Abstract
We previously reported that a combination of β-glucan and indomethacin (IND), a non-steroidal anti-inflammatory drug, was lethal to mice. This lethality was strongly related to translocation of enterobacterial flora to various organs and the development of a systemic inflammation. In this study, we examined expression of microsomal cytochrome P450 (CYP), a drug-metabolizing enzyme mostly found in the liver. Normal ICR mice and endotoxin-low responder C3H/HeJ mice were employed to assess effects of endotoxin on impairment of CYP. In the ICR mice, CYP3A11 expression was decreased by β-glucan or IND. In the early stage of β-glucan + IND-treatment, 3A11 expression decreased more significantly; when shock was induced, CYP was dramatically decreased. 3A11 expression was also decreased in C3H/HeJ mice, but the effect was milder. In contrast, in both strains, CYP2E1 expression did not vary due to β-glucan or IND, but decreased during sepsis. To clarify the molecular mechanisms of induced sepsis in C3H/HeJ mice, the reactivity of other pathogen-associated molecular patterns (PAMPs) was assessed. Those studies showed cooperative effects between Pam3CSK4 (Pam3) and CpG ODN (CpG-oligodeoxynucleotide) on the induction of IL-6 synthesis by C3H/HeJ spleen cells. The findings here suggest that the β-glucan + IND combination influenced hepatic cytochrome P450 expression, particularly in the late stage of sepsis. The results also indicate that this change may be associated with not only endotoxin but other PAMPs as well, and could be affected by the integrity of a host’s drug metabolism function.
| Abbreviations: | ||
| CLP, | = | cecal ligation and puncture, |
| CpG, CpG-ODN, | = | oligodeoxynucleotides (ODNs) with CpG motifs; |
| GOT, | = | glutamate oxaloacetate transaminase; |
| GPT, | = | glutamate pyruvate transaminase; |
| IL-6, | = | interleukin-6; |
| IND, | = | indomethacin; |
| LPS, | = | lipopolysaccharide; |
| MODS, | = | multiple-organ dysfunction syndrome; |
| NSAID, | = | non-steroidal anti-inflammatory drug; |
| Pam3, | = | synthetic bacterial lipopeptide (Pam3CSK4); |
| PAMPs, | = | pathogen-associated molecular patterns; |
| PEC, | = | peritoneal exudates cells; |
| SPG, | = | Sonifilan; |
| TLR, | = | Toll-like receptor; |
| TNF-α, | = | tumor necrosis factor-α |
Introduction
Sepsis can be caused by infection with Gram-negative bacteria, Gram-positive bacteria, fungi, or viruses. In addition to the development of sepsis, septic shock and the ensuring multiple organ failure are the most common causes of morbidity and mortality in intensive care units (Baue et al., Citation1998). It is well known that the outcome of sepsis correlates with the number of dysfunctional organ systems, and hepatic failure in particular is an important indicator of a poor outcome (Tu et al., Citation2003).
Cytochrome P-450 (CYP) enzymes are a superfamily of heme-proteins responsible for the metabolic activation of most clinically used drugs and many toxins. CYP isoforms can be found in most tissues, but the largest concentrations of CYP are found in the liver. The expression of CYP enzymes is regulated by a variety of factors, such as genetic polymorphism, drugs, hormones, and diet (Halpert et al., Citation1994; Morgan, Citation2001). The liver plays an important role in the occurrence of the multiple organ dysfunction syndrome (MODS) under septic conditions. Hepatic metabolism is depressed with endotoxemia or sepsis (Kraemer et al., Citation1982).
We have previously shown that repeated administration of indomethacin (IND) to mice treated with the β-glucan, Sonifilan (SPG) induced severe lethality (Yoshioka et al., Citation1998; Takahashi et al., Citation2001; Moriya et al., 2002a and b; Nameda et al., Citation2005). This model used two drugs, not lipopolysaccharide (LPS) directly, to induce bacterial translocation. That means that lethality is related to endogenous toxin which is contained in enteric bacteria. LPS is thought to be associated with lethality in sepsis. It is well known that LPS causes fever or lethal toxicity with the production of inflammatory cytokines by macrophages, multiple organ failure, and death; however, we have noted that lethality was enhanced by treatment with SPG and IND in C3H/HeJ mice, an LPS-low responder strain. This suggested that not only LPS but other pathogens (and their products) significantly contributed to septic shock.
In this study, we examined the liver function (i.e., GOT and GPT levels), expression of an inflammatory cytokine (i.e., interleukin (IL)-6), and the time-associated expression of CYP in SPG- ± IND-treated mice. To investigate the effect of LPS, we used C3H/HeJ mice with a mutation in TLR4, a receptor for LPS.
Materials and methods
Animals
Male ICR and C3H/HeJ mice (5–8-wk-of-age) were purchased from Japan SLC, Inc. (Shizuoka, Japan) and maintained under specific pathogen-free conditions. The breeding and handling of all animals in this experiment were approved by the Committee on Animal Experiments of the School of Pharmacy, Tokyo University of Pharmacy and Life Sciences.
Reagents
Sonifilan (SPG) was purchased from Kaken Pharmaceutical Co. (Tokyo, Japan) and dissolved in physiologic saline. Indomethacin (IND) was obtained from Wako Pure Chemical Co. (Tokyo) and suspended with 2% polyoxyethylene (20) sorbitan monooleate (Tween 80) in 0.5% sodium carboxymetylcellulose (CMC).
Administration of SPG/Indomethacin
The mice were administered SPG (100 μg/mouse) intraperitoneally once every other day (i.e., Day -5, -3, and -1) and IND (5 mg/kg) per os daily (from Day 0 to 14). Mortality, body weight, and rectal temperature were monitored.
Measurement of GOT and GPT
Sera were assayed for GOT and GPT levels using a Transaminase C II-test kit (Wako) according to the manufacturer’s directions.
Cell cultures
Spleen cells were collected from naive C3H/HeJ mice and peritoneal exudates cells (PEC) were collected from C3H/HeJ mice who had been administered 4.05% thioglycolate more than 3 d prior. Cells were maintained in RPMI1640 medium (supplemented with gentamycin sulfate (50 μg/ml) and 10% heat-inactivated fetal bovine serum) and incubated with Pam3CSK4 (Pam3) and/or CpG ODN (CpG) for 24 hr at a density of 5×106 cells/ml in a 5% CO2 incubator at 37°C. After the incubation, culture supernatants were collected and frozen at −80°C until analyzed.
Hepatic microsome preparation
The liver was obtained on the indicated days, and homogenized at 4°C using a tightly sealed homogenizer (25 strokes) using 1.15% KCl, 1 mM ethylendiamine tetraacetic acid (EDTA), and 0.25 mM phenylmethylsulfonylfluoride. The homogenate was then centrifuged at 1,700 rpm for 10 min at 4°C. The resulting supernatant was isolated and further centrifuged at 85,000 rpm for 10 min at 4°C; this supernatant was then centrifuged at 32,000 rpm for 1 hr to obtain the microsomal fraction. The obtained pellet was suspended in 0.1 M phosphate buffer containing 1 mM EDTA and 30% (v/v) glycerol. The protein concentration of the microsomal fraction was measured using a BCA-200 protein assay kit (Pierce, Rockford, IL). The fraction was kept at −80°C until analysis.
Western blot analysis for microsomal CYP3A11 and CYP2E1
Protein (0.5 mg/ml) was separated by electrophoresis over 10% polyacrylamide gels containing 0.1% sodium dodecyl sulfate (SDS) and then transferred to a nitrocellulose membrane (Pall Corporation, Tokyo). The membrane was subsequently blocked with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (i.e., PBS-T) and 1% bovine serum albumin (Sigma, St. Louis, MO), and detected by rabbit polyclonal antibody to rat CYP3A2 (Daiichi Pure Chemicals, Tokyo) that can recognize CYP3A11 in the mouse liver microsome, and goat polyclonal antibody to rat CYP2E1 (Daiichi). After several washes to remove all unbound primary antibody from the membranes, immune complexes were visualized using horseradish peroxidase-labeled secondary antibodies anti-rabbit IgG (Sigma) and anti-goat IgG (Cell Signaling Technology, Danvers, MA), and ECL+ Western blot detection reagent (Amersham, Piscataway, NJ).
Statistics
Statistical significance was analyzed using the Student’s t-test.
Results
SPG/IND administration modulated IL-6 production by spleen and peritoneal exudates cells and caused damage to the liver
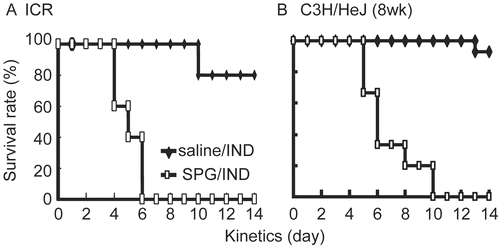
We have previously reported SPG/IND administration induced lethal toxicity through sepsis in mice. First we examined whether LPS-hyporesponsive C3H/HeJ mice evidenced a similar physiological change after SPG/IND-administration. Briefly, ICR mice, (normal LPS-sensitive strain) and C3H/HeJ mice were administered SPG (100 μg/mouse) or saline intraperitoneally on Day -5, -3, and -1, and IND (5 mg/kg) per os daily from Day 0 to 14. Both ICR and C3H/HeJ SPG/IND-administered mice displayed 100% mortalities, although there was a delay in reaching the level of total mortality (Day 10 vs. Day 7) by C3H/HeJ mice compared to ICR mice (). These results strongly suggested that LPS sensitivity was not the sole molecular mechanism for septic death in this model.
Figure 1. Survival of ICR and C3H/HeJ mice administered SPG/IND. (A) Eight-week-old ICR mice (saline/IND; n = 6, SPG/IND; n = 5) were administered SPG (100 mg/mouse) intraperitoneally (IP) on Days -5, -3, and -1, and indomethacin (IND; 5 mg/kg) per os from days 0 to 14. Mortality was monitored. (B) Eight-week-old C3H/HeJ mice (saline/IND; n = 14, SPG/IND; n = 15) were administered SPG (100 mg/mouse) or saline IP on Days -5, -3, and -1, and indomethacin (IND; 5 mg/kg) per os from Days 0 to 14. Mortality was monitored.
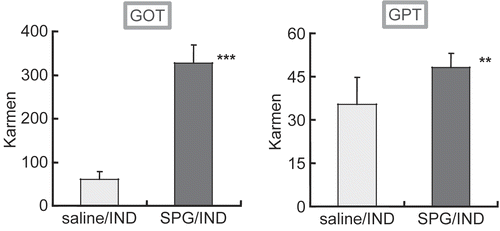
In the previous study, liver dysfunction was shown in ICR mice administered SPG/IND; thus, to examine hepatic damage in C3H/HeJ mice, GOT and GPT levels in sera were assessed. On Day 5 or 7, groups of mice were euthanized and serum samples were collected. As shown in , the release of GOT and GPT increased in the mice treated with SPG/IND.
Figure 2. GOT and GPT levels in sera from SPG/IND-treated C3H/HeJ mice. Eight-week-old C3H/HeJ mice (saline/IND, n = 4; SPG/IND, n = 3) were administered SPG (100 μg/mouse) or saline IP on Day -5, -3, and -1, and indomethacin (IND, 5 mg/kg) per os from Day 0 to 7. Sera were prepared on Day 5 and 7. GOT and GPT levels were measured with kits using sera obtained on Day 7 (serum of one mouse in saline/IND group could not be obtained, thus used serum on Day 5). Results shown are the mean (± S.D) Karmen activity levels; a karmen unit is a formerly-used expression of aminotransferase activity (based on a 0.001 change in absorbance of NADH/min). Significance (**p < 0.05, ***p < 0.01), was evaluated using a student’s t-test.
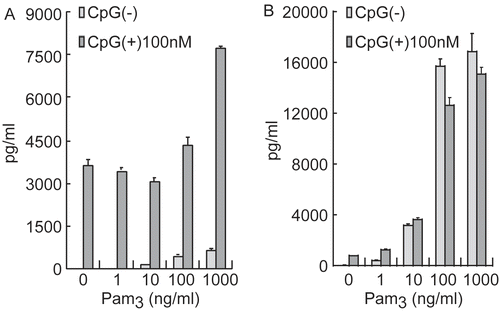
To better understand any underlying mechanisms for these outcomes, effects of PAMPs Pam3 or CpG on interleukin (IL)-6 production by spleen cells and PEC were investigated. As shown in , IL-6 production was dose-relatedly enhanced by stimulation of either cell type with Pam3 or CpG. As expected, LPS stimulation did not enhance production of this cytokine in this strain (data not shown). To examine the cooperative effect of PAMPs, the mixed administration of Pam3 and CpG on IL-6 production by spleen cells and PEC was assessed. As shown in , IL-6 production was enhanced greatly in spleen cells but not in the PEC. These results indicated that bacterial components could work synergistically and, at least in part, amplify inflammatory cytokine production. These results also suggested that bacterial PAMPs other than LPS could act to synergistically affect the host immune system and result in death among the SPG/IND-administered C3H/HeJ mice.
Figure 3. Effect of Pam3 or CpG on IL-6 production of splenocytes and peritoneal exudate cells. (A, B) Spleen cells were collected from naive C3H/HeJ mice, and (C, D) peritoneal exudates cells (PEC) were collected from thioglycolate-broth administered C3H/HeJ mice. Cells were incubated with Pam3 or CpG for 24 hr at 37°C in 5% CO2 (a, c; Pam3, b, d; CpG). IL-6 presence in the supernatant was measured by ELISA. Results are shown as the mean ± SD.
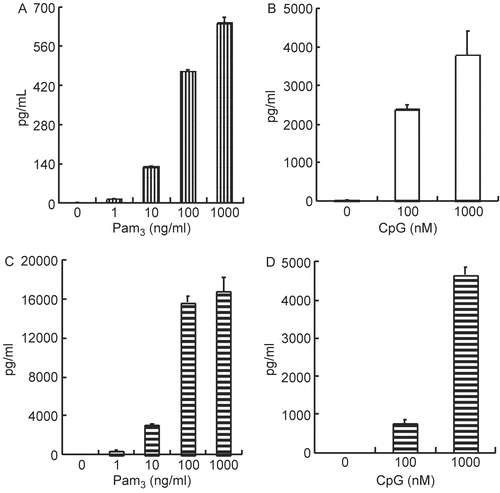
Figure 4. Synergistic effect of Pam3/CpG on IL-6 production of splenocytes and peritoneal exudates cells. (A) Spleen cells were collected from naive C3H/HeJ mice, and (B) peritoneal exudates cells (PEC) were collected from thioglycolate-broth administered C3H/HeJ mice. Cells were incubated with Pam3 in the presence or absence of CpG for 24 hr at 37°C in 5% CO2. IL-6 presence in the supernatant was measured by ELISA. Results are shown as the mean ± SD.
Effect of SPG administration on CYP3A11 expression
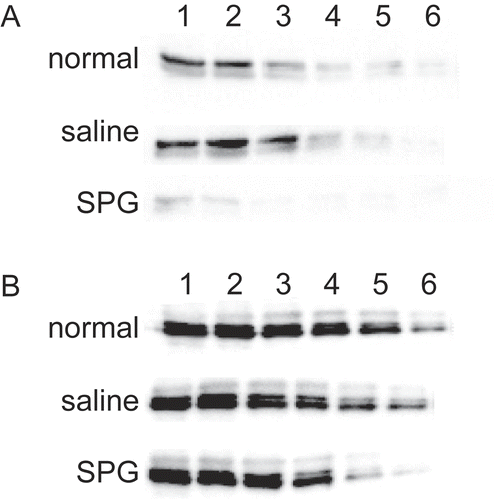
From the data that GOT (as well as GPT) in sera increased after SPG/IND administration in the C3H/HeJ mice (see ), the livers of these mice might also display an induced dysfunction. Therefore, the effect of SPG or IND on CYP expression (at protein level) was next examined. First, whether SPG changed CYP3A11 in mouse liver microsomes was investigated. ICR mice were administered SPG three times (Day -5, -3, and -1) and CYP protein levels were then measured on Day 0. In ICR mice, a significant decrease in CYP3A11 protein level due to SPG treatment was detected compared with that seen in control mice. The rate of decrease was calculated as 1/16, as non-administered and saline-administered mice expressed protein at a 16-fold dilution (lane 5) but SPG-administered mice expressed protein only with the undiluted solution (Lane 1; ). Conversely, C3H/HeJ mice responded very weakly and showed about a 1/2 decrease in CYP3A11 after the SPG administration ().
Figure 5. CYP3A11 protein expression in liver microsomes from SPG-administered mice. Five-week-old ICR mice (A) and eight-week-old C3H/HeJ mice (B) were administered SPG (100 μg/mouse) or saline IP on Days -5, -3, and -1. On Day 0, liver microsomes were obtained and CYP3A11 protein expression was measured by Western blotting. Lane 1, 500 μg/ml; Lane 2, 250 μg/ml; Lane 3, 125 μg/ml; Lane 4, 62.5 μg/ml; Lane 5, 31.25 μg/ml; Lane 6, 15.625 μg/ml.
Effect of IND administration and SPG/IND administration on CYP3A11 protein expression
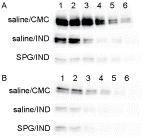
Next experiments, protein expression in SPG/IND-treated mice were tested on Day 2 (). In ICR mice, CYP3A11 expression fell with IND administration and fell further with SPG/IND treatment. The rate of decrease was calculated as 1/2 when comparing saline/IND vs. saline/CMC, and 1/32 when comparing SPG/IND with saline/CMC. Protein was expressed at a 32-fold dilution of saline/CMC (Lane 6), with a 16-fold dilution of saline/IND (Lane 5), and with the undiluted solution of SPG/IND (Lane 1). In C3H/HeJ mice, protein expression also fell with IND and SPG/IND administration, but it did not fall more with SPG/IND administration. The rate of decrease was calculated as 1/4 when comparing saline/IND vs. saline/CMC administration, and comparing SPG/IND vs. saline/CMC administration. Mice that received saline/CMC expressed protein at a 16-fold dilution (Lane 5), while saline/IND- and SPG/IND- treated mice expressed protein with a 4-fold dilution (Lane 3).
Figure 6. CYP3A11 protein expression in liver microsomes from SPG/IND-administered mice. Five-week-old ICR mice (A) and eight-week-old C3H/HeJ mice (B) were administered SPG (100 μg/mouse) or saline IP on Days -5, -3, and -1, and IND (5 mg/kg) per os from Day 0 to 2. On Day 2, liver microsomes were obtained and CYP3A11 protein expression was measured by Western blotting. Lane 1, 500 μg/ml; Lane 2, 250 μg/ml; Lane 3, 125 μg/ml; Lane 4, 62.5 μg/ml; Lane 5, 31.25 μg/ml; Lane 6, 15.625 μg/ml.
Effect of SPG/IND-induced sepsis on CYP3A11 expression
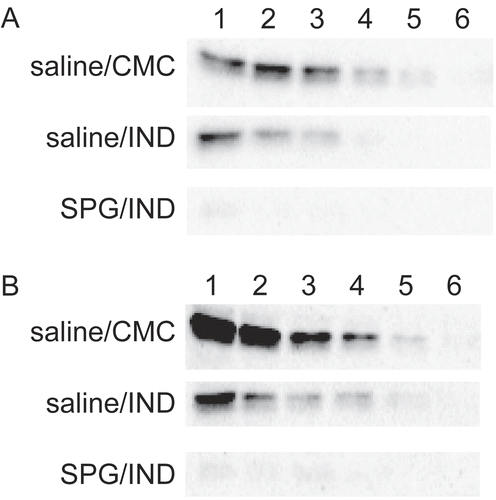
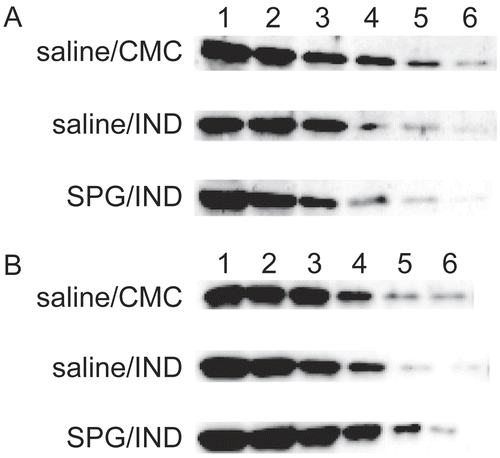
CYP3A11 expression when SPG/IND-administered mice became septic over a longer experimental period (i.e., on Day 3 or 4) was then examined. CYP3A11 protein levels fell more significantly in ICR mice and C3H/HeJ mice affected by sepsis and the rate of reduction was the same. The rate of reduction was calculated as 1/16 when comparing SPG/IND vs. saline/CMC administration. In both ICR mice and C3H/HeJ mice, saline/CMC-administered mice expressed protein at a 16-fold dilution (Lane 5), but SPG/IND-induced septic mice could not express protein even with undiluted solution ().
Figure 7. CYP3A11 protein expression of liver microsomes from SPG/IND-administered mice just before disease. Five-week-old ICR mice (A) and eight-week-old C3H/HeJ mice (B) were administered SPG (100 μg/mouse) or saline IP on Days -5, -3, and -1, and indomethacin (IND, 5 mg/kg) per os from Day 0 to 4. On Day 3 and 4, liver microsomes were obtained and CYP3A11 protein expression was measured by Western blotting. Lane 1, 500 μg/ml; Lane 2, 250 μg/ml; Lane 3, 125 μg/ml; Lane 4, 62.5 μg/ml; Lane 5, 31.25 μg/ml; Lane 6, 15.625 μg/ml.
Effect of SPG/IND administration on CYP2E1 expression
The study here also examined how another CYP species, CYP2E1, might have been affected in response to SPG administration. It was found that SPG alone did not affect CYP2E1 protein expression (). Similarly, IND administration alone and SPG/IND administration also did not effect CYP2E1 protein expression ().
Figure 8. CYP2E1 protein expression in liver microsomes from SPG-administered mice. Five-week-old ICR mice (A) and eight-week-old C3H/HeJ mice (B) were administered SPG (100 μg/mouse) or saline IP on Days -5, -3, and -1. On Day 0, liver microsomes were obtained and CYP2E1 protein expression was measured by Western blotting. Lane 1, 500 μg/ml; Lane 2, 250 μg/ml; Lane 3, 125 μg/ml; Lane 4, 62.5 μg/ml; Lane 5, 31.25 μg/ml; Lane 6, 15.625 μg/ml.
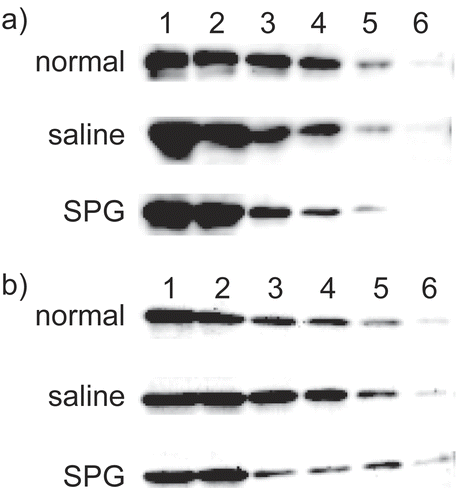
Figure 9. CYP2E1 protein expression in liver microsomes from SPG/IND-administered mice. Five-week-old ICR mice (A) and 8-week-old C3H/HeJ mice (B) were administered SPG (100 μg/mouse) or saline IP on Days -5, -3, and -1, and indomethacin (IND, 5 mg/kg) per os from Day 0 to 2. On Day 2, liver microsomes were obtained and CYP2E1 protein expression was measured by Western blotting. Lane 1, 500 μg/ml; Lane 2, 250 μg/ml; Lane 3, 125 μg/ml; Lane 4, 62.5 μg/ml; Lane 5, 31.25 μg/ml; Lane 6, 15.625 μg/ml.
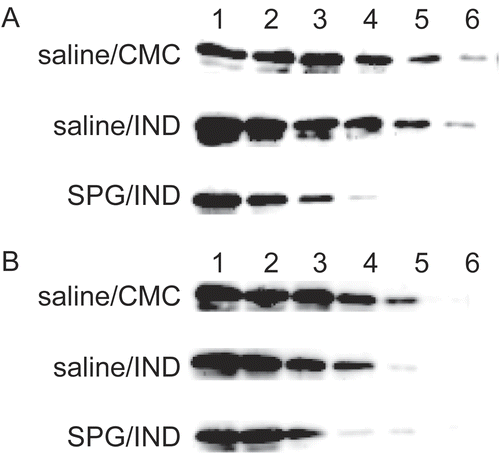
Finally, CYP2E1 protein expression in SPG/IND-induced sepsis was examined in ICR and C3H/HeJ mice. CYP2E1 protein expression slightly decreased during sepsis. The rate of decrease was calculated as 1/4 when comparing SPG/IND vs. saline/IND administration. In ICR mice, saline/CMC-treated mice expressed the protein at a 32-fold dilution (Lane 6), SPG/IND-induced septic mice expressed protein at an 8-fold dilution (Lane 4), C3H/HeJ mice and saline/CMC-treated mice expressed protein (Lane 5) at a 16-fold dilution, and SPG/IND-induced septic mice expressed protein with a 4-fold dilution (Lane 3; ).
Figure 10. CYP2E1 protein expression in liver microsomes from SPG/IND-administered mice just before disease. Five-week-old ICR mice (A) and 8-week-old C3H/HeJ mice (B) were administered SPG (100 μg/mouse) or saline IP on Days -5, -3, and -1, and indomethacin (IND, 5 mg/kg) per os from Day 0 to 4. On Days 3 and 4, liver microsomes were obtained and CYP2E1 protein expression was measured by Western blotting. Lane 1, 500 μg/ml; Lane 2, 250 μg/ml; Lane 3, 125 μg/ml; Lane 4, 62.5 μg/ml; Lane 5, 31.25 μg/ml; Lane 6, 15.625 μg/ml.
Discussion
The cytochrome P450 (CYP) superfamily is a family of enzymes that catalyze numerous reactions, including fatty acid metabolism, xenobiotic biotransformation, and endogenous substrate synthesis and metabolism (Guengerich, Citation1993; Estabrook, Citation1996). It is known that the mediators involved in inflammation and infection can cause changes in the activities and expression of the CYP enzyme system. A large number of reports have shown that CYP and their activities are mostly suppressed in animal models of endotoxemia (Shedlofsky et al., Citation1994; Monshouwer et al., Citation1996; Iber et al., Citation1999; McKindley et al., Citation2002).
We have previously shown that a combination of β-glucan and an NSAID was lethal to mice. This model induces endogenous septic shock in mice without administering endotoxin directly by the microbial translocation of the intestinal flora. To investigate the relationship between lethality and liver function, expression of CYP was examined in this model. Moreover, to investigate the effect of endotoxin on the liver function in this model, we compared CYP expression using C3H/HeJ mice LPS (low responders) to ICR mice (normal responders).
We have reported that administration of Sonifilan (SPG) enhanced responses of mice to LPS and resulted in production of a large amount of inflammatory cytokines, such as tumor necrosis factor (TNF)-α. Our studies have found that the magnitude of enhanced mortality in LPS-resistant C3H/HeJ mice was similar to that in ICR mice, although the timing was slightly delayed. This fact strongly suggested that LPS—capable of inducing toll-like receptor (TLR)-4-mediated inflammatory signaling—might not be the sole molecule responsible for septic shock in this model. Thus, we tested other PAMPs for the capacity to induce inflammatory cytokine synthesis in vitro and found that either Pam3 or CpG could induce IL-6 in spleen cell and PEC cultures. This indicated that PAMPs could stimulate inflammatory responses through other TLRs on cells. We also tested the cooperative/synergistic effects of these PAMPs, When Pam3 and CpG, were used to treat cells from C3H/HeJ mice, synergistic effects on induction of IL-6 by splenocytes were noted. This suggested that endogenous septic shock induced by the microbial translocation of intestinal flora might also have occurred in the C3H/HeJ mice, with a resulting enhanced response to PAMPs stimulating TLR-based activities that ultimately contributed to host death.
The various CYP isoforms primarily involved in drug metabolism are grouped into CYP1, CYP2, and CYP3 subfamilies (Watkins, Citation1990; Gonzalez and Gelboin, Citation1994). We examined the change in CYP3A11 and CYP2E1 expressions in this model. Both CYP3A11 and CYP2E1 activities decreased by LPS stimulation but they have different kinetics of protein expression by inflammatory cytokine modulation (Warren et al., Citation1999; Siewert et al., Citation2000). In this study, we noted that both CYP3A11 and CYP2E1 were decreased, but the effect was much more severe for CYP3A11. Eum et al. (Citation2006) reported that CYP2E1 was decreased in the CLP model and that this reduction was reversed by administration of a nitric oxide (NO) inhibitor.
We have previously reported that NO production was increased in SPG/IND-treated ICR mice (Nameda et al., Citation2005). As NO plays a role as a protective factor in bacterial translocation, NO production in the mice here would be expected to have induced a reduction in CYP2E1. Last, we noted here that the sensitivities of CYP3A11 and CYP2E1 were seen to differ between the ICR and C3H/HeJ hosts. These differences might be regulated by various genetic background (directly and indirectly, such as polymorphism of promoter and coding region sequences) factors that warrant further investigation.
In this study, different subtypes of CYP, CYP3A11 and CYP2E1, were significantly decreased during the septic shock induced by SPG/IND, and that this reduction was inducible not only by LPS. The changes in host mortality also appeared to be related to differences in inducibility of at least one inflammatory cytokine, IL-6. In this model, we have shown that mortality was strongly related to the concentration of IND, i.e., the higher the IND concentration, the shorter the survival period. We believe that because IND might be, at least in part, metabolized by CYP, failure of CYP (due to decreases in levels or activity) might result in longer periods of high blood levels of IND that, in turn, resulted in enhancement of mortality from sepsis. To clarify if this is a mechanism of toxicity in this model, more precise analyses is needed, i.e., measurements of serum IND levels during the sepsis timeframe.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baue, A.E., Durham, R., and Faist, E. 1998. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): Are we winning the battle? Shock 10:79–89.
- Estabrook, R. 1996. The remarkable P450s: A historical overview of these versatile hemeprotein catalysts. FASEB J. 10:202–204.
- Eum, H.A., Yeom, D.H., and Lee, S. M. 2006. Role of nitric oxide in the inhibition of liver cytochrome P450 during sepsis. Nitric Oxide 15:423–431.
- Gonzalez, F., and Gelboin, H. 1994. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab. Rev. 26:165–183.
- Guengerich, F. 1993. Cytochrome P450 enzymes. Am. Sci. 81:440–447.
- Halpert, J.R., Guengerich, F.P., Bend, J.R., and Correia, M.A. 1994. Contemporary issues in toxicology: Selective inhibitors of cytochromes P450. Toxicol. Appl. Pharmacol. 125:163–175.
- Iber, H., Sewer, M.B., Barclay, T.B., Mitchell, S.R., and Morgan, E.T. 1999. Modulation of drug metabolism in infectious and inflammatory diseases. Drug Metab. Rev. 31:29–41.
- Kraemer, M.J., Furukawa, C.T., Koup, J.R., Shapiro, G.G., Pierson, W.E., and Bierman, C.W. 1982. Altered theophylline clearance during an influenza B outbreak. Pediatrics 69:476–480.
- McKindley, D.S., Boulet, J., Sachdeva, K., Wang, P., and Chichester, C. 2002. Endotoxic shock alters the pharmacokinetics of lidocaine and monoethylglycinexylidide. Shock 17:199–204.
- Monshouwer, M., McLellan, R.A., Delaporte, E., Witkamp, R.F., van Miert, A.S., and Renton, K.W. 1996. Differential effect of pentoxifylline on lipopolysaccharide-induced down-regulation of cytochrome P450. Biochem. Pharmacol. 52:1195–1200.
- Morgan, E.T. 2001. Regulation of cytochrome P450 by inflammatory mediators: Why and how? Drug Metab. Dispos. 29:207–212.
- Moriya, K., Miura, N.N., Adachi, Y., and Ohno, N. 2002. Systemic inflammatory response associated with augmentation and activation of leukocytes in Candida/indomethacin-administered mice. Biol. Pharm. Bull. 25:816–822.
- Moriya, K., Ohno, N., Miura, N.N., Adachi, Y., and Yadomae, T. 2002. Septic shock induced by microbial products and indomethacin. Drug. Dev. Res. 55:139–148.
- Nameda, S., Saito, M., Miura, N.N., Adachi, Y., and Ohno, N. 2005. Effect of nitric oxide on β-glucan/indomethacin-induced septic shock. Biol. Pharm. Bull. 28:1254–1258.
- Shedlofsky, S.I., Israel, B.C., McClain, C.J., Hill, D.B., and Blouin, R.A. 1994. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J. Clin. Invest. 94:2209–2214.
- Siewert, E., Bort, R., Kluge, R., Heinrich, P.C., and Jover, R. 2000. Hepatic cytochrome P450 down-regulation during aseptic inflammation in the mouse is interleukin-6-dependent. Hepatology 32:49–55.
- Takahashi, H., Ohno, N., Adachi, Y., and Yadomae, T. 2001. Association of immunological disorders in lethal side effect of NSAIDs on β-glucan-administered mice. FEMS. Immunol. Med. Microbiol. 31:1–14.
- Tu, W., Satoi, S., Zhang, Z., Kitade, H., Okumura, T., Kwon, A.H., and Kamiyama, Y. 2003. Hepatocellular dysfunction induced by nitric oxide production in hepatocytes isolated from rats with sepsis. Shock 19:373–377.
- Warren, G.W., Poloyac, S.M., Gary, D.S., Mattson, M.P., and Blouin, R.A. 1999. Hepatic cytochrome P450 expression in tumor necrosis factor-α receptor (p55/p75) knockout mice after endotoxin administration. J. Pharmacol. Exp. Ther. 288:945–950.
- Watkins, P. 1990. Role of cytochromes P450 in drug metabolism and hepatotoxicity. Sem. Liver Dis. 10:235–250.
- Yoshioka, S., Ohno, N., Miura, T., Adachi, Y., and Yadomae, T. 1998. Immunotoxicity of soluble β-glucans induced by indomethacin treatment. FEMS. Immunol. Med. Microbiol. 21:171–179.