Abstract
Acute cytokine release syndromes are associated with some therapeutic antibodies in man, leading to a spectrum of clinical signs from nausea, chills and fever to more serious dose limiting hypotension and tachycardia. When anticipated this syndrome is typically manageable, however this adverse reaction recently became headline news when a massive and unexpected cytokine release syndrome occurred within a few hours of dosing six healthy volunteers with a therapeutic antibody, putting their lives at risk due to multiple organ failure. Preclinical studies did not predict this adverse event, emphasising the need to compare the relative potency of the product in man and the chosen toxicology species, so that additional margins of safety can be applied when conducting first in man (FIM) studies if there is uncertainty over the predictability of the toxicology species. In vitro human PBMC and whole blood cultures may be useful for predicting cytokine release. However since cytokine release arises through at least two distinct mechanisms, it should be emphasised that the utility of these in vitro methods needs to be established for each antibody product.
INTRODUCTION
In March 2006, the now familiar term “cytokine storms” became headline news as a result of the adverse events observed in six healthy volunteers following their infusion with TeGenero's monoclonal antibody product TGN1412. The severity of this life threatening reaction, which required the volunteers to be admitted to an intensive care unit (Suntharalingam et al., Citation2006), has sparked a wide ranging international debate on the adequacy of drug development guidelines for biotechnology derived pharmaceuticals and the predictability of preclinical studies. This article will review the mechanisms behind monoclonal antibody dependent cytokine release syndrome which has been recognized for at least 15 years, and discusses whether we can predict the likelihood of their occurrence prior to first in man (FIM) studies.
Clinical Syndrome
The first administration of some monoclonal antibodies in man has for some time been associated with an acute adverse event typified by the appearance of plasma cytokines within a few hours of infusion. The clinical symptoms range in their severity from fever, chills and nausea to, potentially lethal, hypotension and tachycardia. The activation of endothelial cells by the cytokines is most likely responsible for leukocyte adhesion and margination, leading to transient leukocytopenia and thrombocytopenia that, in severe cases, may be evident for several days. The relationship between symptoms and cytokines has been confirmed by the ability of corticosteroids to prevent both the cytokine release and associated symptoms, stimulating “molecular engineering” approaches to reduce the immunostimulatory properties of the antibodies.
The syndrome was first described in the early 1990s with the mouse anti-CD3 monoclonal antibody muromonab-CD3 (ORTHOCLONE OKT® 3) that was being used to prevent allograft rejection by depleting T-lymphocytes (Chatenoud et al., Citation1990). Other antibodies known to be associated with first dose syndromes include the chimeric Rituximab (Rituxan® and MabThera®) (Winkler et al., Citation1999) and humanised Alemtuzumab (Campath and Campath-1H) (Uppenkamp, Citation2002) that recognise CD20 and CD52 on lymphocytes respectively (). Although some patients receiving these antibodies experienced severe reactions, these were clearly distinguishable from the life-threatening situation experienced by the six healthy volunteers who recently received the CD28 “superagonist” antibody TGN1412, which resulted in their administration to intensive care due to the severity of the syndrome which, ultimately, included multiple organ failure (Suntharalingam et al., Citation2006).
TABLE 1 Monoclonal antibody drugs known to be associated with first dose cytokine release syndromes in man
Mechanisms
The signature cytokines for the syndrome are TNFα, IFNγ, and IL-6 but may include others dependent upon the mechanism and hence the cellular source of the cytokines. The ligation of the CD3 molecule by muromonab-CD3 is thought to transiently cause T-lymphocyte activation and cytokine release, which in addition to the above cytokines includes IL-2, presumably a reflection of the fact that the T-lymphocyte is the principle source of the cytokines for this drug. In the case of Rituximab and Alemtuzumab, since ligation of CD20 and CD52 are not known to cause cytokine release through activation of the targeted cell, an alternative mechanism and cellular source of cytokine to that targeted by the antibody needs to be sought. In the case of Alemtuzumab, natural killer (NK) cells have been identified as the source of cytokines, which are secreted following ligation of CD16, the low affinity Fc receptor for IgG, by antibody opsonised lymphocytes (Wing et al., Citation1996).
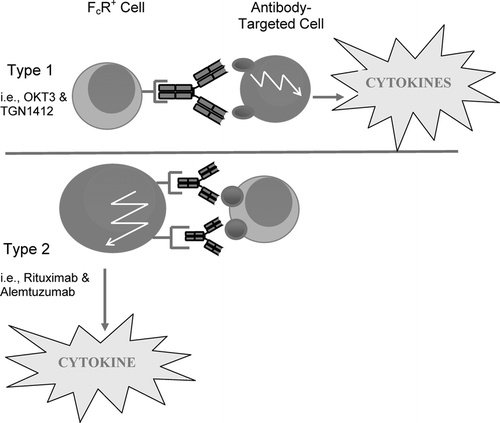
This second (or Type 2) mechanism is clearly different from the Type 1 agonist antibody reaction exhibited by muromonab-CD3. In this respect, the massive release of cytokines seen with TGN1412 is more likely to be due to a targeted receptor-dependent Type 1 mechanism rather than Type 2 Fc receptor-dependent, since CD28 is a known activation receptor and the antibody intentionally exhibits an agonist mechanism of action ().
FIG. 1 In Type 1 reactions, cytokines are released from the targeted cell, following drug binding to “activation” receptors via the Fab region of the antibody. Receptor binding by the agonist drug results in the activation of signal transduction pathways leading to cytokine synthesis. For Type 2 reactions, cytokine release occurs following ligation of low affinity Fc receptors (FcR+) by antibody opsonised target cells. Activation of signal transduction pathways, this time associated with the FcR, results in cytokine synthesis in the Type 2 reaction.
In vitro Prediction
From the above it is apparent that there are at least two mechanisms responsible for cytokine release syndromes seen following the administration of some monoclonal antibody products to man. Given the uncertainty of whether such a reaction will occur with a novel product there is a need to predict the likelihood of such a reaction. In vitro human whole blood or peripheral blood mononuclear cell cultures have been successfully used to accurately predict the extent of cytokine release in man with some antibody products. In one published study, a series of humanised monoclonal antibodies which all recognized CD52 but differed in their constant region isotype, were incubated in heparin whole blood revealing a good correlation between in vitro and in vivo cytokine release see clinically () (Wing et al., Citation1995).
TABLE 2 Comparison of cytokine release seen with therapeutic antibodies using human whole blood cultures in vitro compared to the clinical finding
Importantly, cytokine release was detected in vitro within a few hours, demonstrating similar kinetics to their appearance in vivo, with cytokine release associated with antibody isotypes known to interact with this human Fc receptor. Of note, a humanised antibody that recognised the lymphocyte antigen CD4, which had a human IgG1 isotype and therefore had the potential to interact with Fc receptors in vitro and in vivo, did not stimulate cytokine release clinically or experimentally in the whole blood cultures. This observation indicated that although Fc receptor interaction was a prerequisite for Type 2 reactions, it was not the sole factor responsible for cytokine release, and that receptor number for instance might influence cytokine release by affecting the density of target cell opsonisation and subsequently the strength of interaction with low affinity Fc receptors.
In a series of further experiments using T-lymphocytes or NK cells purified by negative selection, the mechanism of cytokine release observed using Alemtuzumab, the human IgG1 antibody recognizing CD52, was clearly Type 2-mediated, arising through CD16 ligation on NK cells (Wing et al., Citation1995). Whole blood cultures are also able to predict cytokine release following incubation with Rituximab, which like Alemtuzumab, stimulates cytokine release via a Type 2 mechanism. It is noticeable in these studies that the absolute level of cytokine release varies between individuals, which may in part reflect Fc receptor polymorphisms that influence antibody-dependent cell cytotoxicity (ADCC) reactions to the extent that they significantly influence product potency and clinical outcome clinically (Treon et al., Citation2005).
As part of the investigation by the UK National Institute for Biological Standards and Controls (NIBSC) which was prompted by the clinical findings in the FIM TGN1412 study, this humanized IgG4 antibody was incubated in human whole blood and lymphocyte cultures. However unlike the clinical situation where massive cytokine release was detected, cytokines could not be detected in vitro unless the antibody was immobilised by “dry coating” the plates with the product (Stebbings et al., Citation2007). The in vivo mechanism that in vitro dry coating replicates is as yet unclear, but it is intriguing that a human endothelial cell line was also able to present the product in these in vitro cultures in such a way as to stimulate cytokine release. Because TGN1412 stimulates cytokine release clinically, it is reasonable to explore any culture system to replicate this property in vitro, however the challenge, going forward, is to use in vitro cultures which can predict the likelihood that a product which has not undergone clinical testing will elicit unacceptable levels of cytokine release. A concern over the dry coating technique is that this screen will result in too many false positives, because the non-physiological presentation of product at such high density will stimulate cytokine release in vitro by products that do not pose a threat to human safety.
A review of the antibodies in the public domain which cause clinically significant levels of cytokine release and their mechanism of action have identified a number of properties which may alert investigators to their potential to cause adverse events in man. These include products which target lymphocyte activation receptors which are at risk of mediating cytokine release through Type 1 mechanisms, and antibodies at risk of eliciting Type 2 reactions would be those targeting highly expressed receptors regardless of their activation potential or antibody products which recruit cytotoxic Fc effector function. For these at risk products, it seems reasonable to utilize any human culture system to study cytokine release in vitro and then use comparable animal cultures to identify a relevant toxicology species. For low risk antibodies, non-physiological cultures should be avoided to prevent the generation of irrelevant data.
Preclinical Assessments
The measurement of acute cytokine release on preclinical toxicology studies was variably being undertaken prior to TGN1412 for a range of biopharmaceutical products that have included recombinant cytokines, antisense products and monoclonal antibodies. For the development of biopharmaceuticals where toxicity is typically associated with “on target” exaggerated pharmacology, a central principle of the ICHS6 guideline for the development of biologically derived pharmaceutical products is the importance of choosing a toxicology species in which the product has appropriate pharmacological activity. For a monoclonal antibody whose activity is dependent on both binding to its target receptor through the Fab region and Fc effector activities recruited through interaction with Fc receptors or complement proteins, full activity will only be achieved if both interactions/pathways are active in the animal species.
There is an increasing expectation by the regulatory authorities to undertake relative potency assessments between potential toxicology species and man. This was most recently expressed in the EMEA's guidance on requirements for first-in-man clinical trials for high-risk medicinal products (EMEA/CHMP/ SWP/28367/07), which was drafted in response to the TGN1412 clinical findings. This guidance requests that, for products where there are concerns for an adverse reaction in man that the preclinical package is unable to address, a formal demonstration of relative product potency in the toxicology species compared to man is performed to assist with choosing a safe starting dose in man.
Dose Selection for FIM Studies
Since, for some depleting monoclonal antibodies, cytokine release is an expected or indeed essential component of their intentional mechanism of action, then cytokine release should be detected in vitro and/or in vivo if the correct species and dose has been selected during the safety assessment. It has been our experience with monoclonal antibody products at risk of eliciting cytokine release in man because they targeted lymphocyte activation receptors, that when there has been good product potency in vitro using cells derived from the toxicology species, then dose dependent cytokine release and associated symptoms have been detected in vivo in rodents and cynomolgus monkeys. In the case of the cynomologus monkey study, elevated IL-6, interferon-γ and soluble p-selectin were observed within 2 hr of dosing and associated with reversible hypotension, hypomotility, and piloerection (unpublished observation).
In situations where good potency cannot be obtained in the toxicology species, such that the expected cytokine release is not detected, then this uncertainty needs to be factored into the starting dose used for the FIM study, which post TGN1412 is likely to be one based on the minimum anticipated biological effect level (MABEL) in man as well as the traditional no adverse effect level (NOAEL) derived from the preclinical studies. In situations where there are differences the MABEL and NOAEL, then the EMEA guidance suggests that the calculation giving the lowest value is used as the basis of the FIM starting dose. Such a cautious approach for dose selection will provide an additional margin of safety for a product whose cytokine releasing potential was not predicted on theoretical grounds or detected in vitro or in vivo.
Future Development of Biopharmaceuticals
Finally, it is important to recognize that acute cytokine release is not necessarily a fatal blow to a product's development, since as already mentioned, many depleting antibodies intentionally stimulate cytokine release as part of their mechanism of action. Antibodies in this class include Rituximab and Alemtuzumab, where the good clinical management of the anticipated cytokine symptoms by dose fractionation, use of corticosteroids and patient evaluation has enabled these marketed products to be successfully used to treat lymphoma. The situation of TGN1412 was clearly different and unacceptable, in that unexpected cytokine release put the lives of healthy volunteers at risk. It is hoped that the lessons of this tragedy have been learnt and that this does not prejudice the development of future biopharmaceutical products, which have with only a few exceptions, a good safety record.
CONCLUSIONS
In conclusion, at least two mechanisms are known to be involved in monoclonal antibody cytokine release syndromes, the risk of which is increased when the antibody activates lymphocytes or binds to Fc receptors and targets abundant receptors. Both in vitro assays and preclinical animal studies are already being used by some companies developing monoclonal antibodies to successfully predict the risk of seeing cytokine release syndromes in man, though the utility of these approaches need to be carefully evaluated for each antibody. Importantly the relative potency of the product in human and animal cultures should be measured to allow a safe starting dose in man to be calculated.
REFERENCES
- Chatenoud L., Ferran C., Legendre C., Thouard I., Merite S., Reuter A., Gevaert Y., Kreis H., Franchimont P., Bach J. F. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation 1990; 49: 697–702
- Fietz T., Thiel E. Antibody therapy in non-Hodgkin's lymphoma: The role of rituximab, 90Y-ibritumomab tiuxetan, and alemtuzumab. Recent Results Cancer Res. 2007; 176: 153–163
- Plosker G. L., Figgitt D. P. Rituximab: A review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs 2003; 63: 803–843
- Stebbings R., Findlay L., Edwards C., Eastwood D., Bird C., North D., Mistry Y., Dilger P., Liefooghe E., Cludts I., Fox B., Tarrant G., Robinson J., Meager T., Dolman C., Thorpe S. J., Bristow A., Wadhwa M., Thorpe R., Poole S. “Cytokine storm” in the Phase I trial of monoclonal antibody TGN1412: Better understanding the causes to improve preclinical testing of immunotherapeutics. J. Immunol. 2007; 179: 3325–3331
- Suntharalingam G., Perry M. R., Ward S., Brett S. J., Castello-Cortes A., Brunner M. D., Panoskaltsis N. Cytokine storm in a Phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. New Engl. J. Med. 2006; 10: 1–11
- Treon S. P., Hansen M., Branagan A. R., Verselis S., Emmanouilides C., Kimby E., Frankel S. R., Touroutoglou N., Turnbull B., Anderson K. C., Maloney D. G., Fox E. A. Polymorphisms in Fcχ RIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenström's macroglobulinemia. J. Clin. Oncol 2005; 23: 474–481
- Todd P. A., Broden R. N. Muromonab CD3. A review of its pharmacology and therapeutic potential. Drugs 1989; 37: 871–899
- Uppenkamp M., Engert A., Diehl V., Bunjes D., Huhn D., Brittinger G. Monoclonal antibody therapy with CAMPATH-1H in patients with relapsed high- and low-grade non-Hodgkin's lymphomas: A multicenter Phase I/II study. Ann. Hematol. 2002; 81: 26–32
- Wing M. G., Waldmann H., Isaacs J., Compston D. A. S., Hale G. Ex-vivo whole blood cultures for predicting cytokine-release syndrome: Dependence on target antigen and antibody isotype. Ther. Immunol. 1995; 2: 183–190
- Wing M. G., Moreau T., Greenwood J., Smith R., Hale G., Isaacs J., Waldmann H., Lachmann P. J., Compston D. A. S. Mechanism of first-dose cytokine release by CAMPATH 1-H: Involvement of CD16 (FcχR111) and CD11a/18 (LFA-1) on NK cells. J. Clin. Invest. 1996; 98: 2819–2826
- Winkler U., Jensen M., Manzke O., Schulz H., Diehl V., Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8). Blood 1999; 94: 2217–2224