic activity of oligopeptide related to IgE. Pharmacol. Res. 1998; 37: 353–356
- Stassen M., Muller C., Arnold M., Hultner L., Klein-Hessling S., Neudorfl C., Reineke T., Serfling E., Schmitt E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-κ B is decisively involved in the expression of IL-9. J. Immunol. 2001; 166: 4391–4398
- Udupa S. L., Udupa A. L., Kulkarni D. R. Studies on the anti-inflammatory and wound healing properties of Moringa oleifera and Aegle marmelos. Fitoterapia 1994; 65: 119–23
- Varier V. P. Indian Medicinal Plants Compendium of 500 species, P. K. Warrier, V. P. Nambiar, C. Ramankutty. Orient Longman Ltd., Madras 1997; Vol. 4: 58–62
- Villasenor I. M. Bioactive metabolites from Moringa oleifera Lam. Kimika 1994; 110: 47–52
- Villasenor I. M., Dayrit F., Lim-Sylianco C. Y. Studies on M. oleifera seeds, Part II. Thermal degradation of roasted seeds. Phil. J. Sci 1990; 119: 33–39
- Villasenor I. M., Lim-Sylianco C. Y., Dayrit F. Mutagens from roasted seeds of Moringa oleifera. Mutat. Res 1989; 224: 209–212
- Anaphylaxis in Allergy: Principles and Practice, 3rd Edition, S. I. Wasserman, D. L. Marquardt. Mosby, St. Louis 1988
- Wershil B. K., Mekori Y. A., Murakami T., Galli S. J. 125I-Fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin: Demonstration of role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J. Immunol. 1987; 139: 2605–2614
- Yun Y., Han S., Park E., Yim D., Lee S., Lee C. K., Cho K., Kim K. Immunomodulatory activity of betulinic acid by producing pro-inflmmatory cytokines and activation of macrophages. Arch. Pharmacol Res. 2003; 26: 1087–1095
- Yurt R. W., Leid R. W., Austen K. F. Native heparin from rat peritoneal mast cells. J. Biol. Chem 1977; 252: 518–521
