Abstract
Both immune and non-immune cells express an extensive array of scavenger receptors that bind a variety of ligands including bacterial cell-wall components and lipoproteins. Over the past several years, significant advances have been made in elucidating the role of scavenger receptors, predominantly Class A scavenger receptors SR-A I/II and MARCO, on macrophages in the binding of environmental particles such as crystalline silica and titanium dioxide. Recent evidence indicates that the binding of crystalline silica to scavenger receptors leads to apoptosis of macrophages and release of mediators (e.g., proinflammatory cytokines) contributing to lung inflammation and fibrosis. In this review, we examine the evidence for the role of SR-A I/II and MARCO in binding of the environmental particles and signaling initiated by particle-receptor interaction. Emerging concepts on the molecular details of signaling cascades by engagement of scavenger receptors by the environmental particles are also discussed.
INTRODUCTION
Environmental and occupational exposures to respirable inorganic particles, such as asbestos or crystalline silica, pose a huge threat to human health (Green and Vallyathan, Citation1996; Calvert et al., Citation2003). Since these inhaled particles cannot be cleared easily from the lung, long-term exposure to these particles may lead to accumulation in the lungs and initiation of inflammatory reactions which eventually culminate in lung diseases such as pulmonary fibrosis, cancer, and autoimmune diseases (Ulm et al., Citation1999; Park et al., Citation2002; Parks et al., Citation2002; Brown et al., Citation2005). For example, inhalation of silica causes chronic bronchitis (inflammation of air passages of the lung) and a progressive development of pulmonary fibrosis characterized by thickening of alveolar interstitium, hyalinized nodules and deposition of collagen (Huaux, Citation2007). Silica exposure has also been attributed to development of various autoimmune diseases (e.g., scleroderma) (Englert et al., Citation2000; Pfau et al., Citation2004). Various forms of asbestos, such as chrysotile, crocidolite, and amosite, are known to be toxic to mammals when inhaled (O'Reilly et al., Citation2007). Inhalation of asbestos can induce inflammation and asbestosis, or pulmonary fibrosis, and in some cases promote development of malignant pleural mesotheliomas (Leigh et al., Citation2002; Becklake et al., Citation2007).
In contrast, another inorganic particle titanium dioxide (TiO2) is relatively inert and is widely used in many industrial applications, as well as in medical and dental prosthesis (Lindenschmidt et al., Citation1990). While a few studies have shown that TiO2 exposure in murine models leads to an inflammatory reaction in the lung, the exposure does not progress to fibrosis (Lindenschmidt et al., Citation1990; Lardot et al., Citation1998). This contrasting observation raises an important question as to why certain inorganic particles like crystalline silica induce a fibrotic response in the lung while another inorganic particle like TiO2 does not. The apparent paradox in the fibrotic outcome in response to these two inorganic particles may, at least in part, be related to differences in binding of these particles to cellular receptors and the signaling events triggered by these particle-receptor interactions.
In this review we summarize the current knowledge on the class A Scavenger Receptors (SR) particularly SR-A I/II (newly designated as CD204) and MAcrophage Receptor with COllagenous domain (MARCO) functioning in the binding of mainly silica and TiO2 to macrophages. We also summarize the role of these receptors in signaling events triggered following inhalation exposure to various inorganic particles, particularly silica and TiO2.
ENVIRONMENTAL PARTICLES AND LUNG INFLAMMATION
Although the ultimate immunological disorders due to the exposure of the above-mentioned inflammatory environmental particles is well-known, a large gap remains in understanding the cellular and molecular mechanisms that initiate and propagate the process of injury, inflammation, and fibrosis. It has been established that the lung responds to inhaled particulate matter by enrollment of alveolar macrophages (AM) and other immune cells, triggering an inflammatory cascade of reactions (Rimal et al., Citation2005; Lalmanach et al., Citation2006). The AM are cells that are primarily responsible for binding, ingestion, and ultimate clearance of inhaled particulates (Benson et al., Citation1986; Rimal et al., Citation2005). For example, upon inhalation of silica particles, AM engulf silica particles and undergo apoptosis (Iyer and Holian, Citation1997).
This apoptotic material and free crystalline silica can then be re-engulfed by other AM that then release mediators like oxygen radicals, proteases, and proinflammatory cytokines, or undergo apoptosis (Beamer and Holian, Citation2005; Rimal et al., Citation2005). This cycle of engulfment, apoptosis, and release of inflammatory mediators would lead to prolonged inflammation that is considered to be the pathway in pathogenesis of acute and chronic lung injury (Srivastava et al., Citation2002; Rimal et al., Citation2005). In contrast, TiO2, a non-fibrogenic dust, does not induce macrophage apoptosis (Thibodeau et al., Citation2003). Considering the differential response in the development of fibrosis following pulmonary exposure, the dissimilar apoptotic outcome initiated by silica and TiO2 is an appealing avenue to be explored.
Other studies indicate the fibrogenic potential of a particle correlates with its ability to induce apoptosis in AM, which highlights the concept that apoptosis may be an important determining factor in initiation of a fibrotic response (Iyer et al., Citation1996; Rimal et al., Citation2005). Although AM plays a central role in initiation of particle-induced lung injury, the receptors on AM that bind and engulf these inorganic particles were until recently not known.
Scavenger Receptors
The term SR was first coined based on the high affinity binding of some receptor(s) on macrophages for acetylated low-density lipoprotein (AcLDL) (Dresel et al., Citation1985; Moore and Freeman, Citation2006). Modified LDLs are considered to be causative agents of vascular diseases (Brown et al., Citation1979). For a long time, the major focus of studies with SR had been exploring the role of SR in uptake of LDL and vascular diseases, such as atherosclerosis. However, over the past several years, it has become apparent that SR play a significant role in particle-induced pulmonary pathologies.
The SR are germline-encoded receptors that are also referred to as pattern recognition receptors due to their ability to recognize conserved pathogen-associated molecular patterns (Murphy et al., Citation2005; Moore and Freeman, Citation2006). In fact, the SR bind a variety of ligands like gram-positive and gram-negative bacteria, lipopolysaccharide (LPS), lipotechoic acid, polynucleotides, and apoptotic eukaryotic cells (Elomaa et al., Citation1995; van der Laan et al., Citation1999; Kunjathoor et al., Citation2002).
Examination of the SR ligands illustrate that they are polyanionic in nature (Platt and Gordon, Citation1998). The SR are expressed on macrophages and dendritic cells, but are also found on non-immune cells like epithelial cells, endothelial cells and hepatocytes (Murphy et al., Citation2005). The SR are classified into 8 different subclasses based on structure and function (Classes A-H) (Murphy et al., Citation2005; Moore and Freeman, Citation2006). Recent studies involving SR have opened up an entirely new era in understanding of the molecular events that initiate the inflammatory response following exposure to environmental particles.
Structure of Scavenger Receptor A Family Receptors
The class A SR are expressed predominantly on macrophages and dendritic cells, mast cells, epithelial cells, and endothelial cells (Murphy et al., Citation2005; Brown et al., Citation2006). Five members have been identified to date namely: SR-A (I, II, III), MARCO, CSR1 (Cellular Stress Response 1), SRCL (SR with C-type lectin) (Moore and Freeman, Citation2006), and SCARA5 (Class A SR 5) (Jiang et al., Citation2006). All five polypeptides are Type II transmembrane proteins and exist as homotrimers, except SR-A III, which is trapped in the endoplasmic reticulum. All of the polypeptides are extracellular and have been shown to bind a wide variety of ligands including proteins, polyribonucleotides, polysaccharides, and environmental particles and play a significant role in host-defense. A prototypic example of Class A SR family, SR-A I is comprised of six regions: a relatively short N-terminal cytoplasmic domain, a transmembrane domain, and its extracellular domain comprised of: a spacer; an α -helical coiled-coil; a collagenous domain; and, a cysteine rich C-terminal domain (Matsumoto et al., Citation1990). Despite the extensive similarity between the structures of SR polypeptides, some of the polypeptides lack one or more of the above-mentioned domains.
Two of the central structural features of SR include a collagenous domain of varying length in the extracellular region and a short N-terminal cytoplasmic domain ≈40–60 amino acids long. Furthermore, three of the members of Class A SR, including SR-A I, MARCO, and SCARA5, belong to a family of a large group of receptors called SR Cysteine Rich (SRCR) superfamily that have an evolutionarily conserved SRCR domain of ≈ 100–110 amino acid residues (Sarrias et al., Citation2004; Jiang et al., Citation2006). A large number of cell surface proteins with diverse functions possess the SRCR domain as an integral part of their structure. However, at present, a common function of SRCR domain has not been determined (Sarrias et al., Citation2004). In a few proteins, including MARCO, the SRCR domain has been found to play a major role in ligand binding and cell adhesion (Brannstrom et al., Citation2002).
Recently, a positively-charged arginine-based RXR motif in the SRCR domain of MARCO has been reported to mediate the binding of gram-positive and gram-negative bacteria (Brannstrom et al., Citation2002). The SRCR domain of MARCO was also found to be the ligand-binding domain for AcLDL (Chen et al., Citation2006). Although the features that govern the association of ligands to their SRCR domain are not fully understood, SR-A and MARCO avidly bind polyanionic molecules (Platt and Gordon, Citation1998; van der Laan et al., Citation1999). In SRA II, which lacks the SRCR domain, the binding site for ligands is reported to be the positively-charged lysine cluster in the collagenous domain of the receptor (Doi et al., Citation1993). Whether the lysines play a role in binding of environmental particles still remains to be determined.
Taking into consideration the negative surface charge of some of the environmental particles like silica and TiO2, it can be postulated that the positively-charged lysines and arginines in the collagenous and SRCR domain of Class A SR might be the binding site for these particles. In order to account for the full complexity of binding behavior of SR, understanding the role of all other structural and physical factors of the particles, such as size and surface charge distribution, is of importance.
Role of Scavenger Receptor Class A in Binding and Signaling by Environmental Particles
During the early 1990s, a study suggested that crocidolite asbestos that causes asbestosis and mesothelioma, bound efficiently to recombinant SR (SR-A I and II) (Resnick et al., Citation1993). The binding of crocidolite asbestos to these receptors was efficiently inhibited by the well-established SR ligands like polyinosinic acid (poly I) and polyguanylic acid (poly G) (Resnick et al., Citation1993). Additional studies identified other particles (viz., TiO2, silica, iron oxide, and diesel exhaust particles) as possible ligands for SR (Iyer et al., Citation1996; Palecanda et al., Citation1999). The results published in these studies showed potent inhibition of human and hamster AM binding of latex beads, TiO2, silica, and iron oxide by poly I and dextran sulfate.
Consistent with the in vitro experiments, in vivo uptake of silica and latex beads by hamster AM was significantly diminished by poly I (Palecanda et al., Citation1999). The study by Iyer et al. (Citation1996) showed that pre-treating human AM with poly I completely inhibited silica-induced apoptosis while polycytidylic acid (poly C), which does not bind SR, had no effect. Another study demonstrated that a blocking antibody specific for SRA I/II (2F8) inhibited silica-induced caspase activation and apoptosis (Chao et al., Citation2001). In contrast to silica, TiO2-treated CHO cells expressing murine SRA I/II did not undergo apoptosis (Hamilton et al., Citation2000). Thus, it might be anticipated that silica and TiO2 bind to SR as a result of their negative surface charge, but with important differences. Upon binding, silica may trigger clustering of the SR and activate one or more apoptotic pathways, resulting in cell death. Another possibility is that the phagocytosis of silica particles by the SR might lead to apoptosis.
However, the lack of cellular toxicity following TiO2 exposure is intriguing and requires further study (Iyer et al., Citation1996; Hamilton et al., Citation2000). It can be theorized that differences in binding of silica and TiO2, may lead to unique conformational changes in SR that may impact the subsequent signaling resulting in contrasting apoptotic outcome. Moreover, while the results of these studies identified a role for SR in binding of various environmental particles and silica-induced apoptosis of human AM, the data did not allow further analysis of the role of individual SR that were involved. Recent studies however have clearly demonstrated the role of SR-A I/II and MARCO in binding and cell signaling by environmental particles (Arredouani et al., Citation2005; Hamilton et al., Citation2006).
SR-A I/II and MARCO
SR-A I and II are expressed primarily on macrophages and have been extensively studied in the context of atherosclerosis; these were initially known as macrophage receptors for oxidatively modified lipoprotein (Dhaliwal and Steinbrecher, Citation1999. MARCO was discovered more recently and has significant structural similarity with SR-A I, except that it lacks the α -helical coiled-coil domain in the extracellular region (Elomaa et al., Citation1995). Both SR-A I/II and MARCO have somewhat overlapping and extensive ligand recognition capacity (Sarrias et al., Citation2004). However, only a limited number of studies have focused on addressing the individual ligand binding properties of the two isoforms.
It was reported that LPS inhibited binding of AcLDL to SR-A II to a different extent than SR-A I (Resnick et al., Citation1996). Also, Escherichia coli was found to bind more efficiently to SR-A II than SR-A I (Peiser et al., Citation2000). An analysis of any such binding differences of environmental particles to SR-A I and SR-A II respectively, will be important to elucidate the molecular basis of particle binding by these receptors.
As stated earlier, the first direct evidence that SR-A I/II plays a role in environmental particle induced signaling emerged when Chao et al. (Citation2001) reported that silica-induced caspase activation and apoptosis in a murine cell line, which was inhibited by 2F8, a monoclonal antibody to SR-A I/II. Recent in vivo studies have provided support for the role of SR-A I/II on AM in the innate immune response against inhaled environmental particles. SR-A I/II(−/−) mice showed an augmented inflammatory response to silica and TiO2, which included increased levels of pro-inflammatory agents like tumor necrosis factor (TNF-α), and mRNA levels of CXCL3 chemokine, and a significantly increased neutrophilia (Beamer and Holian, Citation2005; Arredouani et al., Citation2006).
Strikingly similar observations were obtained with in vivo exposure of TiO2 in MARCO(−/−) mice. The MARCO(−/−) mice showed a dramatic increase in polymorphonuclear leukocyte trafficking into the lungs, increased levels of TNF-α, and increased mRNA levels of CXCL3 chemokine (Arredouani et al., Citation2006). Although the direct link between SR and enhanced neutrophilia remains uncertain, it has been attributed to increased expression of CXCL3, a potent neutrophil chemoattractant (Arredouani et al., Citation2006). These studies clearly highlight the similar increase in cytokine levels and inflammation in SR-A I/II(−/−) and MARCO(−/−) mice initiated in response to environmental particle exposure, advocating an anti-inflammatory role of these receptors. Additionally, the in vivo experimental results also suggest a protective role of SR-A I/II and MARCO in the lungs against particulate exposure. However, in vitro and ex vivo exposure of the lavaged MARCO(−/−) AM to TiO2 did not lead to altered TNFα levels (Arredouani et al., Citation2004). This observation suggests the importance of crosstalk between AM and other cells in the SR-mediated cytokine release.
In contrast to the similarity of in vivo cytokine release related to these receptors, a recent study demonstrated that only MARCO(−/−) showed AM impaired binding of silica as compared to the AM (Hamilton et al., Citation2006). Whereas there was no significant difference in particle binding by AM from SRA I/II(−/−) mice. On further investigation, the group found that the SRA I/II(−/−) mice showed an increased surface expression of MARCO, which might explain binding of TiO2 to SR-A I/II(−/−) AM. Thus, the presence of structurally and functionally similar cellular receptors and their interdependent expression levels pose challenges in the interpretation for the individual roles of SR in particle-induced signaling. However, this observation strongly advocated MARCO as a predominant receptor in silica binding and the consequent silica-induced cytotoxicity in C57Bl/6 AM. The level of MARCO expression on these AM are highly correlated with the amount of silica uptake. However, the MARCO antibody did not completely inhibit the silica binding to AM, which suggests the role of other SR or a non-receptor mediated uptake mechanisms for these particles (Hamilton et al., Citation2006). Interestingly, unlike silica uptake, the silica-induced cyto-toxicity was completely inhibited by pretreatment of the murine AM with MARCO antibody suggesting a particular role of MARCO in silica-induced cell death. Also, AM isolated from MARCO(−/−) mice did not undergo apoptosis in response to silica exposure (Hamilton et al., Citation2006). Additionally, this study competently ruled out the role of Class B SR (CD36), known to play a significant role in atherosclerosis in silica binding and cytotoxicity, at least in C57Bl/6 mice (Hamilton et al., Citation2006).
It has been reported that fibrogenic particulates (e.g., silica) alter the antigen-presenting activity of AM isolated from humans or murine sources (Hamilton et al., Citation2001; Migliaccio et al., Citation2005). Since it was shown that MARCO was involved in silica uptake and toxicity, Hamilton et al. (Citation2006) also explored the role of MARCO in macrophage antigen presenting activity. In contrast to the normal T-cell stimulation using silica-treated wild type AM, silica-treated MARCO(−/−) AM failed to initiate a robust T-cell cytokine response compared to the AM following CD3 or ovalbumin stimulation in vitro. Nevertheless, based on these published reports both MARCO and SR-A I/II play an important role in the inflammatory response to non-fibrogenic particles like TiO2. However, MARCO seems to be playing a predominant role in binding of environmental particles and possibly even in the inflammatory response, at least in C57Bl/6 mice.
The report by Hamilton et al. (Citation2006) demonstrated that there is a strain-specific variation in the basal expression and functional use of the different SR on the AM. The study addressed the differential expression and role of SR using AM from 129/SvJ and Balb/c mouse models along with the C57Bl/6 mice. The Authors found that MARCO played a predominant role in silica binding and cytotoxicity in 129/SvJ mice, whereas the Balb/c AM did not show significant expression of SR-A I/II and MARCO (Hamilton et al., Citation2006). This report is consistent with the observation reported by Palecanda et al. (Citation1999) that the MARCO antibody did not inhibit the TiO2 binding in Balb/c AM. The Balb/c AM however showed significant silica and TiO2 binding (Hamilton et al., Citation2006). This observation suggests alternative mechanisms, either involving different receptors or non-receptor mediated pathway for uptake of environmental particles in Balb/c AM.
Apart from the role of SR in the AM response to environmental particle exposure, a recent study reported the role of SR on mast cells in silica-induced pulmonary inflammation (Brown et al., Citation2006). The authors for the first time reported the expression of SR on mast cells. Importantly, mice deficient in mast cells did not develop silicosis three months after silica exposure emphasizing the role of mast cells in silicosis. In particular, this study supported the role of SR in silica-induced activation of mast cells. The Authors reported an increase in mRNA levels of SR-A II following silica exposure. There was a significant decrease in apoptosis, reactive oxygen species, and TNF-α production in SRA(−/−), MARCO(−/−), and SRA/MARCO(−/−) bone marrow-derived mast cells following silica exposure (Brown et al., Citation2006). Therefore, consistent with the studies in the macrophages, it appears that SR mediates silica binding and responses in mast cells.
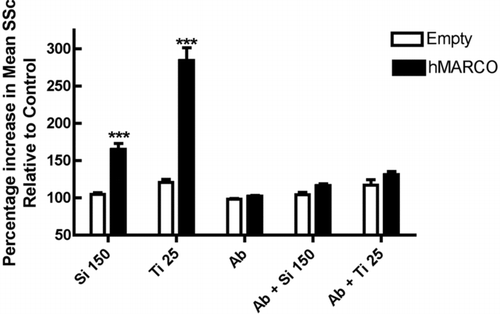
An important question to consider is the relevance of these data using mice to model human biology. In this context, MARCO has also been shown to play a central role in environmental particle binding in human AM. A monoclonal antibody (Plk-1) against human MARCO blocked TiO2 and latex beads binding by human AM (Arredouani et al., Citation2005). Also, transfection of Human Embryonic Kidney (HEK) 293 cells with human MARCO cDNA caused a significant increase in silica and TiO2 binding (). This binding was significantly blocked by Plk-1 monoclonal antibody against human MARCO. Similar observations were obtained using CHO and COS cells transfected with human MARCO (Arredouani et al., Citation2005; Hamilton et al., Citation2006). Blocking antibodies against human SR-A I/II did not inhibit TiO2 binding to human AM (Arredouani et al., Citation2005). Thus, the roles of human SR-A I/II and MARCO in particle binding and AM stimulation are of extreme interest and require further study.
FIG. 1 The effect of human MARCO transfection on silica and TiO2 binding by HEK 293 cells. CHO cells were transfected using Lipofectamine 2000 (Life Technologies, Inc.) as per manufacturer's instructions with empty vector (Empty) or full length MARCO (hMARCO). Following 36–40 hr of transient transfections, CHO cells were harvested by using trypsin; the cells were then resuspended in 1 ml of PAB (0.1% sodium azide, 2% BSA in PBS) and counted. Cells (1 × 106) were treated with or without 10 μ g/ml of monoclonal antibody against SRCR domain of human MARCO (Plk-1) or 10 μ g/ml isotype control (IgG3) on ice for 15 min. The cells were then treated with either silica (150 μ g/ml) or TiO2 (25 μ g/ml) for 30 min at 37°C on the rotator. The particle binding was then measured as an increase in mean Side Scatter (SSc) by FACS Aria Flow cytometer as a marker of increase in granularity of cells due to silica or TiO2 binding Results represent mean ± SEM percent side scatter relative to unstimulated control cells following 30 min particle exposure in suspension culture. Open bars indicate empty vector control transfection. Solid bars indicate human MARCO transfection. ***p < 0.001 compared to corresponding ‘empty vector’ control by Bonferroni's post-hoc test. Sample size n = 7.
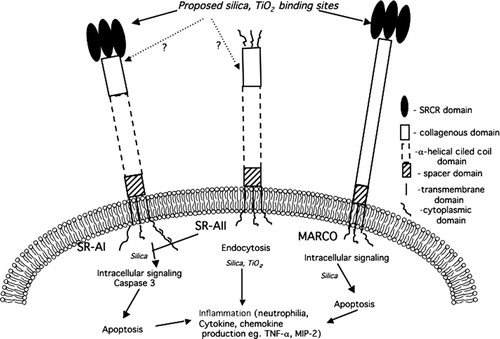
Finally, although the precise physical properties that enable particles to bind and initiate differential signaling through common receptors (SRA I/II and MARCO) remain to be determined, the cytotoxic outcomes and cytokine release by these particle-SR interactions have been discovered (). Particles, owing to their negative surface charge, may bind the positive region in the SRCR domain of MARCO. Alternatively, as in the case for SRA I/II, particles could bind to the collagenous domain. This might lead to a unique conformational change in these receptors initiating downstream signaling. With reference to silica, the signaling that is initiated results in induction of apoptosis in AM that might play a role in the inflammatory response, such as the release of cytokines (TNF-α). Caspase 3 activation has been observed following SRAI/II activation, but apoptotic signaling activated by silica-MARCO interactions have not yet been identified (). An important point that needs to be stressed here is the case of TiO2, which binds to the same receptor (MARCO) as silica, but there is no loss of cell viability. These particle-MARCO interactions thus seem to result in differential cellular outcomes dependent on either the physical properties of the particle and/or the differences in binding of these two particles to MARCO. Consequently, regulating the binding of individual particles to MARCO might be an efficient way of regulating different signaling events.
FIG. 2 Role of Class A Scavenger Receptors in Silica and TiO2 signaling. The SRCR region of MARCO and SRA I is implicated as silica and TiO2 binding domain. The collagenous domain of SRA I/II may also play a role in binding of particles. The signaling cascades downstream of SRA-I/II and MARCO initiated by silica have been minimally defined. It has been reported that SRA-I/II-mediate caspase 3 activation and apoptosis following silica binding. Whereas, the intracellular signaling activated following silica-MARCO interaction are as yet unknown. TiO2 is reported to mediate the release of inflammatory mediators through SRA I/II and MARCO. To date, the endocytosis and inflammatory pathways initiated by particle-SR interaction remain largely undefined.
CONCLUSIONS
At present, there is emerging evidence supporting the notion that MARCO is a major receptor for uptake of environmental particles like silica and TiO2. However, there is substantial evidence that SR-A I/II also seems to play a significant role in regulating the inflammatory response to environmental particles especially in murine models. Silica- and TiO2-induced signaling events via SR are illustrated in . The specific role that SR-A I/II plays in particle binding is less well defined, but may well depend on improving the validation of receptor expression. Despite certain structural differences in SR-A I/II and MARCO receptors, both seem to recognize and play a role in inflammatory responses against common environmental ligands. However, the ultimate outcome (e.g., fibrosis) differs with respect to different particles. Characterizing the molecular details of these contradicting outcomes will help answer the role of SR in disease pathology and may provide new targets to therapeutic strategies.
Nonetheless, results of these studies have provided important new evidence that SR functions in initiating cell signaling pathways following particle exposure. A future challenge is to understand the additive or cumulative effect of the individual SR mediated cellular signaling on particle-induced toxicity. MARCO, SR-A I and SCARA 5 all have SRCR structure as an integral part of the extracellular domain. Exploring the role of SRCR in particle binding will add to discovering this highly conserved motif. The environmental particle-binding aspect of SR biology has only recently become apparent and opens the door for further research in elucidating the mechanisms involved in particle-induced lung pathology.
Our work was supported by National Institute of Health and National Center for Research Resources grant PR017670.
REFERENCES
- Arredouani M. S., Palecanda A., Koziel H., Huang Y. C., Imrich A., Sulahian T. H., Ning Y. Y., Yang Z., Pikkarainen T., Sankala M., Vargas S. O., Takeya M., Tryggvason K., Kobzik L. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J. Immunol. 2005; 175: 6058–6064
- Arredouani M. S., Yang Z., Imrich A., Ning Y., Qin G., Kobzik L. The macrophage Scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell Mol. Biol. 2006; 35: 474–478
- Arredouani M., Yang Z., Ning Y., Qin G., Soininen R., Tryggvason K., Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J. Exp. Med. 2004; 200: 267–272
- Beamer C. A., Holian A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am. J. Physiol. Lung Cell Mol. Physiol. 2005; 289: 186–195
- Becklake M. R., Bagatin E., Neder J. A. Asbestos-related diseases of the lungs and pleura: Uses, trends and management over the last century. Int. J. Tuberc. Lung Dis. 2007; 11: 356–369, Review
- Benson S. C., Belton J. C., Scheve L. G. Regulation of lung fibroblast proliferation and collagen synthesis by alveolar macrophages in experimental silicosis. I: Effect of macrophage-conditioned medium from silica instilled rats. J. Environ. Pathol. Toxicol. Oncol. 1986; 7: 87–97
- Brannstrom A., Sankala M., Trygvasson K., Pikkarainen T. Arginine residues in Domain V have a central role for bacteria-binding activity of macrophage scavenger receptor MARCO. Biochem. Biophys. Res. Comm. 2002; 290: 1462–1469
- Brown J. M., Schwanke C. M., Pershouse M. A., Pfau J. C., Holian A. Effects of rottlerin on silica-exacerbated systemic autoimmune disease in New Zealand mixed mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2005; 289: 990–998
- Brown J. M., Swindle E. J., Kushnir-Sukhov N. M., Holian A., Metcalfe D. D. Silica-directed mast cell activation is enhanced by scavenger receptors. Am. J. Respir. Cell Mol. Biol. 2006; 36: 43–52
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 1979; 82: 597–613
- Calvert G. M., Rice F. L., Boiano J. M., Sheehy J. W., Sanderson W. T. Occupational silica exposure and risk of various diseases: An analysis using death certificates from 27 states of the United States. Occup. Environ. Med. 2003; 60: 122–129
- Chao S. K., Hamilton R. F., Pfau J. C., Holian A. Cell surface regulation of silica-induced apoptosis by the SR-A scavenger receptor in a murine lung macrophage cell line (MH-S). Toxicol. Appl. Pharmacol. 2001; 174: 10–16
- Chen Y., Sankala M., Ojala J. R., Sun Y., Tuuttila A., Isenman D. E., Tryggvason K., Pikkarainen T. A phage display screen and binding studies with acetylated low density lipoprotein provide evidence for the importance of the scavenger receptor cysteine-rich (SRCR) domain in the ligand-binding function of MARCO. J. Biol. Chem. 2006; 281: 12767–12775
- Dhaliwal B. S., Steinbrecher U. P. Scavenger receptors and oxidized low density lipoproteins. Clin. Chim. Acta. 1999; 286: 191–205
- Doi T., Higashino K., Kurihara Y., Wada Y., Miyazaki T., Nakamura H., Uesugi S., Imanishi T., Kawabe Y., Itakura H., Yazakij Y., Matsumoto A., Kodama T. Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J. Biol. Chem. 1993; 268: 2126–2133
- Dresel H. A., Friedrich E. A., Otto I., Waldherr R., Schettler G. The low density lipoprotein and low density lipoprotein receptors and their possible importance in the pathogenesis of atherosclerosis. Arzneimittelforschung 1985; 35: 1936–1940
- Elomaa O., Kangas M., Sahlberg C., Tuukkanen J., Sormunen R., Liakka A., Thesleff I., Kraal G., Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 1995; 80: 603–609
- Englert H., Small-McMahon J., Davis K., O'Connor H., Chambers P., Brooks P. Male systemic sclerosis and occupational silica exposure: A population-based study. Aust N Z J Med. 2000; 30: 215–220
- Green F. H. Y., Vallyathan V. Pathologic responses to inhaled silica: Silica and silica-induced lung diseases. CRC Press, Boca Raton, FL 1996; 39–59
- Hamilton R. F., de Villiers W. J., Holian A. Class A Type II scavenger receptor mediates silica-induced apoptosis in Chinese hamster ovary cell line. Toxicol. Appl. Pharmacol. 2000; 162: 100–106
- Hamilton R. F., Jr, Pfau J. C., Marshall G. D., Holian A. Silica and PM1648 modify human alveolar macrophage antigen-presenting cell activity in vitro. J. Environ. Pathol. Toxicol. Oncol. 2001; 20(S1)75–84
- Hamilton R. F., Jr, Thakur S. A., Mayfair J. K., Holian A. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J. Biol. Chem. 2006; 281: 34218–34226
- Huaux F. New developments in the understanding of immunology in silicosis. Curr. Opin. Allergy Clin. Immunol. 2007; 7: 168–173
- Iyer R., Holian A. Involvement of the ICE family of proteases in silica-induced apoptosis in human alveolar macrophages. Am. J. Physiol. 1997; 273: L760–767
- Iyer R., Hamilton R. F., Holian A. Silica-induced apoptosis mediated via scavenger receptor in human alveolar macrophages. Toxicol. Appl. Pharmacol. 1996; 141: 84–92
- Jiang Y., Oliver P., Davies K. E., Platt N. Identification and characterization of murine SCARA5, a novel class A scavenger receptor that is expressed by populations of epithelial cells. J. Biol. Chem. 2006; 281: 11834–11845
- Kunjathoor V. V., Febbraio M., Podrez E. A., Moore K. J., Andersson L., Koehn S., Rhee J. S., Silverstein R., Hoff H. F., Freeman M. W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002; 277: 49982–49988
- Lalmanach G., Diot E., Godat E., Lecaille F., Herve-Grepinet V. Cysteine cathepsins and caspases in silicosis. Biol. Chem. 2006; 387: 863–870
- Lardot C. G., Huaux F. A., Broeckaert F. R., Delos P. J. D. M., Fubini B., Lison D. F. Role of urokinase in the fibrogenic response of the lung to mineral particles. Am. J. Respir. Crit. Care Med. 1998; 157: 617–628
- Leigh J., Davidson P., Hendrie L., Berry D. Malignant mesothelioma in Australia, 1945–2000. Am. J. Ind. Med. 2002; 41: 188–201
- Lindenschmidt R. C., Driscoll K. E., Perkins M. A., Higgins J. M., Maurer J. K., Belfiore K. A. The comparison of a fibrogenic and two non-fibrogenic dusts by bronchoalveolar lavage. Toxicol. Appl. Pharmacol. 1990; 102: 268–281
- Matsumoto A., Naito M., Itakura H., Ikemoto S., Asaoka H., Hayakawa I., Kanamori H., Aburatani H., Takaku F., Suzuki H., Kobari Y., Miyai T., Takahashi K., Cohen E. H., Wydro R., Housman D. E., Kodama T. Human macrophage scavenger receptors: Primary structure, expression, and localization in atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1990; 87: 9133–9137
- Migliaccio C. T., Hamilton R. F., Jr, Holian A. Increase in a distinct pulmonary macrophage subset possessing an antigen-presenting cell phenotype and in vitro APC activity following silica exposure. Toxicol. Appl. Pharmacol. 2005; 205: 168–176
- Moore K. J., Freeman M. W. Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 2006; 26: 1702–1711
- Murphy J. E., Tedbury P. R., Homer-Vanniasinkam S., Walker J. H., Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 2005; 182: 1–15
- O'Reilly K. M., Mclaughlin A. M., Beckett W. S., Sime P. J. Asbestos-related lung disease. Am. Family Physician. 2007; 75: 683–688
- Palecanda A., Paulauskis J., Al-Mutairi E., Imrich A., Qin G., Suzuki H., Kodama T., Tryggvason K., Koziel H., Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 1999; 189: 1497–1506
- Park R., Rice F., Stayner L., Smith R., Gilbert S., Checkoway H. Exposure to crystalline silica, silicosis, and lung disease other than cancer in diatomaceous earth industry workers: A quantitative risk assessment. Occup. Environ. Med. 2002; 59: 36–43
- Parks C. G., Cooper G. S., Nylander-French L. A., Sanderson W. T., Dement J. M., Cohen P. L., Dooley M. A., Treadwell E. L., St Clair E. W., Gilkeson G. S., Hoppin J. A., Savitz D. A. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: A population-based, case-control study in the southeastern United States. Arthritis Rheum. 2002; 46: 1840–1850
- Peiser L., Gough P. J., Kodama T., Gordon S. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: Role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect. Immun. 2000; 68: 1953–1963
- Pfau J. C., Brown J. M., Holian A. Silica-exposed mice generate autoantibodies to apoptotic cells. Toxicology 2004; 195: 167–176
- Platt N., Gordon S. Scavenger receptors: Diverse activities and promiscuous binding of polyanionic ligands. Chem. Biol. 1998; 5: 193–203
- Resnick D., Chatterton J. E., Schwartz K., Slayter H., Krieger M. Structures of class A macrophage scavenger receptors. Electron microscopic study of flexible, multidomain, fibrous proteins and determination of the disulfide bond pattern of the scavenger receptor cysteine-rich domain. J. Biol. Chem. 1996; 271: 26924–26930
- Resnick D., Freedman N. J., Xu S., Krieger M. Secreted extracellular domains of macrophage scavenger receptors form elongated trimers which specifically bind crocidolite asbestos. J. Biol. Chem. 1993; 268: 3538–3545
- Rimal B., Greenberg A. K., Rom W. N. Basic pathogenetic mechanisms in silicosis: Current understanding. Curr. Opin. Pulm. Med. 2005; 11: 169–173
- Sarrias M. R., Gronlund J., Padilla O., Madsen J., Holmskov U., Lozano F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: an ancient and highly conserved protein module of the innate immune system. Crit. Rev. Immunol. 2004; 24: 1–37
- Srivastava K. D., Rom W. N., Jagirdar J., Yie T. A., Gordon T., Tchou-Wong K. M. Crucial role of interleukin-1β and nitric oxide synthase in silica-induced inflammation and apoptosis in mice. Am. J. Respir. Crit. Care Med. 2002; 165: 527–533
- Thibodeau M., Giardina C., Hubbard A. K. Silica-induced caspase activation in mouse alveolar macrophages is dependent upon mitochondrial integrity and aspartic proteolysis. Toxicol. Sci. 2003; 76: 91–101
- Ulm K., Waschulzik B., Ehnes H., Guldner K., Thomasson B., Schwebig A., Nuss H. Silica dust and lung cancer in the German stone, quarrying, and ceramics industries: results of a case-control study. Thorax 1999; 54: 347–351
- van der Laan L. J., Dopp E. A., Haworth R., Pikkarainen T., Kangas M., Elomaa O., Dijkstra C. D., Gordon S., Tryggvason K., Kraal G. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J. Immunol. 1999; 162: 939–947