Abstract
Interleukin-1 receptor antagonist (IL-1Ra) is a naturally occurring inhibitor of the pro-inflammatory interleukin-1-mediated activation of the interleukin-1 receptor (IL-1R). Although wild-type IL-1Ra is used for treatment of inflammatory diseases, its effect is moderate and/or short-lived. The objective of this study was to generate IL-1Ra mutants with enhanced antagonistic activity for potential therapeutic use. Using a directed evolution approach in which libraries of IL-1Ra gene mutants were generated and screened in functional assays, mutants with desired properties were identified. Initially, diversity was introduced into the IL-1Ra using random mutagenesis. Mutations resulting in enhanced antagonistic activity were identified by screening in a reporter cell assay. To further enhance the antagonistic activity, selected mutations were recombined using the DNA recombination technology Fragment-INduced Diversity (FIND®). Following three rounds of FIND® recombination, several mutants with up to nine times enhanced antagonistic activity (mean IC50 ± SEM value: 0.78 ± 0.050 vs. 6.8 ± 1.1 ng/ml for mutant and wild-type, respectively) were identified. Sequence analysis identified the mutations D47N, E52R and E90Y as being most important for this effect, however, the mutations P38Y, H54R, Q129L and M136N further enhanced the antagonistic function. Analysis of identified mutations in protein models based on the crystal structure of the IL-1Ra/IL-1R complex suggested that mutations found to enhance the antagonistic activity had a stabilizing effect on the IL-1Ra mutants or increased the affinity for the IL-1R. Finally, the therapeutic effect of one mutant was compared to that of wild-type IL-1Ra in collagen-induced arthritis in mice. Indeed, the enhanced antagonistic effect of the mutants observed in vitro was also seen in vivo. In conclusion, these results demonstrate that directed evolution of IL-1Ra is an effective means of generating highly potent therapeutic proteins.
INTRODUCTION
Interleukin-1 (IL-1) is a potent pro-inflammatory cytokine that can be produced by a variety of cell types, including mononuclear phagocytes, in response to infection and inflammation (summarized in Dinarello, 2000, 2002). The IL-1 family consists of two agonists, IL-1α and IL-1β and a naturally occurring antagonist, the IL-1 receptor antagonist (IL-1Ra) (Dinarello, Citation1996). Upon interaction with the IL-1 receptor type I (IL-1RI) / IL-1R accessory protein (IL-1RAcP) complex, IL-1 induces cellular stimulation resulting in the activation of an inflammatory cascade mediated in part by NFκ B (Heguy et al., Citation1992; Stylianou et al., Citation1992). This results in the production and expression of adhesion molecules, cytokines and other inflammatory mediators such as prostaglandin E2 and nitric oxide (NO) (Dinarello, Citation1996, Citation2000). In addition, IL-1 has been shown to promote bone and cartilage degradation in rheumatic diseases (Paget, Citation2002).
IL-1Ra is structurally related to IL-1 and is capable of binding to IL-1RI but fails to interact with IL-1RAcP and is thus incapable of inducing cellular activation (Dinarello, Citation1996; Arend and Gabay, Citation2000). Thus, IL-1Ra inhibits the pro-inflammatory effects of IL-1 by functioning as a competitive inhibitor in receptor binding.
IL-1 has been implicated in the pathogenesis of several autoimmune and auto-inflammatory diseases. In rheumatoid arthritis (RA), treatment with recombinant N-methionyl-IL-1Ra (Anakinra) has been shown to reduce disease severity in several studies (Bresnihan et al., Citation1998; Joosten et al., Citation1999; Cohen et al., Citation2002, Citation2004; Nuki et al., Citation2002). In addition, IL-1RI blockade using Anakinra has been shown to effectively reduce disease severity in a number of disease conditions including systemic onset juvenile idiopathic arthritis (Pascual et al., Citation2005), adult onset Still's disease (Rudinskaya and Trock, Citation2003; Fitzgerald et al., Citation2005; Vasques Godinho et al., Citation2005; Ruiz et al., Citation2007), hyper-IgD syndrome (Bodar et al., Citation2005), and Type 2 diabetes mellitus (Larsen et al., Citation2007).
Finally, IL-1 blockade has also shown dramatic effects on disorders caused by over-production of IL-1 due to mutations in the CIAS1/NALP3/cryopyrin gene, i.e., familial cold auto-inflammatory syndrome, Muckle-Wells disease, and neonatal onset multi-system inflammatory disease (Hawkins et al., Citation2003, Citation2004a, Citation2004b; Hoffman et al., Citation2004; Goldbach-Mansky et al., Citation2006; Hoffman and Firestein, Citation2006), or due to mutations in the pyrin gene, i.e., familial Mediterranean fever and pyogenic arthritis pyoderma gangrenosum and acne syndrome (Dierselhuis et al., Citation2005; Chae et al., Citation2006).
Although IL-1Ra treatment is capable of ameliorating disease, daily injections are required for clinical effects and withdrawal of treatment results in a rapid disease flare in several conditions (Burger et al., Citation2006). In addition, in most RA patients, IL-1Ra treatment is significantly less effective than blockade of other inflammatory mechanisms, e.g., tumor necrosis factor (TNF) (Nixon et al., Citation2007). In RA, the poor efficacy of IL-1Ra in controlling disease severity, compared to, for example, TNF blockers, could potentially be due to that the IL-1 mechanism contributes less to pathogenicity. Alternatively, it could be due to an insufficient receptor affinity or a too short plasma half-life of IL-1Ra (Burger et al., Citation2006).
Thus, an IL-1Ra mutant with improved functional activity or enhanced in vivo half-life could be expected to result in improved clinical effects. Although drug withdrawal would still be expected to result in a disease flare, the therapeutic effect may be more long-lived. In addition, an IL-1Ra variant with enhanced functional effect may be a useful candidate for pegylation that is used to prolong the half-life of therapeutic proteins, but is known to often reduce the functional activity (Bowen et al., Citation1999).
Genetic engineering of proteins is capable of generating mutants with enhanced therapeutic efficacy (Patten et al., Citation1997; Yuan et al., Citation2005). However, predicting the mutations that should be introduced into a gene to generate the optimal therapeutic protein is difficult. To circumvent that difficulty, a random directed evolution approach using selection of beneficial mutations from a randomly mutated library followed by genetic recombination of the selected gene sequences may be used. Such an approach generates libraries comprising clones with all possible combinations of mutations contained in the selected gene sequences. Clones with mutations working in synergy to create the desired properties can thus be identified (Patten et al., Citation1997; Yuan et al., Citation2005). Fragment-INduced Diversity (FIND®) is a genetic recombination technology in which single-stranded DNA fragments generated by exonuclease digestion are recombined in a rapid and efficient manner (described in International Patents No. EP 1 341 909 and EP 1 504 098). In this study, FIND® recombination was used in an effort to generate IL-1Ra mutants with enhanced antagonistic function compared to wild-type IL-1Ra.
MATERIALS AND METHODS
Reagents
The chemicals MnCl2, NaPO4, (NH4)2PO4, MgSO47H2O, CuCl22H2O, CoCl26H2O, Zn(CH3COO)22H2O, citric acid, gelatin, and chloramphenicol were obtained from Sigma-Aldrich (St. Louis, MO). MgCl2, NaCl, KH2PO4, H3BO4, MnCl24H2O, Na2MoO42H2O, EDTA, Imidazole, bovine serum albumin (BSA), glucose, and Tween-20 were obtained from VWR (Stockholm, Sweden). dATP, dGTP, dTTp, and cCTP were obtained from Invitro (Stockholm, Sweden). Ampicillin was obtained from Calbiochem (San Diego, CA). Vector-specific PCR primers were obtained from MWG Biotech (Ebersberg, Germany). Anakinra, produced by Amgen, Thousand Oaks, CA, was obtained from the pharmacy at Lund University Hospital (Lund, Sweden).
Generation of Genetic Libraries by Random Mutagenesis and FIND®
Wild-type human interleukin-1Ra (IL-1Ra) was used as a starting material for subsequent generation of IL-1Ra mutants. The human IL-1Ra wild-type protein was retrieved from a liver cDNA library (Clontech, Mountain View, CA) by PCR amplification. Wild-type or mutant IL-1Ra with or without signal peptide was cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA) or pET22b(+) (Novagen, San Diego, CA) vectors. To facilitate detection and purification, all IL-1Ra variants were cloned with N- or C-terminal 6xhis tags, in pET22b(+) and pcDNA3.1 vectors, respectively.
For the introduction of diversity into wild-type or mutant IL-1Ra gene sequence, random mutagenesis using error-prone PCR or the GeneMorphII PCR mutagenesis kit (Stratagene, La Jolla, CA) was performed according to the manufacturer's instructions. In the GeneMorphII reactions 1 ng wild-type IL-1Ra or 100 pg IL-1Ra mutant gene sequences were used as starting material. In error-prone PCR, the reactions, the PCR reactions contained 1, 10 or 100 pg cDNA, 7 mM MgCl2, 0.01% gelatine, 0.2 mM dATP, 0.2 mM dGTP, 1 mM dTTP, 1 mM dCTP, 0.3 μ M forward primer, 0.3 μ M reverse primer, 0.5 mM MnCl2, and 0.025 U AmpliTaq DNA polymerase (Applied Biosystems, Foster city, CA).
Library generation by random recombination of mutated IL-1Ra cDNA was performed using the FIND® technology according to the method disclosed in International Patents No. EP 1 341 909 and EP 1 504 098. Potential beneficial mutations, as indicated by molecular modeling, were introduced into wild-type or mutant IL-1Ra gene sequences using the QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. Sequence analysis of wild-type or mutant IL-1Ra clones was performed by MWG Biotech using vector-specific primers.
Protein Expression and Purification
For expression of IL-1Ra mutant libraries a eukaryotic expression system was used. pcDNA3.1 plasmid preparation from 10,000–20,000 individual clones from mutant libraries were isolated by GATC (Konstanz, Germany) from overnight cultures of transformed BL21(DE3)pLysS cells (Invitrogen). COS-7 cells (LGC Promochem, Teddington, UK) were transfected with isolated plasmid according to standard protocols (Ausubel et al., 2002). Briefly, 10 μ l plasmid preparation (≈ 0.4 μ g/ml) was mixed with 0.3 μ l Lipofectamine 2000 (Invitrogen) and 16 μ l Opti-MEM1 (Invitrogen) according to the manufacturer's instructions. The mixture was added to 30000 COS-7 cells in 50 μ l DMEM medium (Cambrex, East Rutherford, NJ) supplemented with 5% heat inactivated (56°C, 30 min) fetal bovine serum (Cambrex) and incubated for 72 hr at 37°C in 5% CO2.
For expression of larger amounts of wild-type or mutant IL-1Ra protein that was subsequently purified, a prokaryotic expression system was used. Escherichia coli BL21(DE3)pLysS cells (Stratagene) were transformed with the pET22b(+) vector containing the individual wild-type or mutant IL1-Ra gene sequences containing an N-terminal 6xhis tag according to standard molecular biology techniques (Ausubel et al., 2002). Aseptic cultures were incubated overnight in LB medium supplemented with 50 μ g/ml ampicillin (amp) and 34 μ g/ml chloramphenicol (cam) at 37°C with shaking. Overnight cultures were diluted 30-fold in LB supplemented with amp and cam and 35 μ l diluted culture was added to 965 μ l LB supplemented with amp and cam in deep well plates. Plates were incubated at 37°C with shaking to OD600 0.5, whereupon expression of wild-type or mutant IL-1Ra was induced by the addition of 1 mM IPTG. Plates were incubated for a further 3 hr at 37°C with shaking, after which cells were centrifuged at 3500 rpm for 10 min. Supernatant fluids and cell pellets were retained for later use.
The IL-1Ra mutants expressed in E. coli were purified prior to further in vitro analyses. This was performed by using the His-Select iLAP HC Nickel system (Sigma-Aldrich) or for larger scale purification, HisTrap columns (Amersham Bioscience, Little Chalfont, UK). Briefly, cells were lysed using a lysis buffer (Complete EDTA-free (Roche Diagnostics, Basel, Switzerland), 0.048 U Benzonase (Sigma-Aldrich) and 0.002U Lysozyme (Novagen) in PBS. The lysate was transferred to His-Select iLAP HC Nickel plates (Sigma-Aldrich) and the plates incubated for 3 hr at room temperature (RT) with shaking. After washing four times with PBS supplemented with 0.05% Tween-20, and four times with H2O, the samples were eluted with 50 μ l of 50 mM Na-phosphate (pH 8.0), 300 mM NaCl, and 250 mM Imidazole during 60 min at RT with shaking. The samples were then collected and filtered using MultiScreen Ultracel-10 10,000 NMWL (Millipore) and saved for further analyses.
For large scale purification of protein samples, to be used for in vivo experiments, homogenates were prepared from transformed E. coli BL21(DE3)pLysS cultured in a fed batch protocol in a synthetic medium (16.6 g/l KH2PO4, 4 g/l (NH4)2PO4, 1.5 g/l MgSO4 × 7H2O, 2.1 g/l citric acid, 74.3 mg/l Fe-citrate, 3.7 mg/l H3BO4, 18.9 mg/l MnCl24H2O, 10.6 mg/l EDTA, 1.9 mg/l CuCl2 · 2H2O, 3.1 mg/l Na2MoO4 · 2H2O, 3.1 mg/l CoCl2 · 6H2O, 10 mg/l Zn(CH3COO)2 · 2H2O and 26 g/l glucose) and induced with 1 mM IPTG. Expressed protein was purified using HisTrap HP columns according to the manufacturer's instructions.
Quantification of Wild-type and Mutant IL-1Ra
Levels of expressed protein samples were quantified using a standard sandwich ELISA. Briefly, Maxisorp 96-well plates (Nunc, Albertslund, Denmark) coated with anti-His antibody (Novagen) in PBS overnight were used to capture IL-1Ra variants with 6xhis tags following blocking with PBS, 1% BSA, 0.05% Tween-20. Wild-type IL-1Ra with C-terminal 6xhis tag was used as a protein standard. The detection antibody used was anti-Anakinra rabbit antiserum (Antibody AB, Södra Sandby, Sweden), which was detected using swine-anti-rabbit-HRP (DAKO, Copenhagen, Denmark) followed by chromogenic substrate SIGMA-FAST o-phenylenediamine dihydrachloride tablets (SIGMA-Aldrich) according to the manufacturer's instructions. The reaction was stopped using 2 M H2SO4 and the absorbance at 492 nm was measured in a SpectraMax plate reader using the Softmax pro software (Molecular Devices, Sunnyvale, CA). All steps were performed at room temperature. Washing solution consisted of PBS supplemented with 0.05% Tween-20 and all reagents were diluted in PBS 1% BSA 0.05% Tween-20 unless otherwise stated.
Reporter Cell Assay
IL-1Ra mutants with enhanced antagonistic activity were identified in a step-wise screening process utilizing a reporter cell system consisting of NIH/3T3 cells naturally expressing IL-1RI and stably transfected with a 5xNFkB driven luciferase reporter construct (Radons et al., Citation2002). The reporter cells were cultured overnight in DMEM medium (Cambrex, Walkersville, MD) supplemented with 5% fetal bovine serum (heat inactivated at 56°C for 30 min) (Cambrex) at 37°C, 5% CO2.
For primary screening of 10,000–20,000 mutant clones transiently expressed in COS-7 cells as described above, single point measurements were performed. Following identification of 200–400 clones with improved activity, more thorough analyses with quantified samples were performed using replicates and/or dilution series of the respective clones.
For analysis of 20–40 top clones, the sequences were cloned into the pET22b(+) vector and expressed and purified as described above. Briefly, samples containing expressed IL-1Ra mutants or control wild-type IL-1Ra was added to the reporter cells in the presence or absence of IL-1 (R&D, Minneapolis, MN) at a final concentration of 0.1 ng/ml and plates were incubated at 37°C in 5% CO2 for 4 hr. Plates were further incubated in darkness for 5 min at 37°C following the addition of 16 μ l Steady Glo Luciferase Assay system substrate (Promega). Luminescence was recorded using a FLUOstar OPTIMA (BMG Labtech, Offenburg, Germany) and EC50 values were calculated.
Collagen-Induced Arthritis
Collagen-induced arthritis (CIA) was induced in male DBA-1 Janvier mice by intravenous injection of 100 μ l complete Freunds adjuvant (Difco 263810, Detroit, MI) supplemented with 1 μ g/ul Type II collagen and 3 μ g/μ l Mycobacterium tuberculosis (Difco H37RA, 231141) at the base of the tail. On Day 21, mice were boosted with an intraperitoneal injection of 100 μ g Type II collagen in saline. Mice were scored macroscopically on each paw for arthritis development three times per week, according to the following system: 0 = No visual inflammation; 0.25 = one inflamed toe; 0.5 = two or three inflamed toes; 0.75 = inflamed ankle or wrist; 1.5 = more than three toes and ankle or wrist moderately inflamed; 2.0 = severely inflamed toes and ankle or wrist. Thus, a maximum score of eight could result for each animal.
When mice reached a mean score of 2, groups of eight animals received one of the following treatments: treatment with citrate buffer (5 mM citric acid, 90 mM NaCl, 0.35 mM EDTA, 0.07% Tween 80) constituting the control group; Anakinra (20mg/ml); wild-type IL-1Ra with N-terminal 6xhis tag (1, 5, or 20 mg/ml); or, mutant IL-1Ra with N-terminal 6xhis tag (1, 5, or 20 mg/ml). Proteins were diluted in citrate buffer and administered using mini-osmotic pumps type 1007D (Alzet, Cupertino, CA) implanted intraperitoneally. A release rate of 0.5 μ l/hr from the pumps resulted in an Anakinra dose of 240 μ g/d and doses of 240, 60, and 12 μ g/d of the wild-type and mutant IL-1Ra proteins. Mice were scored macroscopically on each paw for arthritis development at Days 0, 1, 4, 6 and 7 post-implantation of the pump.
Following 7 days of treatment, the experiment was terminated and disease development from the start of the treatment until termination of the experiment determined as a macroscopic score.
Modeling of IL-1Ra – IL-1RI Interaction
Structural analysis and modeling were made based on the crystal structure (PDB code: 1IRA) of the complex between IL-1Ra and IL-1RI (Schreuder et al., Citation1997) using the PyMOL molecular graphics program (Delano, 2002).
Statistical Analysis
For statistical analysis of the differences between the various wild-type and mutated IL-1Ra clones in the reporter assay, a balanced ANOVA (which in all cases resulted in p < 0.05) followed by Student's t-test were used. Due to the relatively low number of repetitions (the clones were analyzed in 2–4 independent experiments) the variance of all samples was pooled and used for statistical calculations. For statistical analysis of the differences between wild-type and mutant IL-1Ra in each dose in CIA, Mann-Whitney U-test was used. All calculations were made in GraphPad.
RESULTS
Generation of IL-1Ra Mutants with Enhanced Antagonistic Function
Diversity was introduced into the IL-1Ra gene by random mutagenesis as described in Methods. Protein samples representing 10,000 clones were expressed and screened in an NFκ B-driven luciferase gene reporter cell assay. Eleven clones, comprising a total of 18 mutations (, ), with a slightly enhanced antagonistic activity compared to wild-type IL-1Ra were identified.
FIG. 1 Mutations present in clones selected from indicated libraries. IL-1Ra mutant libraries were generated by random mutagenesis and/or FIND® recombination and expressed protein libraries were analyzed in reporter assay. Indicated mutations were present in clones selected from the randomly mutated library (A), first FIND® library (B) and the second FIND® library (C). Grey indicates mutations present in the starting material but absent in selected clones (A) and green indicates mutations that were introduced by random mutagenesis (C).
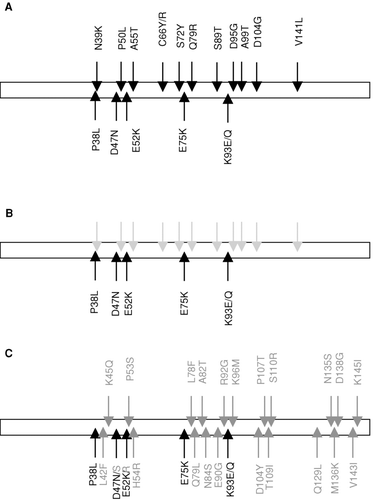
TABLE 1 Mutations present in clones selected from IL-1Ra libraries
To generate IL-1Ra mutant clones with further improved antagonistic activity, the 18 mutations were recombined using the FIND® technology. As shown in , three clones with approximately two times enhanced antagonistic activity were identified. Of the 18 mutations included in the FIND® library from which these clones were identified, the six mutations P38L, D47N, E52K, E75K, K93E and K93Q had been selected (). In addition, it was observed that the E52K mutation was present in all three clones, suggesting that this position is critical for the enhanced functional activity of the IL-1Ra mutants.
FIG. 2 IC50 values of wild-type IL-1Ra and selected mutants as determined in the reporter assay. Results from the first FIND® library (A) and the second FIND® library (B) are shown. Results are shown as mean ± standard deviation of data collected from three individual experiments (A) or between two and four experiments (B). In (B), clone 1.3 selected from the previous round was included as a reference and novel clones are designated 2.10–2.20. Exact sequence of each mutant is shown in . Statistical analysis determining statistically significant differences between wild-type IL-1Ra and mutated clones was performed as described in Methods. * p < 0.05, ** p < 0.01, *** p < 0.001.
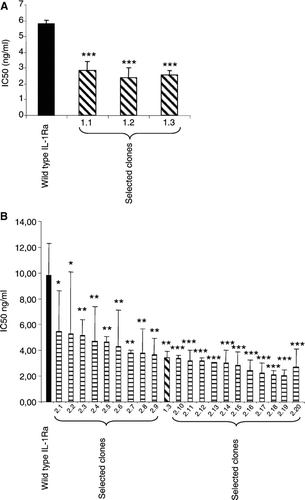
Although clones with clearly improved antagonistic function compared to wild-type IL-1Ra were identified, the diversity of the clones was limited. To determine whether additional mutations could contribute to the antagonistic function, and thereby generate clones with superior inhibitory effect, a second round of modified FIND® mutagenesis was performed. Based upon results from the primary screening (performed as described in Method) 20 clones were selected for more thorough analysis in the reporter assay. Although a variation in absolute IC50 values between individual experiments could be observed (explaining the large error bars as well as the difference in IC50 values between results shown in and ) several clones with potent antagonistic activity were identified. Among these clones, all mutations that were selected in the previous optimization round were retained. In addition, a number of novel mutations with potential beneficial effect on the performance of the IL-1Ra mutants were identified ( and ).
Increased Therapeutic Efficacy of IL-1Ra Mutant
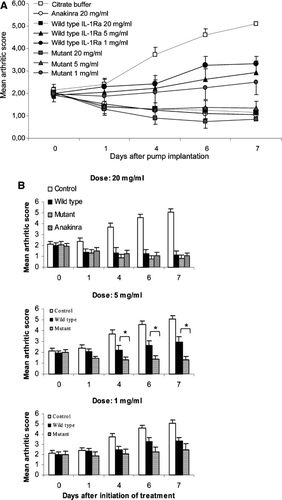
We next investigated whether the improved antagonistic activity of IL-1Ra mutants observed in vitro correlates with an enhanced therapeutic efficacy in vivo. This was done in a murine model for RA, CIA. CIA was induced and allowed to progress to score 2, at which time treatment with wild-type or the mutant IL-1Ra clone 2.20 (according to the designation in ) was initiated. Commercially available IL-1Ra (Anakinra) was included as a positive control. Indeed, the improved function of the IL-1Ra mutant seen in vitro translated into the in vivo situation, and a 4–5 times lower dose of the mutant was required to obtain a similar effect on disease as seen with wild-type IL-1Ra (). Thus, 5 mg/ml of the mutant resulted in similar effect on disease as 20 mg/ml of wild-type IL-1Ra; similarly, 1 mg/ml of the mutant was comparable or even superior to 5 mg/ml of the wild-type (). In , a direct comparison between wild-type IL-1Ra and clone 2.20 in each dose is shown. A statistically significant difference between the wild-type and mutant can only be observed at the 5 mg/ml dose.
FIG. 3 Therapeutic effect of wild-type and mutant IL-1Ra in collagen-induced arthritis. CIA was induced in mice (n = 8) as described in Methods. Treatment with citrate buffer (control), Anakinra (20 mg/ml, wild-type IL-1Ra with 6xhis tag (20, 5 or 1 mg/ml) or the IL-1Ra mutant clone 2.20 with 6x his tag (20, 5 or 1 mg/ml) using osmotic mini-pumps was initiated once an arthritic score of 2 was reached. Arthritic score was determined on days 0, 1, 4, 6 and 7. Results are shown as mean ± SEM. Statistical analysis determining statistically significant differences between wild-type IL-1Ra and clone 2.20 in each dose was performed as described in Methods. * p < 0.05.
Structural Analysis and Modeling of the IL-1Ra-IL-1RI Complex for Explanation of Functional Effects and Suggestion of Alternative Beneficial Mutations
To provide a structural explanation of the functional effects observed in the selected IL-1Ra mutants, selected mutations were analyzed using the crystal structure (PDB code: 1IRA) of the complex between IL-1Ra and IL-1RI (Schreuder et al., Citation1997). In the complex (shown in ), the antagonist makes contacts with all three domains of the IL-1 receptor. The region consisting of residues L25, R26, N27, Q29, L42 and P130 together with the region consisting of residues Y34, L35, Q36, G37, P38, N39 both interact with domain 1.
FIG. 4 Molecular model of the complex between a mutated IL-1Ra variant (green) and IL-1RI (grey) based on the IL-1Ra – IL-1RI crystal structure (PDB code: 1IRA) (Schreuder et al., Citation1997). Two sides of the molecule are viewed rotated 180° from each other. The seven selected mutations found in top clones (magenta) and additional positions 42 and 109 (cyan) are indicated. The beneficial effects of the indicated mutations could be explained by structure-based analysis of the model enabling suggestions of new alternative amino acids in those positions.
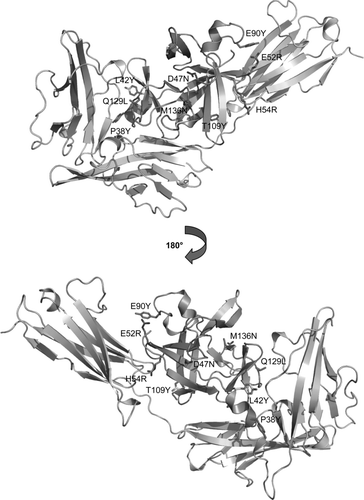
Residues R14, W16, V18, N19, Q20, A127, D128, Y147, Q149 interact with domain 2 and residues S8, K9, P50, I51, P53, H54, L56, E150, D151 interact with domain 3. Thus, of the beneficial substitutions, only position P38 and positions in the I51-A55 loop involve regions interacting with IL-1RI. Interestingly, the J51-A55 loop makes large conformational changes when interacting with IL-RI (Schreuder et al., Citation1997). The P38L substitution probably enhances the interaction towards IL-1RI by making favorable contacts with a hydrophobic pocket in the junction between domain 1 and 2.
In the complex, H54 moves towards the third IL-1RI domain while E52 moves away compared to un-complexed IL-1Ra (Schreuder et al., Citation1997). This raises the hypothesis that the H54R substitution finds favorable interactions with the receptor. The substitution E52K, which was present in almost all clones, change the electrostatic properties of the surface of IL-1Ra. E52 is located close to E90 on a neighboring loop and the charge reversal by the E52K substitution may increase stability. D47 is semi-buried and its side chain makes a hydrogen bond to G62. This structure is maintained in the D47N substitution and apart from that Asn is less polar than Asp little has changed. Therefore, the presence of this mutation in clones with enhanced antagonistic function cannot be explained from a structural point of view.
The substitutions E75K and K93E/Q occurring in several clones with enhanced antagonistic activity are located on exposed loops in IL-1Ra. The presence of these mutations could not be explained by the structure model. Furthermore, the Q129L mutation is located next to A127 and D128, which interacts with domain 2 of IL-1RI. Q129 makes a side chain—main chain hydrogen bond with A127. Removal of this hydrogen bond by mutation of Q129 might allow the loop to move and find a more favorable conformation for interaction with IL-1RI.
The final rounds from the FIND® recombination were analyzed and for some of the substituted positions alternative mutations were suggested based on molecular modeling. A larger Tyr residue was suggested at position 38. The long hydrophobic side chain might make more favorable contacts with the hydrophobic cleft on IL-1RI. In addition, the side-chain hydroxyl group could make enhanced interactions with the side-chains of K92 and Q105 on IL-1RI. Similarly, if L42 is mutated to a bulky hydrophobic residue (for example, Tyr) favorable interactions with K9 and I10on IL-1RI could be gained. The combined substitution of E52R and E90Y was predicted to increase stability of the two loops by stabilization of their conformation.
The mutation to Arg rather than Lys at position E52 will facilitate favorable interactions with the aromatic moiety of Tyr at position E90 and perhaps also with K295 and Y239 on IL-1RI. Thus, the clones must contain both mutations in order to increase activity. In order to stabilize the S105-T108 loop T109 could be substituted to a residue with a bulky side-chain. This effect was achieved with the T109Y variant. M136 is located on an exposed loop, which does not interact with IL-1RI. The initial selected Lys residue was suggested to be substituted to an Asn residue to better fit the local environment.
Generation of Several IL-1Ra Mutants with up to 10 Times Enhanced Antagonistic Function in vitro
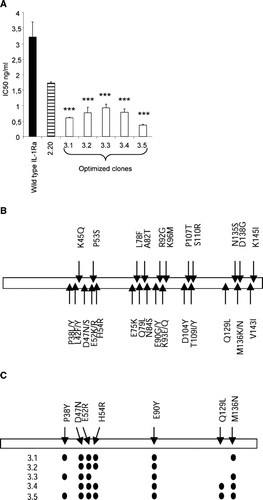
A final attempt to generate IL-1Ra mutants with further antagonistic function was done by combining the sequence information received from the clones identified in the 2nd FIND® round () with that obtained from structure-based analysis and modeling of the IL-1Ra-IL-1RI complex (). Thus, FIND® recombination of clones containing the mutations shown in as well as those suggested by molecular modeling was performed and the resulting library was screened in the reporter cell assay. As shown in , five clones with 4–9-times improved antagonistic activity compared to wild-type IL-1Ra were identified. The IC50 value of the top clone, 3.5, was 0.78 ± 0.050 vs 6.8 ± 1.1 ng/ml for wild-type (mean ± SEM). Sequence analysis of the five clones revealed that out of the 34 mutations that were included in the library, seven were selected ( and ). In addition, it was shown that three mutations were present in all clones, namely D47N, E52R and E90Y. The most potent clone contained all seven amino acid substitutions.
FIG. 5 IC50 values and mutations in selected mutants. IC50 values were determined in the reporter assay. Results are shown as mean ± SEM of data collected from three individual experiments. Clone 2.20 was included as a reference and statistical analysis determining statistically significant differences between 2.20 and the clones 3.1–3.5 was performed as described in Methods. *** p < 0.001 (A). 34 mutations in 26 positions were included in the final FIND® recombination (B). Of these, seven mutations were selected and combined in the top clones as shown (C).
DISCUSSION AND CONCLUSIONS
In this study, we have shown that directed evolution of IL-1Ra can generate mutant clones with up to nine times enhanced antagonistic activity. Evolution occurred in a stepwise manner, with the initial step being introduction of diversity into the gene sequence by screening of a randomly mutated IL-1Ra library in a functional reporter gene assay and selection of clones with increased antagonistic activity. This resulted in the identification of clones with only modestly enhanced functional activity compared to wild-type IL-1Ra. This was expected since most mutations have no effect or a negative effect on protein performance. In addition, introduction of single or few amino acid substitutions is often insufficient to generate major alterations in protein function. However, this approach did provide us with information on 18 mutations of potential importance for the antagonistic function of the protein.
Therefore, to generate IL-1Ra mutant clones with improved antagonistic activity, these mutations were recombined using the FIND® technology. In FIND® recombination, the mutations included in the recombination process are combined in all possible ways, thereby generating a genetic library of immense diversity and allowing the most optimal combination of mutations to be created. Depending on the size of the library, i.e., the number of possible combinations of mutations, all clones may not be possible to screen in a timely manner. However, beneficial mutations will be enriched, thereby providing information about which mutations are of greatest importance and should be recombined for generation of the optimal clone in a following evolution round.
Following genetic recombination of the 18 mutations and screening of the resulting library, only three clones with significantly enhanced antagonistic activity, containing five mutations, were selected. Thus, the process of genetic recombination had resulted in clones with improved function by generating beneficial combinations of mutations and at the same time omitting mutations not contributing to protein function. Therefore, unnecessary mutations were lost, whereas mutations critical for improved protein function were retained. However, although mutants with clearly enhanced antagonistic function had been generated, the diversity was limited and there was a chance that additional, yet unidentified, mutations could potentially work in an additive or synergistic manner with those already identified.
As such, a further round of modified FIND® mutagenesis was performed and resulting libraries were screened in the reporter assay. This resulted in the identification of several clones with further enhanced antagonistic activity compared to the clones identified in the previous evolution round, as well as a number of novel mutations potentially capable of enhancing IL-1Ra function even further. Since we reasoned that the low diversity in the previous library had limited the performance of the resulting clones, we wanted to maximize the diversity in the final evolution round. Therefore, all mutations present in clones with enhanced antagonistic function compared to wild-type IL-1Ra were included. In addition, the potential effect on molecular function of the mutations identified was analyzed using molecular models of IL-1Ra variants interacting with IL-1RI. Based upon this analysis, alternative amino acids in some of the identified positions were suggested, and were included in the final library. Indeed, this approach turned out to be effective, since clones with up to nine times enhanced antagonistic activity compared to wild-type IL-1Ra were identified.
The seven positions finally selected as contributing to improved antagonistic function were identified from the first randomized library followed by the first FIND® round (P38, D47, E52) and from the second FIND® round (H54, E90, Q129, M136). The actual amino acid present in these positions remained the same as initially generated by random mutagenesis for D47N, E52R (although this was a mutation of E52K), H54R and Q129L. For the positions P38, E90 and M136, the substitution initially generated by random mutagenesis was not retained in the final clones.
In contrast, the alternative amino acids P38Y, E90Y and M136N suggested by the structure-based analysis were shown to be superior to P38L, E90G and M136K initially selected. Thus, although modeling of the IL-1Ra-IL-1RI interaction could not initially be used to suggest mutations to be introduced into the sequence for enhancing antagonistic function, once beneficial mutations had been identified, the model could be used to suggest alternative amino acids in the selected positions. Therefore, combining genetic recombination using the FIND® technology with random mutagenesis and protein modeling efficiently generated IL-1Ra mutants with enhanced functional activity.
In vivo testing of one IL-1Ra mutant (clone 2.20 according to designation in ) demonstrated that the improved antagonistic function seen in vitro correlated with an improved therapeutic effect in vivo. Then, 1 mg/ml of the mutant had a similar effect on disease score as 5 mg/ml of wild-type IL-1Ra, whereas 5 mg/ml mutant was comparable to 20 mg/ml wild-type. The interpretation of that is that a similar, or even slightly increased, inhibitory effect compared to wild-type IL-1Ra was seen in vivo compared to in vitro. A statistically significant difference between wild-type and mutant at each dose could be demonstrated at Days 4, 6 and 7 for the 5 mg/ml dose. The lack of significant differences at 20 mg/ml could be explained by the potent inhibition by the wild-type as well as by the mutant at this high dose. For the dosage 1 mg/ml, there was a tendency of a greater effect of the mutant compared to the wild-type; however, this did not reach statistical significance. Based upon the fact that the improved antagonistic effect of clone 2.20 compared to wild-type IL-1Ra was similar in vitro and in vivo, it could be expected that the clone with the greatest antagonistic activity in vitro, clone 3.5 (according to designation in ) exhibiting a 9-fold enhanced function, would have a similarly enhanced therapeutic activity in vivo, i.e., 9-fold enhanced effect compared to wild-type IL-1Ra.
In conclusion, we have demonstrated that directed evolution of IL-1Ra using FIND® recombination efficiently generates IL-1Ra mutants with up to 9 times increased antagonistic activity, and that the effect seen in in vitro systems correlates with therapeutic efficacy in CIA in vivo.
Current address of Eva Bäckman: Dynamic code AB, Westmansgatan 47, 58216 Linköping, Sweden.
REFERENCES
- Arend W. P., Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Res. 2000; 2: 245–248
- Short Protocols in Molecular Biology, F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, K. Struhl. Wiley, Hoboken, NJ 2002
- Bodar E. J., van der Hilst J. C., Drenth J. P., van Der Meer J. W., Simon A. Effect of etanercept and anakinra on inflammatory attacks in the hyper-IgD syndrome: Introducing a vaccination provocation model. Neth. J. Med. 2005; 63: 260–264
- Bowen S., Tare N., Inoue T., Yamasaki M., Okabe M., Horii I., Eliason J. F. Relationship between molecular mass and duration of activity of polyethylene glycol conjugated granulocyte colony-stimulating factor mutein. Exp. Hematol. 1999; 27: 425–432
- Bresnihan B., Alvaro-Gracia J. M., Cobby M., Doherty M., Domljan Z., Emery P., Nuki G., Pavelka K., Rau R., Rozman B., Watt I., Williams B., Aitchison R., McCabe D., Musikic P. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998; 41: 2196–2204
- Burger D., Dayer J. M., Palmer G., Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis?. Best. Pract. Res. Clin. Rheumatol. 2006; 20: 879–896
- Chae J. J., Wood G., Masters S. L., Richard K., Park G., Smith B. J., Kastner D. L. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc. Natl. Acad. Sci. USA 2006; 103: 9982–9987
- Cohen S., Hurd E., Cush J., Schiff M., Weinblatt M. E., Moreland L. W., Kremer J., Bear M. B., Rich W. J., McCabe D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002; 46: 614–624
- Cohen S. B., Moreland L. W., Cush J. J., Greenwald M. W., Block S., Shergy W. J., Hanrahan P. S., Kraishi M. M., Patel A., Sun G., Bear M. B. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin-1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann. Rheum. Dis. 2004; 63: 1062–1068
- Delano. The PyMol Molecular Graphics System. Delano Scientific, San Carlos 2002
- Dierselhuis M. P., Frenkel J., Wulffraat N. M., Boelens J. J. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology 2005; 44: 406–408
- Dinarello C. A. Biologic basis for interleukin-1 in disease. Blood 1996; 87: 2095–2147
- Dinarello C. A. Pro-inflammatory cytokines. Chest 2000; 118: 503–508
- Dinarello C. A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002; 20: S1–13
- Fitzgerald A. A., Leclercq S. A., Yan A., Homik J. E., Dinarello C. A. Rapid responses to anakinra in patients with refractory adult-onset Still's disease. Arthritis Rheum. 2005; 52: 1794–1803
- Goldbach-Mansky R., Dailey N. J., Canna S. W., Gelabert A., Jones J., Rubin B. I., Kim H. J., Brewer C., Zalewski C., Wiggs E., Hill S., Turner M. L., Karp B. I., Aksentijevich I., Pucino F., Penzak S. R., Haverkamp M. H., Stein L., Adams B. S., Moore T. L., Fuhlbrigge R. C., Shaham B., Jarvis J. N., O'Neil K., Vehe R. K., Beitz L. O., Gardner G., Hannan W. P., Warren R. W., Horn W., Cole J. L., Paul S. M., Hawkins P. N., Pham T. H., Snyder C., Wesley R. A., Hoffmann S. C., Holland S. M., Butman J. A., Kastner D. L. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. New Engl. J. Med. 2006; 355: 581–592
- Hawkins P. N., Bybee A., Aganna E., McDermott M. F. Response to anakinra in a de novo case of neonatal-onset multisystem inflammatory disease. Arthritis Rheum 2004a; 50: 2708–2709
- Hawkins P. N., Lachmann H. J., Aganna E., McDermott M. F. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004b; 50: 607–612
- Hawkins P. N., Lachmann H. J., McDermott M. F. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. New Engl. J. Med. 2003; 348: 2583–2584
- Heguy A., Baldari C. T., Macchia G., Telford J. L., Melli M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila toll protein are essential for IL-1R signal transduction. J. Biol. Chem. 1992; 267: 2605–2609
- Hoffman H. M., Firestein G. S. Anakinra for the treatment of neonatal-onset multisystem inflammatory disease. Nat. Clin. Pract. Rheumatol. 2006; 2: 646–647
- Hoffman H. M., Rosengren S., Boyle D. L., Cho J. Y., Nayar J., Mueller J. L., Anderson J. P., Wanderer A. A., Firestein G. S. Prevention of cold-associated acute inflammation in familial cold auto-inflammatory syndrome by interleukin-1 receptor antagonist. Lancet 2004; 364: 1779–1785
- Joosten L. A., Helsen M. M., Saxne T., van De Loo F. A., Heinegard D., van den Berg W. B. IL-1αβ blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNFα blockade only ameliorates joint inflammation. J. Immunol. 1999; 163: 5049–5055
- Larsen C. M., Faulenbach M., Vaag A., Volund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., Donath M. Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New Engl. J. Med. 2007; 356: 1517–1526
- Nixon R., Bansback N., Brennan A. The efficacy of inhibiting tumour necrosis factor-α and interleukin 1 in patients with rheumatoid arthritis: A meta-analysis and adjusted indirect comparisons. Rheumatology 2007; 46: 1140–1147
- Nuki G., Bresnihan B., Bear M. B., McCabe D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: Extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002; 46: 2838–2846
- Paget S. A. Efficacy of anakinra in bone: Comparison to other biologics. Adv. Ther. 2002; 19: 27–39
- Pascual V., Allantaz F., Arce E., Punaro M., Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005; 201: 1479–1486
- Patten P. A., Howard R. J., Stemmer W. P. Applications of DNA shuffling to pharmaceuticals and vaccines. Curr. Opin. Biotechnol. 1997; 8: 724–733
- Radons J., Gabler S., Wesche H., Korherr C., Hofmeister R., Falk W. Identification of essential regions in the cytoplasmic tail of interleukin-1 receptor accessory protein critical for interleukin-1 signaling. J. Biol. Chem. 2002; 277: 16456–16463
- Rudinskaya A., Trock D. H. Successful treatment of a patient with refractory adult-onset still disease with anakinra. J. Clin. Rheumatol. 2003; 9: 330–332
- Ruiz P. J., Masliah E., Doherty T. A., Quach A., Firestein G. S. Cardiac death in a patient with adult-onset Still's disease treated with the interleukin 1 receptor inhibitor anakinra. Ann. Rheum. Dis. 2007; 66: 422–423
- Schreuder H., Tardif C., Trump-Kallmeyer S., Soffientini A., Sarubbi E., Akeson A., Bowlin T., Yanofsky S., Barrett R. W. A new cytokine-receptor binding mode revealed by the crystal structure of the IL-1 receptor with an antagonist. Nature 1997; 386: 194–200
- Stylianou E., O'Neill L. A., Rawlinson L., Edbrooke M. R., Woo P., Saklatvala J. Interleukin-1 induces NF-κ B through its type I but not its type II receptor in lymphocytes. J Biol Chem 1992; 267: 15836–15841
- Vasques Godinho F. M., Parreira Santos M. J., Canas da Silva J. Refractory adult onset Still's disease successfully treated with anakinra. Ann. Rheum. Dis. 2005; 64: 647–648
- Yuan L., Kurek I., English J., Keenan R. Laboratory-directed protein evolution. Microbiol. Mol. Biol. Rev. 2005; 69: 373–392