Abstract
Immune-mediated liver diseases contribute significantly to morbidity and mortality due to liver failure and the need for liver transplantation. The pathogenesis of the immune-mediated chronic liver diseases, primary sclerosing cholangitis, autoimmune hepatitis, and primary biliary cirrhosis, is poorly understood. Genetic susceptibility factors may play a role, but increasing attention is being given to the association between environmental factors and these diseases. The existence of such a relationship is supported by epidemiologic surveys, animal models, and geographic clustering analyses. Unearthing the cause of this association may provide insight into the pathogenesis of immune-mediated chronic liver diseases and autoimmunity.
SUMMARY
Primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), and autoimmune hepatitis (AIH) are often grouped together as immune-mediated liver diseases, though the etiology and pathogenesis of each is poorly understood. Serum anti-nuclear autoantibodies are common to each disease, though more frequent in AIH. Whether this loss of self-tolerance is due to cross-reactivity or self-antigen modification by an environmental toxin is unclear. The prevalence rate for each disease is low but similar (10–40/100,000 population). Overlap syndromes have been reported but account for less than 10% of cases. Despite the low number of cases, together these diseases account for approximately 20% of the patients undergoing liver transplantation each year. Etiological and pathogenic factors associated with these diseases have been difficult to identify, in part, because the diseases generally are diagnosed many years after their presumed onset, which is asymptomatic.
Few epidemiological studies have compared these diseases in the same population. In the same Norwegian population, the incidence and prevalence of PSC (incidence 1.31/100,000 and prevalence 8.5/100,000) was slightly less than that of AIH (incidence 1.9 cases/100,000 and prevalence 16.9/100,000) and PBC (incidence 1.6/100,000 and prevalence 14.6/100,000). The only other population-based epidemiological study of PSC comes from Spain in which the incidence of PSC was 0.068/100,000 with a point prevalence of 0.22/100,000 population. As with other immune-related conditions, it was noted that the incidence and prevalence was lower in a southern European country. Geographical differences in sunlight exposure do affect serum Vitamin D levels, which in turn affect immune function. In addition to its effect on Vitamin D, ultraviolet light induced modification of chemicals or toxins in the skin may also play a role in autoimmunity. An amalgamation of genetics, ultraviolet light and toxin exposure may influence the prevalence rate of autoimmune liver diseases.
Proximity to toxic waste sites has been associated with an increased prevalence of certain cancers and thyroid disease. Recently, we have found that small, but statistically significant clusters of individuals with PSC and PBC exist near certain well-characterized toxic waste sites in New York City. Benzene, a ubiquitous environmental toxin, was a common contaminant found in high concentrations in toxic sites associated with disease clusters. Expansion of these studies to additional areas may be useful in confirming our initial observation and in identifying additional toxins involved in the etiopathogenesis of PSC, PBC, or AIH. Thus, the goal of our ongoing studies is to detect additional toxic sites significantly associated with clusters of individuals with PSC, PBC, or AIH and to identify the toxin(s) that these sites have in common. Using a specialized apparatus, mice will be exposed long-term to these toxins and then analyzed for hepatic and immunologic effects. If available, specific markers of prolonged toxin exposure will be analyzed in tissues obtained from individual patients. A clinical study of the effects of Vitamin D on immune function is also ongoing. In summary, current studies indicate that an amalgamation of genetics, ultraviolet light exposure and environmental toxins may influence the pathogenesis of PSC.
I. INTRODUCTION
Primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), and autoimmune hepatitis (AIH) are often grouped together as immune-mediated liver diseases, though the etiology and pathogenesis of each is poorly understood. Serum anti-nuclear autoantibodies are common to each disease, though more frequent in AIH. Additionally, anti-mitochondrial autoantibodies are specific for PBC, whereas p-ANCA autoantibodies are more prevalent in PSC. Whether loss of self-tolerance is due to cross-reactivity or self-antigen modification by an environmental (xenobiotic) agent is unclear. The autoantibodies are generally not considered pathologic. However, autoreactive T-cells may be involved in tissue damage. Combined examination of serum liver function tests, liver histology, autoantibody specificity, and radiographic features of the biliary tree allows one to discriminate amongst these diseases. Overlap syndromes have been reported but account for less than 10% of cases (Beuers and Rust, Citation2005). The prevalence rate for each disease is low but similar (10-40/100,000 population) (Boberg et al., Citation1994). Despite the low number of cases, together these diseases account for ≈ 20% of the patients undergoing liver transplantation each year. Etiological factors associated with these diseases have been difficult to identify, in part because the diseases generally are diagnosed many years after their presumed onset, which is asymptomatic. PSC is perhaps the least studied of these liver diseases.
II. EPIDEMIOLOGY OF IMMUNE-MEDIATED LIVER DISEASES
Regional differences in prevalence rates suggested that environmental factors may play an important role in the etiology and pathogenesis of these diseases. The majority of epidemiological studies have focused on PBC. An Australian study found a significantly lower incidence and prevalence rate of PBC than those reported in England, despite having a strong British heritage. A change in disease prevalence in emigrant populations may be evidence of an environmental trigger in disease pathogenesis (Watson et al., Citation1995; Anand et al., Citation1996; Prince et al., Citation2001). Prior to the introduction of the Autoimmune Hepatitis Group Revised Scoring System in 1993 (Johnson and McFarlane, Citation1993), there was no standardized way of evaluating patients with suspected AIH and there was an inability to exclude hepatitis C infection in the earlier studies of AIH. Only one study has examined the epidemiology of AIH directly while excluding HCV-infected individuals (Boberg et al., Citation1994). In a Norwegian population, Boberg et al. (Citation1994) found a mean annual incidence of 1.9 cases of AIH per 100,000 population and a point prevalence of 16.9 cases per 100,000 population. Using similar case identification methods, these Authors calculated the incidence and prevalence of AIH, PBC and PSC in the same Norwegian population. They found that the incidence and prevalence of AIH (incidence 1.9 cases/100 000 and prevalence 16.9/100 000) were similar to that of PBC (incidence 1.6/100 000 and prevalence 14.6/100 000) and less than double that of PSC (incidence 1.31/100 000 and prevalence 8.5/100 000). The only other population-based epidemiological study of PSC comes from Spain. Escorsell et al. (Citation1994) found the incidence of PSC to be 0.068/100 000 with a point prevalence of 0.22/100 000 population. Again, it was noted that the incidence and prevalence were lower in a southern European country. A large, international epidemiological study would clearly be helpful, but may be impractical.
The suggestion that the increased prevalence of autoimmune liver disease in northern latitudes is in part due to decreased sun exposure has been proposed for quite a number of chronic inflammatory and autoimmune conditions (Ponsonby et al., Citation2002: Cantorna and Mahon, Citation2004; Holick, Citation2004). Only a small number of studies have clearly demonstrated such an association. Geographical differences in sunlight exposure do affect serum Vitamin D levels, which in turn affect immune function (Bouillon et al., Citation2005). Uncontrolled studies in patients with multiple sclerosis and a small controlled study in patients with rheumatoid arthritis indicated that supplemental Vitamin D reduces symptoms (Cantorna and Mahon, Citation2004). Such studies have not yet been done in those with autoimmune liver disease, but polymorphisms in the Vitamin D receptor have been associated with immune-mediated liver disease (Vogel et al., Citation2002; Jones and Donaldson, Citation2003; Fan et al., Citation2005). Of course, a role for Vitamin D does not preclude a role for an environmental toxin in the etiology of autoimmune liver disease. In addition to its effect on Vitamin D, ultraviolet light induced modification of chemicals or toxins in the skin may also play a role in autoimmune responses. An amalgamation of genetics, ultraviolet light and toxin exposure may influence the prevalence of immune-mediated liver diseases.
A case-control study by Mitchell et al. demonstrated a decreased prevalence of PSC among former and current smokers, regardless of concomitant inflammatory bowel disease (IBD), as well as among those with a history of appendectomy (Mitchell et al., Citation2002). However, the risk of liver cancer is higher in those with PSC who smoke (Bergquist et al., Citation1998). It is difficult to determine whether prevalence differences with regard to race, familial incidence, and geography are environmental or genetic in origin (Takikawa, Citation1999; Mitchell et al., Citation2002; Bambha et al., Citation2003; Feld and Heathcote, Citation2003). No studies of disease comparing monozygotic versus dizygotic twins with at least one twin affected by PSC have been reported.
Infection with Reovirus type 3 (Reo-3) has been associated with cholestatic liver diseases (Minuk et al., Citation1987). Titers of anti-Reo-3 were significantly higher in PSC and PBC sera than in sera of those with other causes of chronic liver disease and healthy controls, but staining for Reo-3 viral markers and cultures of liver biopsy material for Reo-3 virus were negative. Lastly, helicobacter species DNA can be found in the liver of those with PSC, suggesting a possible etiologic role. However, Boomkens et al. (Citation2005) observed no significant difference between the incidence of Helicobacter spp.-specific DNA in PSC livers and a control group. These studies demonstrate the difficulty of studying small numbers of patients. Given its low prevalence, extensive and highly sensitive epidemiologic techniques will be needed to identify specific risk factors for PSC.
III. ANIMAL MODELS OF PSC
Animal models offer an alternative means of investigating the etiology of PSC. Several animal models reproduce the histological features of PSC. Formalin and several other toxins directly damage cholangiocytes or induce ischemia of cholangiocytes (Bedossa et al., Citation1989; Houry et al., Citation1990). Mdr2−/− mice develop lesions similar to PSC (Pauli-Magnus et al., Citation2004). Leakage of bile through disrupted tight junction appears to lead to a pro-inflammatory/fibrotic cascade. However, genetic variation of the human homologue of Mdr2 (MDR3) does not appear to play a role in PSC (Pauli-Magnus et al., Citation2004). Individuals with cystic fibrosis are homozygous for mutation of the cystic fibrosis transmembrane conductance regulator (CFTR), expressed in cholangiocytes as well as the lung, and many develop peribiliary fibrosis similar to PSC. However, increased frequencies of the common CFTR mutations are not found among those with PSC (Gallegos-Orozco et al., Citation2005). Colitis models including those in CFTR−/− mice and models of graft versus host disease (GVHD) may develop biliary inflammation (Nonomura et al., Citation1998; Blanco et al., Citation2004). The former model mimics the association between inflammatory bowel disease and PSC. Murine bacterial overgrowth or LPS exposure in immunodeficient mice similarly may cause peribiliary inflammation (Lichtman et al., Citation1991; Koga et al., Citation2002). The development of peribiliary fibrosis in animal models is often strain specific, which may be due to genetic differences among strains. These models confirm that the etiology of sclerosing cholangitis is both variable and multi-factorial.
A rat model of fibrosing cholangitis induced by administration of the hapten reagent 2,4,6-trinitrobenzenesulfonic acid (TNBS) into a dilated bile duct develops immunologic changes, including p-ANCA autoantibodies, as well as histological abnormalities consistent with PSC (Goetz et al., Citation2003). Mrp2 and Oatp1 protein levels are reduced in this model. Replication of this model by additional groups would help validate the model. Cholangitis can also be induced in rats by low-dose oral administration of the biliary toxin alpha-naphthylisothiocyanate. Hepatic inflammation centered on damaged bile ducts, significant bile duct proliferation, and progressive fibrosis develop in this model, but no autoantibodies are found (Tjandra et al., Citation2000). These animal models demonstrate a potential role for exposure to external toxins in the pathogenesis of PSC. The natural history of known human cases of toxin-induced secondary sclerosing cholangitis reportedly differs from that of PSC (Gossard et al., Citation2005). This finding argues against the hypothesis that PSC is simply a collection of cases of secondary sclerosing cholangitis in which the toxic agents have escaped detection. However, toxin exposure that is difficult to detect or unsuspected may yet be determined to play a role in PSC.
IV. DISEASES ASSOCIATED WITH PROXIMITY TO TOXIC WASTE SITES
Each community has certain sites at which toxic wastes are concentrated. In urban environments, these sites may abut residential areas. Increasingly, sophisticated statistical programs are being used to identify significant geographic clusters (areas of unusually high prevalence of affected individuals) of patients and to determine if these clusters non-randomly associate with the locations of known toxic sites (Buffler et al., Citation1985; Dayal et al., Citation1995; Williamson and Henry, Citation2004; Albert et al., Citation2005). The geographic area included in these analyses can vary considerably, and statistically significant clusters of a very small number of individuals can be identified. For example, a number of oncology studies have shown increased prevalence of individuals with certain cancers, particularly breast and lung, near known toxic waste sites (Burmaster and von Stackelberg, Citation1991; Durant et al., Citation1995; Johnson and DeRosa, Citation1997; Warshawsky, Citation1999; Karagas et al., Citation2002). Recently, Carpenter et al. (Citation2001) linked clustering of women with thyroid disease to the location of Superfund toxic waste sites (SFS) in New York State contaminated primarily with PCBs, dioxins, furans, or persistent pesticides. SFS are toxic waste sites that have been designated for immediate remediation by the New York State Department of Environmental Conservation (NYSDEC) and include all New York State sites placed on the United States Environmental Protection Agency's national priorities list. The statistical programs employed clearly have the potential not only to discover statistically significant clusters of affected individuals, but also to link these clusters to specific toxic waste sites. However, other potential chemical sites in the geographical area such as chemical factories and power plants were not evaluated. The above study was of particular interest in relation to PSC and PBC because many individuals with PSC or PBC have thyroid disease. Of course, thyroid disease is much more common than either PSC or PBC. Detection of clusters is easier for diseases with higher prevalence rates.
V. XENOBIOTICS AND LIVER DISEASE
Xenobiotics are foreign chemicals or toxins not usually found within the body that may accumulate in various tissues typically due to exposure to pollutants. Deactivation and the secretion of xenobiotics primarily occur in the liver. Lipophilic xenobiotic toxins include halogenated hydrocarbons found in pesticides as well as industrial waste. Certain halogenated or aromatic hydrocarbons are known to be toxic to immune cells (Ezendam et al., Citation2005) and may adversely affect hepatic function (Yang, Citation1993; Chen et al., Citation2003; Suh et al., Citation2003; Vezina et al., Citation2004). Occupational exposure to xenobiotics appears to increase the prevalence of non-alcoholic fatty liver disease (NAFLD). The increased prevalence of NAFLD in a large group of workers chronically exposed to several volatile petrochemical products in an industrial area in northeast Brazil (Cotrim et al., Citation1999; Cotrim et al., Citation2004). Among the petrochemical products most frequently found at the industrial area were benzene, toluene, carbon tetrachloride, and chlorinated hydrocarbons. After evaluating other known risk factors for NAFLD, occupational toxin exposure appeared to be an independent risk factor for NAFLD. Increased cholestasis along with hepatitis was noted on biopsies of individuals with toxin associated NAFLD compared to a control NAFLD cohort. The prevalence of the less common immune-mediated liver diseases in this industrial area was not examined. Though many of these toxins are stored primarily in adipose tissues, a common route of excretion for these lipophilic toxins is in bile (Jandacek and Tso, Citation2001). These toxins may induce or exacerbate pre-existing cholestasis. These toxins are detectable in human tissues and may serve as future biomarkers for overall hepatic xenobiotic exposure. Many SFS have particularly high concentrations of these agents according to the NYSDEC. Therefore, the possibility of an association between the location of SFS and clustering of cases of PSC and PBC was examined in a recent study discussed below.
VI. GEOGRAPHIC CLUSTERING OF IMMUNE-MEDIATED CHRONIC LIVER DISEASE
There are no extensive databases listing all individuals with immune-mediated liver diseases in the United States. Referral bias is inherent in the few, single center existing databases. To examine the geographic distribution of individuals in each of the five boroughs of New York City who were listed for liver transplantation with a diagnosis of either PBC or PSC, patient demographic data obtained from the national transplantation registry was employed (Ala et al., Citation2006). The registry records only the residential zip code of the patient at the time of listing. The standardized prevalence ratios (spr) of PSC and PBC patients listed for liver transplantation (sprPSC-OLT and sprPBC-OLT, respectively) between 1995 and 2003 were calculated and plotted for each NYC zip code.
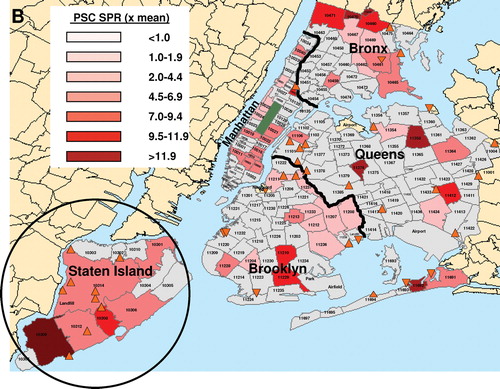
The geographic patterns of distribution of the sprPBC-OLT ( and sprPSC-OLT ( were dissimilar, particularly in Queens and Brooklyn. Individual zip codes with the highest sprPBC-OLT are located in Staten Island and Queens (). To identify an association between disease clusters and toxic waste sites, focused cluster analysis was performed using a validated statistical software package (SaTScan™) that evaluates the distribution of a set of coordinates defined by their longitude and latitude. Focused clustering of PBC-OLT and PSC-OLT cases in association with the location of all SFS listed by the NYSDEC was performed for NYC. Analysis of PSC-OLT revealed a statistically significant large cluster encompassing the borough of Staten Island (p = 0.050) ( and a smaller, overlapping PBC-OLT cluster (p = 0.039) ().
FIG. 1A Standardized Prevalence Ratio of PBC-OLT (A) and PSC-OLT (B) by zip code. Darker shading indicates a higher standardized prevalence ratio (spr). Thick black lines indicate the border between boroughs. Superfund sites are denoted by orange triangles. Statistically significant disease clusters associated with the location of Superfund sites in Staten Island are denoted by circles. PBC-OLT, PBC patients listed for liver transplantation; PSC-OLT, PSC patients listed for liver transplantation.
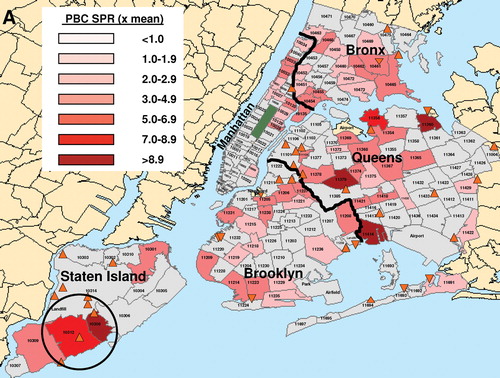
FIG. 1B Standardized Prevalence Ratio of PBC-OLT (A) and PSC-OLT (B) by zip code. Darker shading indicates a higher standardized prevalence ratio (spr). Thick black lines indicate the border between boroughs. Superfund sites are denoted by orange triangles. Statistically significant disease clusters associated with the location of Superfund sites in Staten Island are denoted by circles. PBC-OLT, PBC patients listed for liver transplantation; PSC-OLT, PSC patients listed for liver transplantation.
Four other clusters of PBC-OLT were identified (, solid circles), but were not statistically significant (all p > 0.050). When the prevalence data for PSC-OLT and PBC-OLT were combined, an additional statistically significant cluster (p = 0.023) was identified in NYC in Queens near a SFS (data not shown). None of these clusters contained liver transplantation centers or major medical centers within their borders to suggest referral bias induced clustering. The increase in prevalence of PBC amongst relatives of those with PBC does not significantly affect cluster analysis since only 1.1% of probands have two or more relatives with PBC (Lazaridis et al., Citation2007). No areas with significantly decreased prevalence rates were identified. Statistically significant geographic clusters of PBC and PSC occur in NYC in association with SFS locations.
FIG. 2 Comparison of clusters of PBC patients listed for transplantation (PBC-OLT) and non-listed PBC patients (PBC-MSSM). Clusters of PBC-OLT and PBC-MSSM cases are denoted by solid and dashed circles, respectively. Of the PBC-OLT clusters, only the cluster in Staten Island was statistically significant. Thick black lines indicate the border between boroughs. Superfund sites are denoted by orange triangles.
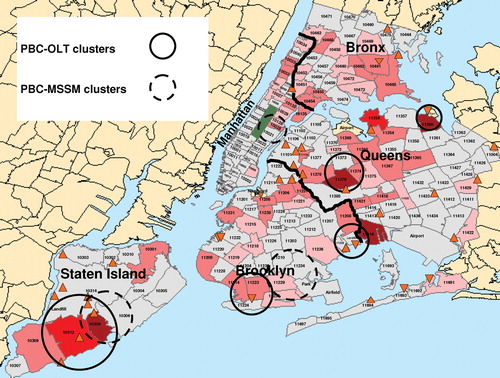
Cluster analysis of the residential zip codes from the time of diagnosis of the Mount Sinai cohort of non-listed PBC patients (PBC-MSSM) (n = 172) identified three statistically significant clusters (, dashed circles). As expected, one cluster was in Manhattan centered on the Mount Sinai School of Medicine, which likely reflected physician referral bias or greater physician awareness and diagnosis of PBC. One PBC-MSSM cluster overlapped with the statistically significant PBC-OLT cluster in Staten Island, and the other PBC-MSSM cluster was near a non-statistically significant PBC-OLT cluster in southern Brooklyn, suggesting clustering may be associated with early disease progression or etiology. Similar data was not available for PSC patients not listed for transplantation. Subdividing the geographic area studied or separating the SFS by the type of site (dump and landfill versus structure), size, class, borough location, or contaminants did not identify any additional statistically significant clusters.
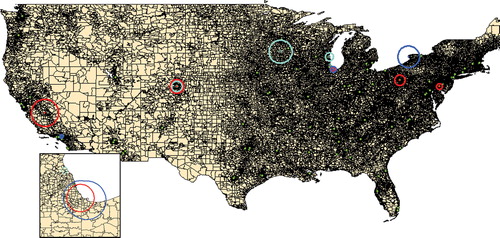
The major contaminants at these sites were volatile organic compounds (e.g., benzene and toluene) and chlorinated hydrocarbons (e.g., tri- and tetrachloroethylene) as noted by the NYSDEC. Water supply contamination is possible, but such volatile compounds may also be disseminated through the air if bound to particulate matter. Though not associated with liver disease, airborne particulate matter exposure is a well-known risk factor for both pulmonary and circulatory disease (Alfaro-Moreno et al., Citation2007). The United States Environmental Protection Agency (U.S. E.P.A.) monitors airborne levels of chlorinated hydrocarbons nationwide. Cluster analysis is not feasible within New York City alone since there are too few monitoring sites in New York City to provide adequate spatial data. However, on a national scale, multiple statistically (p < 0.05) significant clusters of individuals listed for liver transplantation with a diagnosis of either PBC, PSC, and AIH were identified in association with U.S. E.P.A. monitoring sites recording mean daily levels of chlorinated hydrocarbons in the 90th percentile (). The mean relative risk for within the area of the clusters was increased 2.36 fold (range: 1.79 to 5.22 fold). This clustering of cases of immune-mediated chronic liver diseases in areas with high levels of airborne chlorinated hydrocarbons is consistent with a potential role of these environmental toxins in either the etiology or progression of these diseases.
FIG. 3 Association of immune-mediated chronic liver disease and airborne chlorinated hydrocarbons. Focused cluster analysis using U.S. E.P.A. data to identify sites with the highest mean daily concentrations (90th percentile) of airborne chlorinated hydrocarbons was performed for PBC-OLT (blue), PSC-OLT (turquoise), and AIH-OLT (red) cases. Major cities within the cluster areas included Dover (AIH), Pittsburgh (AIH), Buffalo (PBC), Chicago/Gary (PSC, AIH, and PBC), Milwaukee (PSC), St. Paul/Rochester (PSC), Denver (AIH, PSC), Fresno (AIH) and Los Angeles (PBC). Green dots indicate the locations of adult liver transplantation centers. The inset map shows the Chicago/Gary area in greater detail.
VII. SIGNIFICANCE
The above studies suggest environmental factors play a role in the etiology and pathogenesis of immune-mediated liver diseases, including PSC, which account for a significant percentage of liver transplantations and deaths due to cirrhosis each year. Of course identifying an association between an environmental factor and a disease is only the beginning. Evaluation of an appropriate biomarker in patients and control individuals should be done to confirm any association. To establish a pathologic connection animal studies are essential. Identifying environmental factors involved in the etiology and pathogenesis of these diseases should lead to improved prevention and treatment of these diseases, as well as enhance our understanding of other more common immune-mediated conditions, such as thyroid disease and rheumatoid arthritis.
REFERENCES
- Ala A., Stanca C. M., Bu-Ghanim M., Ahmado I., Branch A. D., Schiano T. D., Odin J. A., Bach N. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology 2006; 43: 525–531
- Albert D. A., Albert A. N., Vernace M., Sebastian J. K., Hsia E. C. Analysis of a cluster of cases of Wegener granulomatosis. J. Clin. Rheumatol. 2005; 11: 188–193
- Alfaro-Moreno E., Nawrot T. S., Nemmar A., Nemery B. Particulate matter in the environment: Pulmonary and cardiovascular effects. Curr. Opin. Pulm. Med. 2007; 13: 98–106
- Anand A. C., Elias E., Neuberger J. M. End-stage primary biliary cirrhosis in a first generation migrant south Asian population. Eur. J. Gastroenterol. Hepatol. 1996; 8: 663–666
- Bambha K., Kim W. R., Talwalkar J., Torgerson H., Benson J. T., Therneau T. M., Loftus E. V., Jr., Yawn B. P., Dickson E. R., Melton L. J., 3rd. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003; 125: 1364–1369
- Bedossa P., Houry S., Bacci J., Martin E., Lemaigre G., Huguier M. A longitudinal study of histologic and immunohistologic changes in an experimental model of sclerosing cholangitis. Virch. Arch. A Pathol. Anat. Histopathol. 1989; 414: 165–171
- Bergquist A., Glaumann H., Persson B., Broome U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: A case-control study. Hepatology 1998; 27: 311–316
- Beuers U., Rust C. Overlap syndromes. Semin Liver Dis. 2005; 25: 311–320
- Blanco P. G., Zaman M. M., Junaidi O., Sheth S., Yantiss R. K., Nasser I. A., Freedman S. D. Induction of colitis in cftr−/− mice results in bile duct injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2004; 287: G491–496
- Boberg K. M., Lundin K. E., Schrumpf E. Etiology and pathogenesis in primary sclerosing cholangitis. Scand J Gastroenterol Suppl. 1994; 204: 47–58
- Boomkens S. Y., de Rave S., Pot R. G., Egberink H. F., Penning L. C., Rothuizen J., Zondervan P. E., Kusters J. G. The role of Helicobacter spp. in the pathogenesis of primary biliary cirrhosis and primary sclerosing cholangitis. FEMS Immunol. Med. Microbiol. 2005; 44: 221–225
- Bouillon R., Verlinden L., Eelen G., De Clercq P., Vandewalle M., Mathieu C., Verstuyf A. Mechanisms for the selective action of Vitamin D analogs. J. Steroid Biochem. Mol. Biol. 2005; 97: 21–30
- Buffler P. A., Crane M., Key M. M. Possibilities of detecting health effects by studies of populations exposed to chemicals from waste disposal sites. Environ. Health Perspect. 1985; 62: 423–456
- Burmaster D. E., von Stackelberg K. Using Monte Carlo simulations in public health risk assessments: Estimating and presenting full distributions of risk. J. Expo. Anal. Environ. Epidemiol. 1991; 1: 491–512
- Cantorna M. T., Mahon B. D. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp. Biol. Med. (Maywood) 2004; 229: 1136–1142
- Carpenter D. O., Shen Y., Nguyen T., Le L., Lininger L. L. Incidence of endocrine disease among residents of New York areas of concern. Environ. Health Perspect. 2001; 109(S6)845–851
- Chen S., Nguyen N., Tamura K., Karin M., Tukey R. H. The role of the Ah receptor and p38 in benzo[a]pyrene-7,8-dihydrodiol and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide-induced apoptosis. J. Biol. Chem. 2003; 278: 19526–19533
- Cotrim H. P., Andrade Z. A., Parana R., Portugal M., Lyra L. G., Freitas L. A. Nonalcoholic steatohepatitis: A toxic liver disease in industrial workers. Liver 1999; 19: 299–304
- Cotrim H. P., De Freitas L. A., Freitas C., Braga L., Sousa R., Carvalho F., Parana R., Santos-Jesus R., Andrade Z. Clinical and histopathological features of NASH in workers exposed to chemicals with or without associated metabolic conditions. Liver Int 2004; 24: 131–135
- Dayal H., Gupta S., Trieff N., Maierson D., Reich D. Symptom clusters in a community with chronic exposure to chemicals in two superfund sites. Arch. Environ. Health 1995; 50: 108–111
- Durant J. L., Chen J., Hemond H. F., Thilly W. G. Elevated incidence of childhood leukemia in Woburn, Massachusetts: NIEHS Superfund Basic Research Program searches for causes. Environ. Health Perspect. 1995; 103(S6)93–98
- Escorsell A., Pares A., Rodes J., Solis-Herruzo J. A., Miras M., de la Morena E. Epidemiology of primary sclerosing cholangitis in Spain. Spanish Association for the Study of the Liver. J. Hepatol. 1994; 21: 787–791
- Ezendam J., Kosterman K., Spijkerboer H., Bleumink R., Hassing I., van Rooijen N., Vos J. G., Pieters R. Macrophages are involved in hexachlorobenzene-induced adverse immune effects. Toxicol. Appl. Pharmacol. 2005; 209: 19–27
- Fan L., Tu X., Zhu Y., Zhou L., Pfeiffer T., Feltens R., Stoecker W., Zhong R. Genetic association of Vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the Chinese. J. Gastroenterol. Hepatol. 2005; 20: 249–255
- Feld J. J., Heathcote E. J. Epidemiology of autoimmune liver disease. J. Gastroenterol. Hepatol. 2003; 18: 1118–1128
- Gallegos-Orozco J. F., Yurk C. E., Wang N., Rakela J., Charlton M. R., Cutting G. R., Balan V. Lack of association of common cystic fibrosis transmembrane conductance regulator gene mutations with primary sclerosing cholangitis. Am. J. Gastroenterol. 2005; 100: 874–878
- Goetz M., Lehr H. A., Neurath M. F., Galle P. R., Orth T. Long-term evaluation of a rat model of chronic cholangitis resembling human primary sclerosing cholangitis. Scand. J. Immunol. 2003; 58: 533–540
- Gossard A. A., Angulo P., Lindor K. D. Secondary sclerosing cholangitis: A comparison to primary sclerosing cholangitis. Am. J. Gastroenterol. 2005; 100: 1330–1333
- Holick M. F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004; 80: 1678S–1688S
- Houry S., Languille O., Huguier M., Benhamou J. P., Belghiti J., Msika S. Sclerosing cholangitis induced by formaldehyde solution injected into the biliary tree of rats. Arch. Surg. 1990; 125: 1059–1061
- Jandacek R. J., Tso P. Factors affecting the storage and excretion of toxic lipophilic xenobiotics. Lipids 2001; 36: 1289–1305
- Johnson B. L., DeRosa C. The toxicologic hazard of superfund hazardous-waste sites. Rev. Environ. Health 1997; 12: 235–251
- Johnson P. J., McFarlane I. G. Meeting report: International Autoimmune Hepatitis Group. Hepatology 1993; 18: 998–1005
- Jones D. E., Donaldson P. T. Genetic factors in the pathogenesis of primary biliary cirrhosis. Clin. Liver Dis. 2003; 7: 841–864
- Karagas M. R., Stukel T. A., Tosteson T. D. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int. J. Hyg. Environ. Health 2002; 205: 85–94
- Koga H., Sakisaka S., Yoshitake M., Harada M., Kumemura H., Hanada S., Taniguchi E., Kawaguchi T., Kumashiro R., Sata M. Abnormal accumulation in lipopolysac-charide in biliary epithelial cells of rats with self-filling blind loop. Int. J. Mol. Med. 2002; 9: 621–626
- Lazaridis K. N., Juran B. D., Boe G. M., Slusser J. P., de Andrade M, Homburger H. A., Ghosh K., Dickson E. R., Lindor K. D., Petersen G. M. Increased prevalence of anti-mitochondrial antibodies in first-degree relatives of patients with primary biliary cirrhosis. Hepatology 2007; 46: 785–92
- Lichtman S. N., Keku J., Clark R. L., Schwab J. H., Sartor R. B. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology 1991; 13: 766–772
- Minuk G. Y., Rascanin N., Paul R. W., Lee P. W., Buchan K., Kelly J. K. Reovirus type 3 infection in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J. Hepatol. 1987; 5: 8–13
- Mitchell S. A., Thyssen M., Orchard T. R., Jewell D. P., Fleming K. A., Chapman R. W. Cigarette smoking, appendectomy, and tonsillectomy as risk factors for the development of primary sclerosing cholangitis: a case control study. Gut 2002; 51: 567–573
- Nonomura A., Kono N., Minato H., Nakanuma Y. Diffuse biliary tract involvement mimicking primary sclerosing cholangitis in an experimental model of chronic graft-versus-host disease in mice. Pathol. Int. 1998; 48: 421–427
- Pauli-Magnus C., Kerb R., Fattinger K., Lang T., Anwald B., Kullak-Ublick G. A., Beuers U., Meier P. J. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology 2004; 39: 779–791
- Ponsonby A. L., McMichael A., van der Mei I. Ultraviolet radiation and autoimmune disease: Insights from epidemiological research. Toxicology 2002; 181: 71–78
- Prince M. I., Chetwynd A., Diggle P., Jarner M., Metcalf J. V., James O. F. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology 2001; 34: 1083–1088
- Suh J., Kang J. S., Yang K. H., Kaminski N. E. Antagonism of aryl hydrocarbon receptordependent induction of CYP1A1 and inhibition of IgM expression by di-ortho-substituted polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 2003; 187: 11–21
- Takikawa H. Recent status of primary sclerosing cholangitis in Japan. J. Hepatobiliary Pancreat. Surg. 1999; 6: 352–355
- Tjandra K., Sharkey K. A., Swain M. G. Progressive development of a TH1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology 2000; 31: 280–290
- Vezina C. M., Walker N. J., Olson J. R. Subchronic exposure to TCDD, PeCDF, PCB126, and PCB153: Effect on hepatic gene expression. Environ. Health Perspect. 2004; 112: 1636–1644
- Vogel A., Strassburg C. P., Manns M. P. Genetic association of Vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology 2002; 35: 126–131
- Warshawsky D. Polycyclic aromatic hydrocarbons in carcinogenesis. Environ. Health Perspect. 1999; 107: 317–319
- Watson R. G., Angus P. W., Dewar M., Goss B., Sewell R. B., Smallwood R. A. Low prevalence of primary biliary cirrhosis in Victoria, Australia. Melbourne Liver Group. Gut 1995; 36: 927–930
- Williamson D. M., Henry J. P. Challenges in addressing community concerns regarding clusters of multiple sclerosis and potential environmental exposures. Neuroepidemiology 2004; 23: 211–216
- Yang R. NTP technical report on the toxicity studies of a chemical mixture of 25 groundwater contaminants administered in drinking water to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 1993; 35: 1–I12