Abstract
Here we sought to determine whether prenatal exposure to subtoxic levels of inorganic mercury induced modulations in the fetal immune repertoire. After overnight breeding of female BALB/c and male DBA/1 mice, pregnant females were exposed to 10 mg/l mercuric chloride in drinking water. DBF1 pups were examined at day 16 of gestation for immunophenotypic changes in the fetal thymus and liver. While total thymocyte counts remained comparable to unexposed controls, intrauterine mercury exposure was found to modulate several immune phenotypes in the fetal thymus. Specifically, we saw an increase in the percentages of double negative (DN, CD4−CD8−) cells as well as a reduction in the numbers of cells representing activation phenotypes (CD4+CD25+). In the liver, we observed modulations that suggested skewing of the development of B220+-expressing cells with mercury exposure. Further, we saw increased populations of thymic and splenic lymphocytes reactive with a pathogenic idiotypic determinant IdLNF1, which is associated with spontaneous autoimmune nephritis in (NZB × SWR)F1 (SNF1) mice. Previous studies from our laboratory have shown that dysregulation of the IdLNF1 idiotypic network, particularly the expansion of idiotype-reactive T- and B-lymphocytes, is key in the development of lupus nephritis in the autoimmune SNF1 mouse. These studies show that acute exposure to relatively low levels of inorganic mercury in utero may alter the fetal immune repertoire which could potentially modulate post-natal immune responses.
Keywords :
INTRODUCTION
Mercury is a ubiquitous, highly toxic environmental contaminant that poses a serious threat to public health. Common sources of exposure include amalgams used in tooth fillings, cosmetics, antiseptics and paints (Pollard and Hultman, Citation1997; Bagenstose et al., Citation1999) and occupationally from mining and coal-burning activities (Powell et al., Citation1999). In the United States, a significant source of mercury exposure has been traced to the consumption of certain marine products, notably mackerels and predatory fish, which have been found to contain high levels of methylmercury. A National Health and Nutrition Examination Survey (1999–2000) identified women of childbearing age as having the highest blood mercury levels; as much as 7% had levels above the EPA oral reference dose (RfD) of 5.8 μ g/l, placing at least 300,000 children at significant risk for mercury toxicity as a result of intrauterine exposures (Schober et al., Citation2003).
The immunomodulating effects of mercury in mammalian systems are well known and its propensity to induce an autoimmune-like condition in susceptible mice has been extensively studied (reviewed by Mathieson, Citation1992). A critical parameter known to influence immunotoxic outcome is age of exposure. The intrauterine period, in which decisive events of lymphocytic development take place, is especially vulnerable to the effects of environmental exposures. It has been suggested that disruption of critical events during immune development in utero could result in altered immune functions that persist into postnatal life and could lead to long-term immune dysfunction. There has also been evidence to suggest depression of immune responses (i.e., hypersensitivity) during adulthood may result from disrupted immune development (Gehrs et al., Citation1997).
Further, the range of immune alterations after postnatal or adult exposure may differ since the fetal immune system is especially vulnerable to environmental insults even at lower concentrations (Dietert, Citation2002). In humans, the data documenting immune changes associated with prenatal mercury exposure have been inconsistent; while Bilrha and co-workers (2003) found no evidence of differential expression of activation markers in cord blood lymphocytes from exposed neonates, others have found specific immune alterations including reductions in the numbers of naïve CD4+ cells and decreased mitogen-induced proliferative responses (Belles-Isles et al., Citation2002). In animal models, in utero HgCl2 exposure of BALB/c mice was found to induce alterations in immune functions that persisted long after cessation of exposure (Silva et al., Citation2005).
Here we characterized the changes in immunophenotypes in murine fetal thymus and liver following maternal exposure to 10 mg/l (ppm) mercuric chloride in drinking water. (BALB/c × DBA/1)F1 (DBF1) mice, which carry the H-2q/d haplotype, known to confer sensitivity to the autoimmune-inducing effects of mercury, were exposed to a single concentration of inorganic mercury that is within the range of concentrations recently shown to modulate immune phenotypes in adult BALB/c mice (Kim et al., Citation2003). We found that gestational exposure to mercuric chloride led to changes in lymphocytic phenotypes in the thymus that included increased numbers of CD4−CD8− thymocytes, reduced expression of activation marker CD25 and, in fetal liver, an increase in the numbers of B-lymphocytes. In both compartments, lymphocytic reactivity to a well-characterized biomarker of autoimmune nephritis (IdLNF1) was found to be increased as well. The implications of prenatal mercury exposure on fetal thymic development and its possible role as an environmental trigger for altered postnatal immune response, including autoimmunity, are discussed.
MATERIALS AND METHODS
Animals
Eight-week-old female BALB/c and male DBA/1 mice were obtained from The Jackson Labs (Bar Harbor, ME) and individually housed under standard conditions of temperature, humidity and 12 hr light/dark cycle until acclimated. Harems consisting of three BALB/c females and one DBA/1 male were setup for overnight breeding. Pregnant dams, ascertained by the presence of vaginal plugs, were housed in separate polycarbonate cages. Mice were treated in accordance with animal use and care guidelines set by the Institutional Care and Animal Use Committee at Cornell University.
Reagents
Mercuric chloride (Sigma, St. Louis, MO) at 10 mg/l (10 ppm) was prepared in solution using endotoxin-free water. RPMI 1640 media, 200 mM l-glutamine, fetal bovine serum, and ammonium chloride (NH4cl) were obtained from Sigma. Non-essential amino acids supplement was obtained from Cellgro (Mediatech, Inc., Herndon, VA).
Experimental Design
Two separate timed-breeding exposure experiments were done. Pregnant dams were arbitrarily assigned to either a control or mercury-treated group. Starting at gestation day (gd) 0 (the day after overnight breeding), mercury-treated dams were fed with 10 ppm mercuric chloride in drinking water while a control group was given plain water, both supplied ad libitum. All mice were observed at least once daily and no mortalities or morbidities were noted. At gd 16, dams were sacrificed and all fetuses extracted and freed from adherent placental tissue. Individual fetal bodies were weighed and fetal thymic tissue and liver were dissected for immunophenotypic studies. Fetal liver provided tissue for DNA extraction that was subsequently used in a multiplex PCR-based assay for the Sry gene in order to distinguish males from females (Lambert et al., Citation2000).
Preparation of Single Cell Suspensions
The liver and thymus were aseptically dissected from each fetus and placed in cold media (RPMI 1640 media supplemented with 10% FBS, β -mercaptoethanol, 1 mM l-glutamine and 0.1 M non-essential amino acids). A Dounce homogenizer was used to mash fetal thymus while frosted ends of glass slides were used to macerate fetal liver to prepare a cell suspension. Erythrocytes were lysed with 0.2 M Tris-buffered NH4Cl solution. Cells were then washed, suspended in media and viable cells were counted by trypan blue exclusion.
Immunophenotyping
Flow cytometry was used to identify lymphocyte populations in the fetal thymus or liver. Briefly, cells were washed in phosphate-buffered saline (PBS) buffer containing 0.5% BSA and 0.1% NaN3 and re-suspended in aliquots of 2 × 105 cells. These cells were subsequently stained with fluorochrome or biotin conjugated monoclonal antibodies at optimum concentrations. After a 20-min incubation in the dark, the stained cells were washed in PBS buffer and incubated with APC Cy7-conjugated to streptavidin for 20 min. After a final washing, the cells were fixed with 1% paraformaldehyde and stored at 4°C until analysis. The following monoclonal antibodies were obtained from BD Pharmingen (San Diego, CA): PE,FITC- or APC-conjugated rat anti-mouse CD8; Biotin-conjugated rat anti-mouse CD25; PeCy7-conjugated rat anti-mouse CD3; APC Cy7-,PE- or APC-conjugated rat anti-mouse CD4; APC-conjugated rat anti-mouse CD44; PE-conjugated rat anti-mouse CD45RB and Biotin-conjugated rat anti-mouse B220. FITC-conjugated rabbit anti-mouse IdLNF1was prepared as previously described (Knupp et al., Citation1995).
Flow Cytometry
Twenty thousand cells were acquired using a FACS LSRII Flow Cytometer (Becton Dickinson, Mountain View, CA). Non-viable cells were excluded by gating on forward versus side angle light scatter. For quantitative analysis of specific lymphocyte populations, WinMDI software was used.
Statistical Analysis
All pups were included in the analysis. The dam was considered the statistical unit of comparison. In the first experiment, there were two dams in the control group and three in the mercury-treated group. In the second experiment, three dams received plain water while three others were fed mercury (). The differences between mercury-exposed and unexposed groups in individual experiments were analyzed statistically using the unpaired two-tailed t-test between means. Likewise, gender-based analyses were done using unpaired t-tests. Results were considered statistically significant at p < 0.05.
TABLE 1 Litter statistics from two independent experiments evaluating the effects of intrauterine exposure to 10 ppm HgCl2
RESULTS
Prenatal Exposure to Mercuric Chloride Did Not Modulate Fetal Body Weight
No deaths or significant morbidities were observed in the dams throughout the course of mercury exposure. shows data from both experiments that included 36 DBF1 pups from a total of five control dams and 33 DBF1 pups from a total of 6 mercury-exposed dams. We noted three cases of fetal resorptions in the mercury-treated group only in the first experiment. This was unexpected since embryotoxic effects of mercury have not been consistently demonstrated in mice, especially at the relatively low doses we used (Inouye and Murakami, Citation1975). Initially, we had thought that this might be the result of toxic effects of mercury but resorptions were not seen in the second timed-breeding experiment. There was no significant difference in average fetal weights in water (0.85 ± 0.24 g for Experiment 1 vs. 1.04 ± 0.14 g for Experiment 2) and mercury-treated groups (0.82 ± 0.26 g for Experiment 1 vs. 0.90 ± 0.28 g for Experiment 2). Interestingly, we found that the M:F sex ratio was skewed slightly towards the female sex in the control group, and there were nearly twice as many females as males in the mercury-treated group (M:F equal to 0.56 for control vs. 0.83 for mercury).
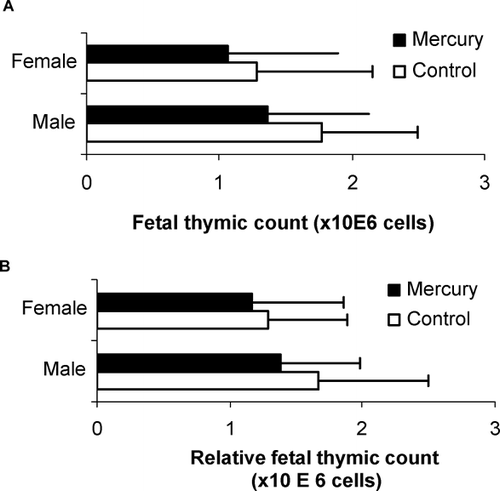
Prenatal Exposure to Mercuric Chloride Did Not Alter Fetal Thymic Cellularity
In order to determine whether mercury exposure affected immune cell development, we first evaluated whether fetal thymic cellularity was altered following in utero mercuric chloride exposure. As shown in , we found that mean thymocyte counts were comparable for both control and mercury-exposed pups among males (1.62 ± 0.85 × 106 for control vs. 1.35 ± 0.85 × 106 for mercury) and females (1.28 ± 0.87 × 106 for control vs. 1.09 ± 0.75 × 106 for mercury). Similar results were found when total thymocyte cell numbers were adjusted for fetal body weight (in males, 1.52 ± 0.73 × 106 for control vs. 1.36 ± 0.73 × 106 for mercury; in females, 1.28 ± 0.82 × 106for control vs. 1.20 ± 0.60 × 106 for mercury) ().
Prenatal Mercury Exposure Modulated Phenotypic Repertoire in the Thymus
We next sought to determine whether in utero exposure to 10 ppm mercuric chloride induced alterations in the phenotypic repertoire of lymphocytes in the developing thymus. In both experiments, immunostaining for CD4 and CD8 antigen expression showed that a majority of gd 16 thymocytes were still in the DN stage. As shown in , the percentage of cells in the DN subset was significantly increased among mercury-exposed pups in both males (73.05 ± 4.51for control vs. 83.72 ± 2.78 for mercury, p = 0.01) and females (78.96 ± 4.34 for control vs. 87.56 ± 5.03 for mercury, p = 0.02). Mercury-exposed mice also showed a striking reduction in the percentages of DP cells in both males (1.91 ± 0.58 for control vs. 0.84 ± 0.22 for mercury, p = 0.01) and females (1.23 ± 0.45 for control vs. 0.55 ± 0.28 for mercury, p = 0.01). Furthermore, total counts of DP cells were also significantly decreased in mercury-exposed pups (in males, 3.73 ± 1.34 × 105 for control vs. 1.41 ± 0.29 × 105 for mercury; in females, 2.14 ± 1.11 × 105 for control vs. 0.79 ± 0.45 × 105 for mercury); however, only a slight increase in the total counts of DN cells was observed ().
TABLE 2 Effects of intrauterine exposure to 10 ppm HgCl2 on CD4/8-expressing gd 16 cells in the thymus
The percentages and total cell counts in the single positive CD4+CD8− compartment were comparable in the mercury-exposed and unexposed groups. On the other hand, the population of thymocytes represented by CD8+CD4− cells was significantly lower in terms of both percentages (in males, 16.26 ± 4.07 for control vs. 8.15 ± 1.89 for mercury, p = 0.01; in females, 12.59 ± 3.34 for control vs. 6.19 ± 2.72 for mercury, p = 0.01) and cell counts for mercury-treated pups. Pup sex did not influence cell phenotype since gender-based analysis showed similar significant trends in the CD4/CD8 thymic populations.
Prenatal Mercury Exposure Down-Modulated Activation Markers in Fetal Thymocytes
We also examined expression of the activation marker CD25+ (α -chain of the IL-2 receptor) in gd 16 CD4+ thymocytes and saw that the percentages of CD4+ cells co-expressing CD25+ were significantly reduced in mercury-exposed male (26.05 ± 3.73 for control vs. 12.74 ± 3.83 for mercury, p = 0.02) and female mice (23.56 ± 9.10 for control vs. 13.45 ± 4.73 for mercury, p = 0.04) (). These effects were also seen when total cell counts were enumerated.
TABLE 3 Effects of intrauterine exposure to 10 ppm HgCl2 on CD25 expression of gd 16 thymic cells
Prenatal Exposure to Mercury-Induced Modulations in the B-cell Compartment
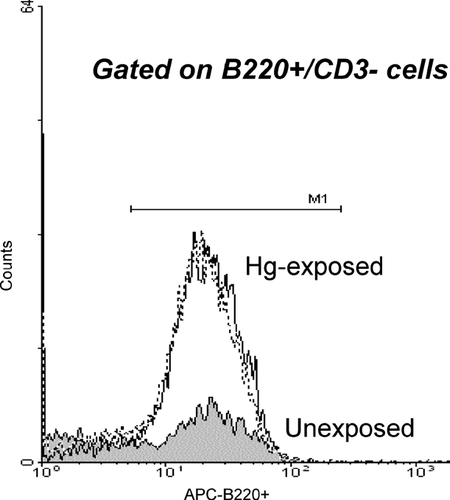
The effect of prenatal mercury exposure on the B-cell compartment was evaluated by examining fetal liver-derived lymphocytes for the expression of B220, while simultaneously excluding for CD3 expression. As shown in , we saw a significant increase in the mean fluorescent intensity of B220+ staining in the liver of mercury-exposed pups.
FIG. 2 Effect of intrauterine exposure to 10 ppm HgCl2 on B220 expression in liver of gd 16 mice measured as mean fluorescence intensity. Dashed line refers to male-mercury-exposed pups; solid line refers to female mercury-exposed pups. Data is representative of two independent timed-breeding experiments.
Mercury Exposure Induced an Increased in Autoimmune-Associated Phenotypes
We further investigated whether immunophenotypic changes resulting from mercury exposure were associated with autoimmunity. To do this, we examined cells for their reactivity against a biomarker for autoreactivity, the IdLNF1 idiotypic determinant, found on autoantibodies deposited in the kidneys of SNF1 mice, which develop a lethal form of immune-complex nephritis that resembles human SLE nephritis. As shown in , IdLNF1-reactivity among CD4+-expressing thymocytes from mercury-treated pups was significantly increased in both the percentages of reactive cells (0.87 ± 0.59 for control vs. 2.06 ± 1.05 for mercury, p = 0.05) and total cell counts (0.64 ± 0.11 × 105 for control vs. 0.36 ± 0.24 × 105 for mercury, p = 0.05). Further, fetal liver-derived B-cells () also showed a significant increase in the percentage of B220+CD3− cells reactive against IdLNF1 (44.47 ± 12.93 for control vs. 62.28 ± 10.83 for mercury, p = 0.05). However, the total cell counts of IdLNF1-reactive B-cells, while generally higher in mercury-exposed pups, did not achieve statistical significance. The high variability of the total cell counts of gd 16 liver were most likely due to differences in the weights of the fetuses, and consequently, organ size, due to differences in the sizes of the litters.
TABLE 4 Effects of intrauterine exposure to 10 ppm HgCl2 on IdLNF1-reactivity in gd 16 (A) thymus and (B) liver
DISCUSSION
Several groups have reported that the developing fetal immune system is especially vulnerable to the immunotoxic effects of various environmental xenobiotics such as mercury (Silva et al., Citation2005) and other heavy metals (Dietert et al., Citation2004). Further, the immune alterations that result from exposure may be irreversible and highly exacerbated compared to adult exposures. Several investigators have reported that perturbations during critical phases of lymphocytic development and maturation persist post-natally (Miller et al., Citation1998) lead to altered adult immune repertoire and function (Gehrs et al., Citation1997; Holladay and Smialowicz, Citation2000). For example, several animal studies have suggested that prenatal xenobiotic exposure may lead to depression of immune responses such as delayed-type hypersensitivity responses (Gehrs et al., Citation1997; Miller et al., Citation1998).
This may, in part, be due to alterations in cytokine production since prenatal exposure of BALB/c mice to mercuric chloride induced distinctive profiles of splenic and lymph node cytokine production in pre-weaned pups (Silva et al., Citation2005). More importantly, the altered cytokine production persisted in adult mice. These data support the hypothesis that gestational exposures to environmental xenobiotics can have long-term effects on postnatal immune function.
In this study, we sought to characterize the effects of mercury exposure on the developing immune repertoire by evaluating changes in specific lymphocyte phenotypes in the fetal thymus and liver after gestational exposure to 10 ppm mercuric chloride (HgCl2). This level of mercury exposure, although slightly higher than some studies (Hultman and Nielsen, Citation2001), falls within the range of concentrations recently shown to induce alterations in immune phenotypes in adult mice (Kim et al., Citation2003) and is less than a derived lowest observed adverse effect level (LOAEL) of 15 ppm for immunotoxicity of mercuric chloride (Dietert et al., 1983).
We found that while intrauterine mercury exposure did not affect fetal thymic and liver cellularity, the modulations in immunophenotypes observed included a pattern of intrathymic dysregulation (i.e., high DN and low DP) that has been described for other known environmental toxicants, including TCDD (Holladay et al., Citation1991; Blaylock et al., Citation1992; Lai et al., Citation1998), DES (Holladay et al., Citation1993), and ethylene glycol monomethyl ether (Holladay et al., Citation1994). Although the long-term immune consequences of these phenotypic changes are unknown, disruptions of lymphocytic maturational events could potentially lead to altered postnatal immune responses. Indeed, HgCl2 exposure has recently been found to induce changes in postnatal immune function as well as sex-specific modulations in cytokine production of adults (Silva et al., Citation2005). On the other hand, exposure of adult CD-1 mice to levels of mercuric chloride that were 10-fold higher (100 ppm) for a more protracted period, did not induce changes in T-cell and B-cell phenotypes (Brunet et al., Citation1993). These data support the hypothesis that the intrauterine period is more vulnerable to the effects of low-level exposure to environmental toxicants.
We also noted other phenotypic modulations resulting from intrauterine mercury exposure, specifically reductions in the numbers of CD4+ cells expressing CD25+, a subset of cells that includes a class of regulatory T-cells (Tregs) (Apostolou et al., Citation2002). Notably, our data showing significantly decreased percentages of CD4+CD25+ T-cells in mice exposed to mercury in utero are consistent with proposed models suggesting that regulatory T-cells may play a role in susceptibility to metal-induced autoimmunity in rats (Kosuda et al., Citation1991). Further evidence supporting an immunoregulatory role for Tregs in mercury-induced autoimmunity has been derived from a study that found adoptive transfer of CD4+CD25+ T-cell fractions into mercury-treated H-2s mice effectively prevented the formation of anti-nuclear autoantibodies (ANA) (Layland et al., Citation2004). However, while there is substantial evidence to date supporting a significant role for immunoregulatory populations in modulating strain-specific responses to mercury-induced autoimmunity, it is likely that other factors such as genetic susceptibility and cytokine secretion profile may influence loss of tolerance mechanisms.
The B-cell compartment has been generally regarded to be more sensitive to the immunotoxic effects of mercury than the T-cell compartment and polyclonal activation of B-cells has been consistently demonstrated in mercury-induced autoimmunity (Nielsen and Hultman, Citation2002). In this study, we found that in utero exposure to mercury altered the fetal B-cell repertoire. However, although the percentages of CD3−B220+ cells in the fetal liver were appreciably increased among mercury-exposed pups, total cell counts were comparable to controls. Taken together, these data suggest that mercury exposure leads to skewing of B-cell development to a particular phenotype rather than expansion of the B-cell compartment. In contrast, and similar to what has been reported for T-cells, adult exposure to oral mercuric chloride at concentrations as high as 37 ppm failed to demonstrate significant changes in B220+-expressing B-cells (Kim et al., Citation2003), a finding that has also been seen in rat models of mercury-induced autoimmunity (Kosuda et al., Citation1998).
Our data also revealed that prenatal mercury exposure resulted in the significant expansion of lymphocytes reactive with the IdLNF1 biomarker that is associated with the pathogenesis of spontaneous autoimmune glomerulonephritis in the SNF1 model for human SLE nephritis. In female SNF1 mice, the numbers of IdLNF1-reactive B-cells and IdLNF1 immunoglobulins increase over time, correlating significantly with the onset of immune complex deposition in the kidneys and ultimately culminating in lethal glomerulonephritis (Knupp et al., Citation1992). Furthermore, adoptive transfer studies have shown that these cells can transfer disease to young pre-nephritic SNF1 mice (Knupp et al., Citation1995). While DBF1 mice do not develop spontaneous autoimmunity, they share the same MHC background as SNF1 mice; indeed, we have found that treatment of DBF1 mice with estrogen led to the development of lupus nephritis like that in the SNF1 mouse, with immunophenotypic changes and clinical manifestations that are very similar to those involved in SNF1 disease (manuscript submitted). Our results demonstrating significantly increased reactivity to the pathogenic IdLNF1 idiotype in T-cells and B-cells after mercury exposure of DBF1 pups offer strong support for the potential role of metals as triggers of autoimmune disease especially in at-risk genetic backgrounds.
Based on studies of developmental immunotoxicity by metals and evidence of autoimmune dysfunction among mercury-exposed populations (Silva et al., Citation2004) it is likely that the immunophenotypic changes that we found to be induced by prenatal mercury exposure will be persistent (Miller et al., Citation1998) and could potentially develop into altered immune responses later in adulthood. Thus, our study demonstrates the potential health risks associated with intrauterine metal exposure to include phenotypic changes related to postnatal immune dysfunction. This is highly relevant in light of recent evidence showing increased risk of mercury exposure among newborns as a result of maternal mercury intake.
The Authors wish to express their gratitude to Michelle Banyai for her selfless assistance during cell harvests. This work was supported by a grant from the Children's Miracle Network Foundation (to JG) and the James A Pucello and Margie Kehoskie Memorial Foundation (to JG).
Author Gavalchin is also affiliated with the Department of Medicine, SUNY Upstate Medical University, 750 East Adams St., Syracuse, NY 13210 and the Department of Animal Science, Cornell University, Ithaca, NY 14853, USA.
REFERENCES
- Apostolou I., Sarukhan A., Klein L., Von Boehmer H. Origin of regulatory T-cells with known specificity for antigen. Nature Immunol. 2002; 3: 756–763
- Bagenstose L., Salgame P., Monestier M. Murine mercury-induced autoimmunity. Immunol. Res. 1999; 20: 67–78
- Belles-Isles M., Ayotte P., Dewailly E., Weber J. P., Roy R. Cord blood lymphocyte functions in newborns from a remote maritime population exposed to organochlorines and methylmercury. J. Toxicol. Environ. Health A 2002; 65: 165–182
- Bilrha H., Roy R., Moreau B., Belles-Isles M., Dewailly E., Ayotte P. In vitro activation of cord blood mononuclear cells and cytokine production in a remote coastal population exposed to organochlorines and methylmercury. Environ. Health Perspect. 2003; 111: 1952–1957
- Blaylock B. L., Holladay S. D., Comment C. E., Heindel J. J., Luster M. I. Exposure to tetrachlordibenzo-p-dioxin (TCDD) alters fetal thymocyte maturation. Toxicol. Appl. Pharmacol. 1992; 112: 207–213
- Brunet S., Guertin F., Flipo D., Fournier M., Krzystyniak K. Cytometric profiles of bone marrow and spleen lymphoid cells after mercury exposure in mice. Int. J. Immunopharmacol. 1993; 15: 811–819
- Dieter M. P., Luster M. I., Boorman G. A., Jameson C. W., Dean J. H., Cox J. W. Immunological and biochemical responses in mice treated with mercuric chloride. Toxicol. Appl. Pharmacol. 1983; 68: 218–228
- Dietert R. R., Le J. E., Hussain I., Piepenbrink M. Developmental immunotoxicology of lead. Toxicol. Appl. Pharmacol. 2004; 198: 86–94
- Dietert R. R., Lee J. E., Bunn T. L. Developmental immunotoxicology: Emerging issues. Human Exp. Toxicol. 2002; 21: 479–485
- Gehrs B., Riddle M., Williams W. Alterations in the developing immune system of the F344 rate after perinatal exposure to 2,3,7, 8-tetrachlorodibenzo-p-dioxin. II: Effects on the pup and adult. Toxicology 1997; 122: 229–240
- Holladay S. D., Blaylock B. L. The mouse as model for developmental immunotoxicology. Human Exp. Toxicol. 2002; 21: 525–531
- Holladay S. D., Smialowicz R. J. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000; 108(S3)463–473
- Holladay S. D. Prenatal immunotoxicant exposure and postnatal autoimmune disease. Environ. Health Perspect. 1999; 107(S5)687–691
- Holladay S. D., Smith B. J. Alterations in murine fetal thymus and liver hemotapoietic cell populations following developmental exposure to 7,12-dimethylbenz(a)anthracene. Environ. Res. 1995; 68: 106–113
- Holladay S. D., Comment C. E., Kwon J., Luster M. I. Fetal hematopoietic alterations after maternal exposure to ethylene glycol monomethyl ether: Prolymphoid cell targeting. Toxicol. Appl. Pharmacol. 1994; 129: 53–60
- Holladay S. D., Blaylock B. L., Comment C. E., Heindell J. J., Fox W. M., Korack W. M., Luster M. I. Selective prothymocyte targeting by prenatal diethylstilbestrol exposure. Cell Immunol. 1993; 152: 131–142
- Holladay S. D., Lindstrom P., Blaylock B. L., Comment C. E., Germolec D. R., Heindell J. J., Luster M. I. Perinatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p-dioxin (TCDD). Teratology 1991; 44: 385–393
- Hultman P., Nielsen J. The effect of dose, gender, and non-H2 genes in murine mercury-induced autoimmunity. J. Autoimmun. 2001; 17: 27–37
- Inouye M., Murakami U. Teratogenic effect of orally administered methylmercuric chloride in rats and mice. Cong. Anom. 1975; 15: 1–9
- Kim S. H., Johnson V. J., Sharma R. P. Oral exposure to inorganic mercury alters T-lymphocyte phenotypes and cytokine expression in BALB/c mice. Arch. Toxicol. 2003; 77: 613–620
- Knupp C. J., Uner A. H., Tatum A., Kakanar J., Gavalchin J. IdLNF1-specific T cell clones accelerate the production of IdLNF1+ IgG and nephritis in SNF1 mice. J. Autoimmun. 1995; 8: 367–380
- Knupp C. J., Uner A. H., Tatum A. H., Gavalchin J. The onset of nephritis in the (NZB × SWR) F1 murine model for systemic lupus erythematosus correlates with an increase in the ratio of CD4 to CD8 lymphocytes specific for the nephritogenic idiotype (IdLNF1). Clin. Immunol. Immunopath. 1992; 65: 167–175
- Kosuda L. L., Whalen B., Greiner D. L., Bigazzi P. E. Mercury-induced autoimmunity in Brown Norway rats: Kinetics of changes in RT6+ T-lymphocytes correlated with IgG isotypes of circulating autoantibodies to laminin 1. Toxicology 1998; 125: 215–231
- Kosuda L. L., Hannigan M. O., Bigazzi P. E., Leif J. H., Greiner D. L. Thymus atrophy and changes in thymocyte subpopulations of BN rats with mercury-induced renal autoimmune disease. Autoimmunity 1996; 23: 77–89
- Kosuda L. L., Hosseinzadeh H., Greiner D. E., Bigazzi P. E. The role of RT6+ lymphocytes in mercury-induced renal autoimmunity: Experimental manipulations of ‘susceptible’ and ‘resistant’ rats. J. Toxicol. Environ. Health 1994; 42: 303–321
- Kosuda L. L., Wayne A., Nuhounou M., Greiner D. L., Bigazzi P. E. Reduction of the RT6.2+ subset of T-lymphocytes in BN rats with mercury-induced renal autoimmunity. Cell Immunol. 1991; 135: 154–167
- Lai Z. W., Fiore N. C., Gasiewicz T. A., Silverstone A. E. 2,3,7,8-Tetra-chlorodibenzo-p-dioxin and diethlystilbestrol affect thymocytes at different stages of development in fetal thymus organ culture. Toxicol. Appl. Pharmacol. 1998; 149: 167–177
- Lambert J. F., Benoit B. O., Colvin G. A., Carlson J., Delville Y., Quesenberry P. J. Quick sex determination of mouse fetuses. J. Neurosci. Meth. 2000; 95: 127–138
- Layland L. E., Wulferink M., Dierkes S., Gleichmann E. Drug-induced autoantibody formation in mice: Triggering by primed CD4+CD25- T-cells, prevention by primed CD4+CD25+ T-cells. Eur. J. Immunol. 2004; 34: 36–46
- Lee J. E., Dietert R. R. Developmental immunotoxicity of lead: Impact on thymic function. Birth Defects Res. (Part A) 2003; 67: 861–867
- Mathieson P. W. Mercuric chloride-induced autoimmunity. Autoimmunity 1992; 13: 243–247
- Miller T. E., Golemboski K. A., Ha R. S., Bun T. L., Sanders F. S., Dietert R. R. Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicol. Sci. 1998; 42: 129–135
- Nielsen J. B., Hultman P. Mercury-induced autoimmunity in mice. Environ. Health Perspect. 2002; 110(S5)877–881
- Pollard K., Hultman P. Effects of mercury on the immune system (Review). Metal Ions Biol. Syst. 1997; 34: 421–440
- Powell J. J., Van de Water J., Gershwin M. E. Evidence for the role of environmental agents in the initiation or progression of autoimmune conditions. Environ. Health Perspect. 1999; 107(S5)667–672
- Schober S. E., Sinks T. H., Jones R. L., Bolger P. M., McDowell M., Osterloh J., Garrett E. S., Canady R. A., Dillon C. F., Sun Y., Joseph C. B., Mahaffey K. R. Blood mercury levels in US children and women of childbearing age, 1999-2000. J. Am. Med. Assoc. 2003; 289: 1667–1674
- Silva I. A., El Nabawi M., Hoover D., Silbergeld E. K. Prenatal HgCl2 exposure in BALB/c mice: Gender-specific effects on the ontogeny of the immune system. Dev. Comp. Immunol. 2005; 29: 171–183
- Silva I. A., Nyland J. F., Gorman A., Perisse A., Ventura A. M., Santos E. C. O., de Souza J. M., Burek C. L., Rose N. R., Silbergeld E. K. Mercury exposure, malaria and serum anti-nuclear/anti-nucleolar antibodies in Amazon populations in Brazil: A cross-sectional study. Environ. Health: A Global Access Science Source 2004; 3: 11–22
- Thuvander A., Sundberg J., Oskarsson A. Immunomodulating effects after perinatal exposure to methymercury in mice. Toxicology 1996; 114: 163–175