Abstract
The exposure of Libby MT residents to amphibole-contaminated vermiculite is well known. To explore the gene-environment interactions in the development of asbestos-related diseases (ARD), a mouse model of asbestos exposure using Six-mix (a combination of amphibole fibers gathered from six sites at the Libby vermiculite mine), crocidolite asbestos, or saline as a negative control was used to determine both gene expression responses by using mouse 10,000 oligonucleotide array and to visualize these changes histologically. Mice were sacrificed and whole lungs harvested for histology and microarray analysis six months following exposure via intratracheal instillation. Using an arbitrary cutoff of 1.25-fold change, genes whose RNA expression levels were specifically altered in response to the different amphibole exposures were grouped into categories by a gene ontology analysis program, GoMiner. Our hypothesis was that assessment of asbestos-responsive genes would provide a better understanding of response mechanisms. These experiments have provided new candidates for genes involved in the asbestos response pathways.
Keywords :
INTRODUCTION
A serious public health situation has been identified in Libby, Montana where miners and town residents were exposed to hazardous levels of asbestos from the local mining operation. Vermiculite was mined, transported, and processed in the area from 1923 to 1990. Most vermiculite ore currently used in many consumer products (including insulation, fireproofing material, and lawn/garden products) is not considered a health hazard. However, the Libby ore is different in that it is contaminated with up to 21–26% asbestos (Atkinson et al., Citation1982). Asbestos contamination has been found extensively in Libby as a result of the mining and milling operations, as well as the distribution of the asbestos-contaminated vermiculite given away as a soil amendment, thus Libby has been designated as an Environmental Protection Agency (EPA) Superfund site.
Asbestos fibers are naturally occurring silicate minerals that have been mined for their usefulness in thermal insulation, chemical and thermal stability, and high tensile strength. Their crystal formation involves the construction of long, thin fibers. These fibers may become airborne when disturbed and then inhaled into the lungs, where they cause serious health problems. Actinolite, tremolite, winchite, and richterite, the primary forms of asbestos found in Libby vermiculite, are amphiboles with a chain-like structure that can become trapped in lung tissue when inhaled (Meeker, Citation2003).
Prolonged exposures to asbestos can manifest in a variety of health problems including asbestosis, lung cancer, and mesothelioma. In December 2000, the Agency for Toxic Substances and Disease Registry (ATSDR, 2000) conducted a mortality study on the Libby population for a 20-yr period encompassing 1979–1998. It was reported that there was a 20–40% increase in malignant and nonmalignant respiratory deaths. In particular, mesothelioma mortality was elevated, asbestosis mortality was 40–80 times higher than expected, and lung cancer mortality was 1.2–1.3-times higher than expected when compared to the rest of the United States (ATSDR, 2000, 2003).
Unfortunately, asbestos exposures are neither limited to Libby residents, nor are they phenomenon of the past. It is estimated that about 2–6 million people in the United States have had significant levels of exposure (Khan et al., Citation2004). The EPA estimates that there are ≈ 30 million homes today that still contain asbestos contaminated vermiculite in their insulation, which equates to many more exposures in the future. Furthermore, in the United States alone, 2,500 to 4,000 new individuals are diagnosed with an asbestos-related disease (ARD) and nearly 10,000 people die from an ARD every year (Nicholson et al., Citation1982; CDC, 2001). Because of the omnipresent nature of contamination in building insulation and the long latent period of disease, ARD will remain an important public health issue for many years to come. For this reason, we used a mouse model of asbestos exposure to begin to understand how this material is causing the ARD seen in this exposed population.
Previous studies in mice have shown that after asbestos exposure, a complex network of cytokines, growth factors, and receptors are involved in initial inflammation and ensuing asbestosis and carcinogenesis (Geist et al., Citation2000; Shukla et al., Citation2003). We have used gene expression studies in a mouse model to identify potential candidate genes involved in asbestos response. A six-month exposure in a mouse model demonstrated gene expression alterations in several categories, primarily those involved with the plasma membrane. Histological changes consistent with fibrosis were seen in animals exposed to both amphiboles, however the changes were much less severe in the Six-mix exposed animals. These observations were confirmed with lucifer yellow quantitation of collagen deposition in exposed mouse lungs. Thus, these studies have confirmed the fibrotic effects of the amphibole fibers from the Libby, Montana vermiculite mine and have indicated specific pathways of response to these fibers for future studies.
MATERIALS AND METHODS
Amphiboles
Fibers used in this study were a sample of the Libby Amphibole obtained from the US Geological Survey (USGS) and crocidolite obtained from the Research Triangle Institute (Research Triangle Park, NC). The Libby Amphibole has been chemically and physically well characterized (Wylie and Verkouteren, Citation2000; Gunter et al., Citation2003; Meeker et al., Citation2003). Several types of amphibole contaminate the vermiculite from the Libby mine; thus, the USGS has provided researchers with a composite sample of the six primary varieties, termed Six-mix. The crocidolite was used as a well-studied control fiber for comparison. The fibers used for the experiments reported here have been well described previously (Blake et al., Citation2007). For reference, size parameters of the Six-mix were 0.61 μ m in diameter, 7.21 μ m in length, with 22.52 for the aspect ratio. Size parameters of the crocidolite were 0.16 μ m in diameter and 4.59 μ m in length, with a 34.05 aspect ratio. Samples were freshly prepared in sterile phosphate-buffered saline (saline) and sonicated before instillation.
Mouse Treatment
C57Bl/6 mice, 6–8 wk-of-age, were divided into three groups of at least seven mice each. Using standard intratracheal instillation protocols employed in our laboratories (Adamson and Bowden, Citation1987), mice were exposed with 100 μ g Six-mix or 100 μ g crocidolite asbestos in 30 μ l of saline. Saline without fiber was instilled in control mice. The mice were sacrificed and the lungs harvested for study 6 mo after instillation.
RNA Isolation
Experimental and control mouse lungs were initially stored in RNAlater (Ambion, Austin, TX). Lungs were subsequently homogenized in the presence of TRIzol reagent and RNA was isolated following the manufacturer's protocol (Invitrogen, Carlsbad, CA). Additional purification of the total RNA was performed using the RNeasy kit (Qiagen, Valencia, CA).
Microarray Analysis
RNA was analyzed by microarray hybridization to a 10K element mouse oligonucleotide array based on set A from MWG-Biotech (High Point, NC). Gene expression was analyzed relative to mouse reference standard RNA (Stratagene, La Jolla, CA), enabling experiment-to-experiment comparison. Alterations of transcript levels were characterized by fold-change, k-means cluster analysis, and principle components analysis. Genes with expression changes were organized into biologically relevant categories and assessed for significance using the GoMiner program (http://discover.nci.nih.gov/gominer/). Assays and analyses were performed in the MicroArray core at The University of Montana Center for Environmental Health Sciences.
Histology
Mouse lung samples were perfused and immersed in Histochoice fixative (Amresco, Solon, OH), embedded in paraffin, and 7-μ m sections analyzed. Routine Gomori's trichrome staining was performed in the Center's Molecular Histology and Fluorescence Imaging core and the sections examined under light microscopy.
Lucifer Yellow (LY) Staining
LY has been shown to bind to collagen, enabling quantification of the extent of fibrosis based on the amount of collagen present (Antonini et al., Citation2000; Taylor et al., Citation2002). Briefly, histologic tissue sections were incubated in Lucifer Yellow-CH (1 mg/ml) (Molecular Probes, Eugene, OR) for one hour at room temperature, washed in deionized (dI) water and cover-slipped with water soluble, anti-fade mounting medium (Immuno Concepts, Sacramento, CA). LY-stained tissue was quantitated using a Laser Scanning Confocal Microscope (LSC), (CompuCyte, Cambridge, MA) in the Fluorescence Cytometry Core fitted with an argon-ion laser. A circular area having a radius of 700 μ m was scanned at approximately the same location on each section using a series of smaller scan areas called phantoms, each having a radius of 8 μ m.
The mean fluorescent intensity for the phantoms in each scan area was recorded and analyzed using Wincyte software. The phantoms that were saturated or below a set threshold were gated out and were not included in the analysis. This eliminated background areas and artifacts from the analysis. Intensity values were obtained from every tenth section (for a total of 15 sections) for each lung and added together for each animal. Wincyte software (CompuCyte Corporation) was used in the analysis.
Statistics
Values obtained from quantitation of the LY-stained sections of amphibole- and saline-exposed mouse lungs (n = 4/treatment group) were compared using one-way analysis of variance (ANOVA) with a Newman-Keuls Multiple Comparison Test, with significance determined at p < 0.05. Both Dixon and Grubbs tests were used to analyze for outliers in all data sets prior to performing the statistical analyses (Taylor, 1990). In testing for outliers among the data collected for each group, both the highest and the lowest values in each data set were tested to see if they fell beyond the 95% confidence level for rejection in either test system, i.e., > 0.765 in the Dixon test or > 1.463 in the Grubbs test.
Genes exhibiting at least 1.25-fold up- or down-regulation in 6-month treated lungs were grouped with GoMiner (NCBI) into functional categories (http://discover.nci.nih.gov/gominer/). lists the GoMiner categories identified by the program as having significant gene changes (p < 0.05).
TABLE 1 GoMiner analysis of RNA expression changes determined by microarray analysis. Genes exhibiting at least 1.25-fold up- or down-regulation in treated lungs (6-mo postexposure) were grouped with GoMiner (NCBI) into functional categories
RESULTS
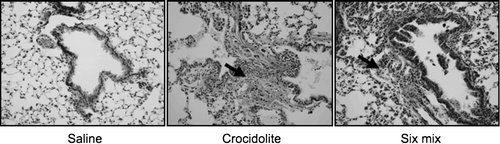
Six months after instillation, lungs from animals instilled with Six-mix or crocidolite asbestos demonstrated fibrosis development with increased cellularity and elaboration of extracellular matrix (collagen appears green in Gomori Trichrome-stained sections, ). Although fibrotic areas were identified in the Six-mix-treated mouse lung sections, it was not to the same extent as the fibrosis seen in crocidolite asbestos-treated lung sections included as positive control. No fibrotic areas were identified in sections from mouse lungs instilled with saline as the vehicle control.
FIG. 1 Gomori Trichrome-stained 7 μ M sections of mouse lungs from animals instilled with saline, Six-mix, and crocidolite asbestos for comparison (200X original magnification). Green staining indicates collagen deposition, also indicated with a bold arrow.
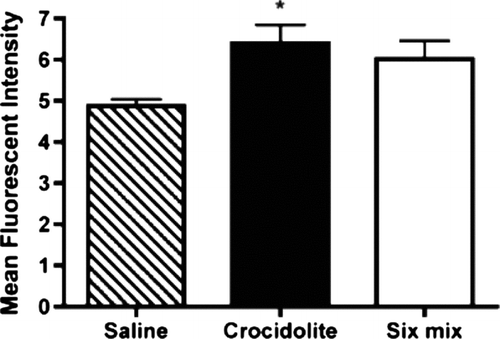
To quantitate mouse lung collagen deposition, an assay using lucifer yellow to stain collagen was performed. This assay allows collagen quantitation without the tissue destruction required by a hydroxyproline assay. demonstrates the mean fluorescence intensities seen in histologic sections of mouse lungs. While mean fluorescence intensity was increased in lungs from both treatment groups, lungs from the crocidolite-treated mice demonstrated a statistically significant (p < 0.05) increase over saline-treated control mice (n = 4 in each group). In these studies, none of the data associated with each treatment group was found to be beyond the 95% confidence level for rejection when tested in either the Dixon or the Grubbs tests.
FIG. 2 Quantitation of LY stained tissue. Stained tissue was scanned using a Laser Scanning Confocal Microscope fitted with an argon-ion laser. Intensity values were obtained from every tenth section (for a total of 15 sections) for each lung and added together for each animal. Statistical analysis was performed using one-way ANOVA with a Newman-Keuls Multiple Comparison test. Significance was determined at the p < 0.05 level (*).
Gene expression analysis was performed on pooled aliquots of RNA from seven to eight animals per exposure and the results analyzed with the GoMiner program from NCBI. Results from Six-mix- and crocidolite exposed animals demonstrated significant gene expression changes in multiple GoMiner categories (), most notably in membrane-associated genes. Shared effects include changes to transport channels and signal transduction genes. Numerous but non-significant expression changes were also seen in genes involved in cell proliferation, regulation of signal transduction, the extracellular matrix, apoptosis, fibrosis, tumor formation, and oxidative stress.
DISCUSSION AND CONCLUSIONS
Despite intensive investigation, the mechanisms of asbestos toxicity have yet to be delineated. The development of lung disease has been related to both the cumulative dose and the time since first exposure, with asbestos-related diseases (ARD) typically arising after a 15-40 year latent period (Browne, 1994). Recent reviews have detailed evidence of various pathogenic pathways of asbestos-induced lung diseases, which include: (1) the chemical and structural properties of the fibers; (2) the lung fiber burden; (3) fiber uptake by lung epithelial cells; (4) iron-catalyzed free radicals; (5) DNA damage; (6) cytokines/growth factors; and, (7) exposure to cigarette smoke and other pulmonary toxicants (reviewed in Kamp, 1999, Manning, Citation2002). No single mechanism has been found to explain the abnormalities caused by asbestos, and the exact pathogenic pathways and their regulation have not been determined. Through the analysis of gene expression changes found in mouse lungs after asbestos exposure, our studies will direct us to determine the contribution of these various mechanisms on the initiation and progression of asbestos-related diseases.
The physiological effects of crocidolite asbestos have been well studied for many years, but the effects of the Libby amphibole (Six-mix) are still under investigation. The asbestos-related diseases seen in the exposed Libby population make it essential to determine the mechanism of action of this material, and to compare the effects of the Six-mix to the effects of other, well-characterized amphiboles. Using crocidolite asbestos as a well-characterized fiber for comparison (Hamilton et al., Citation2004; Wang et al., 2006; Baldys et al., 2007), we studied the effects of the Six-mix in a mouse model of exposure. Crocidolite exposure produced significantly increased collagen deposition in a mouse model of asbestosis, as demonstrated by both Trichrome staining and lucifer yellow (LY) quantitation. In contrast, analyses of the Trichrome-stained tissues from Six-mix-exposed hosts appeared to only be slightly increased; the LY quantitation studies showed that, in fact, these changes in the level of collagen in these lungs were not statistically significant when compared to that in lungs of the saline-exposed controls.
In comparing the histology of the lungs of mice in the crocidolite and Six-mix exposure groups, it was noted that the extent of fibrosis was consistently less in the lungs of the Six-mix mice as compared to that in the crocidolite mice. This disparity is most likely related to the Six-mix composition. In the Six-mix used here, the majority of amphiboles present were in the winchite/richterite series, with tremolite being present as a minor component (∼ 6% of respirable fraction; Meeker et al., 2003). While effects of winchite or richterite on the endpoints examined in our studies have not been assessed in any other studies, the effects of richterite in a hemolysis test and macrophage cultures indicated that this amphibole had a smaller biological effect than seen with traditional asbestos minerals like crocidolite (Collan et al., Citation1986). Unlike for winchite or richterite, previous studies in rats have shown that tremolite instillation gave rise to pulmonary fibrosis within 90 days (Bernstein et al., Citation2005). As such, because the tremolite asbestos is only a minor component of the Six-mix, a low level of fibrosis in the Six-mix-exposed mice (i.e., in general and, in particular, relative to that after crocidolite treatment) is not entirely unexpected and consistent with previously published results.
Gene expression changes after asbestos exposure have been studied in in vitro systems (Nymark et al., Citation2007). The analysis of changes in gene expression in whole lung tissue after exposure to asbestos may provide new directions for research into the mechanisms of fibrosis development. Our results demonstrate that after a six-month exposure to either Six-mix or crocidolite, there are both similarities and differences in the gene expression changes seen as analyzed with the gene ontology analysis program from NCBI, GoMiner. This program uses the gene ontology assignment to place genes in functional categories and assesses the significance of the number of genes altered in their expression in a category versus the total number of genes in a category. This analysis demonstrated that genes associated with the plasma membrane, either intrinsically or as part of signaling pathways, had the most significant changes in gene expression.
Although many GoMiner categories were changed in common between Six-mix- and crocidolite-treated lungs, there were subtle differences in the spectrum of categories represented in the groups with the most significant changes. In particular, the category with the most significant changes in the crocidolite-treated lungs was associated with transition metal ion trans-membrane transporter activity, while this category did not appear as significant in the Six-mix-treated lungs. In addition, as expected after exposure to fibers that cause fibrosis, gene expression changes were seen in the positive regulation of epithelial cell proliferation category after crocidolite exposure. The importance of the changes in the plasma membrane after exposure to Six-mix could provide significant clues to the mechanisms critical to the development of ARD in individuals exposed to the Libby amphibole. It will be important to assess fibrotic and gene expression changes at various times after exposure in order to better understand the mechanisms underlying fibrotic disease development.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Laura Hoerner and Lou Herritt. This project was supported by CCR822092 (CDC) and RR017670 (NCRR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
REFERENCES
- Adamson I. Y., Bowden D. E. Response of mouse lung to crocidolite asbestos. 2. Pulmonary fibrosis after long fibres. J. Pathol. 1987; 152: 109–117
- Antonini J. M., Hemenway D. R., Davis G. S. Quantitative image analysis of lung connective tissue in murine silicosis. Exp. Lung Res. 2000; 26: 71–88
- Atkinson G. R., Rose D., Thomas K., Jones D., Chatfield E. J., Going J. E. Collection, Analysis and Characterization of Vermiculite Samples for Fiber Content and Asbestos Contamination. 1982, MRI report for EPA, project No. 4901-A32 under EPA contract 68-01-5915
- ATSDR. Health Consultation on Mortality from Asbestosis in Libby, Montana. United States Department of Health and Human Services, Atlanta, GA 2000, www.atsdr.cdc.gov/hac/PHA/libby/lib_toc.html
- ATSDR. Public Health Assessment, Libby Asbestos Site, Libby, Lincoln County, Montana. United States Department of Health and Human Services, Atlanta, GA 2003, www.atsdr.cdc.gov/HAC/PHA/libby3/lby_toc.html
- Baldys A., Pande P., Moseih T., Park S. H., Aust A. E. Apoptosis induced by crocidolite asbestos in human lung epithelial cells involves inactivation of Akt and MAPK pathways. Apoptosis 2007; 12: 433–447
- Bernstein D. M., Chevalier J., Smith P. Comparison of Calidria chrysotile asbestos to pure tremolite: Final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal. Toxicol. 2005; 17: 427–449
- Blake D. J., Bolin C. M., Cox D. P., Cardozo-Pelaez F., Pfau J. C. Internalization of Libby amphibole asbestos and induction of oxidative stress in murine macrophages. Toxicol. Sci. 2007; 99: 277–288
- CDC; Centers for Disease Control. National Center for Health Statistics, Multiple Cause of Death Files, 1999–2001. 2001, www.cdc.gov/nchs/deaths/htm
- Collan Y., Kosma V. M., Anttonen H., Kulju T. Toxicity of richterite in hemolysis test and macrophage cultures. Arch. Toxicol. Suppl. 1986; 9: 292–295
- Geist L. J., Powers L. S., Monick M. M., Hunninghake G. W. Asbestos stimulation triggers differential cytokine release from human monocytes and alveolar macrophages. Exp. Lung Res 2000; 26: 41–56
- Gunter M. E., Dyar D. M., Twamley B., Foyt F. F., Jr, Cornelius S. Composition, Fe+ 3/Fe and crystal structure of non-asbestiform and asbestiform amphiboles from Libby, Montana, USA. Am. Mineral. 2003; 88: 1970–1978
- Hamilton R. F., Holian A., Morandi M. A comparison of asbestos and urban particulate matter in the in vitro modification of human alveolar macrophage antigen-presenting cell function. Exp. Lung Res. 2004; 30: 147–162
- Kamp D. W., Weitzman S. A. The molecular basis of asbestos-induced lung injury. Thorax 1999; 54: 638–652
- Khan A., Jones C. Asbestos-related disease. 2004, www.emedicine.com/radio/topic53.htm
- Manning C. B., Vallyathan V., Mossman B. T. Diseases caused by asbestos: Mechanisms of injury and disease development. Int. Immunopharm. 2002; 2: 191–200
- Meeker G. P., Bern A. M., Brownfield I. K., Lowers H. A., Sutley S. J., Hoefen T. M., Vance J. S. The composition and morphology of amphiboles from the Rainy Creek complex, near Libby, Montana. Am. Mineral 2003; 88: 1955–1969
- Nicholson W. J., Perkel G., Selikoff I. J. Occupational exposure to asbestos: Population at risk and projected mortality, 1980–2030. Am. J. Ind. Med. 1982; 3: 259–311
- Nymark P., Lindholm P. M., Korpela M. V., Lahti L., Ruosaari S., Kaski S., Hollmen J., Anttila S., Kinnula V. L., Knuutila S. Gene expression profiles in asbestos-exposed epithelial and mesothelial lung cell lines. BMC Genomics 2007; 8: 62
- Shukla A., Ramos-Nino M., Mossman B. T. Cell signaling and transcription factor activation by asbestos in lung injury and disease. Int. J. Biochem. Cell. Biol. 2003; 35: 1198–1209
- Taylor M. D., Roberts J. R., Hubbs A. F., Reasor M. J., Antonini J. M. Quantitative image analysis of drug-induced lung fibrosis using laser scanning confocal microscopy. Toxicol. Sci. 2002; 67: 295–302
- Wang X., Samet J. M., Ghio A. J. Asbestos-induced activation of cell signaling pathways in human bronchial epithelial cells. Exp. Lung Res. 2006; 32: 229–243
- Wylie A. G., Verkouteren J. R. Amphibole asbestos from Libby, Montana: Aspects of nomenclature. Am. Mineral. 2000; 85: 1540–1542