Abstract
Intratracheal aspiration (IA) exposure to Metarhizium anisopliae crude antigen (MACA), which is composed of equal protein amounts of mycelium (MYC), conidia (CON) and inducible proteases/chitinases (IND) extracts/filtrates, has resulted in responses characteristic of human allergic asthma in mice. The study objective was to evaluate the potential of each component extract to induce allergic/asthma-like responses observed in this mouse model. BALB/c mice received 4 IA exposures to MACA, CON, MYC, IND, or bovine serum albumin (BSA; negative control) or appropriate vehicle control or inflammatory control over a 4-wk period. Mice were assessed by whole-body plethysmography for immediate airway responses and airway hyperresponsiveness to methacholine (Mch) challenge (PenH). Serum and bronchoalveolar lavage fluid (BALF) were collected 3 d after the final exposure. Additionally, BALF neurotrophin levels and extract protease and chitinase activity levels were evaluated. Western blot analysis showed that each component contained different IgE-reactive proteins. All fungal extract exposures resulted in elevated BALF total and differential cell counts, IgE and IgA and total serum IgE compared to HBSS and BSA controls. MYC-exposed mice had the highest responses except for neutrophil influx, which was highest in MACA and IND exposures. However, the MYC-exposed mice had significantly lower PenH values compared to other treatments. By comparison IND and MACA induced significantly higher PenH values. Additionally, IND had substantially higher protease activity levels but induced the lowest neurotrophin levels compared to the other fungal exposures. In this allergic asthma model extract chitinase activity was not associated with allergic responses. In summary, multiple exposures to any of the M. anisopliae component extracts induced allergic/asthma-like responses in BALB/c mice but the response magnitude was different for each component and each appears to contain unique IgE-reactive proteins. Therefore, hazard identification and/or risk assessment for molds must test both mycelia and conidia.
| Abbreviations: | ||
| BAL, | = | bronchoalveolar lavage; |
| BALF, | = | bronchoalveolar lavage fluid; |
| DPIT, HBSS, | = | Hank’s balanced salt solution; |
| IP, | = | intraperitoneal; |
| IA, | = | intratracheal aspiration; |
| MACA, | = | Metarhizium anisopliae crude antigen; |
| Mch, | = | methacholine |
Introduction
The incidence of asthma, a complex chronic respiratory disorder characterized by intermittent airway constriction and airway hyperresponsiveness, has increased dramatically over the past 25–30 years in industrialized nations, particularly among children (Centers for Disease Control, Citation2002; Stafford et al., Citation2003). In addition to the adverse health effects caused by asthma, the economic burden was estimated as 12.7 billion dollars in the United States for 1998 (Weiss and Sullivan, Citation2001). The indoor environment is thought to have some role in this increased incidence because children now spend 90–95% of their time indoors (Platts-Mills, Citation1995) and steps to conserve energy have greatly reduced dilution of indoor air by outdoor air. Furthermore, children exhibiting the most severe manifestations of asthma usually have a clear allergic component, with symptoms that begin in early life, and continue through adolescence and into adulthood. The estimates of adult asthmatics that are allergic asthmatics range from 50% (Pearce et al., Citation1999) to more than 90% (Holt et al., Citation1999). The cause(s) of this increased incidence are unknown, but lifestyle and/or environment changes are implicated.
A number of well-characterized protein allergens, including common indoor air contaminants such as house dust mite and cockroach antigens, have been shown to produce sensitization and respiratory allergic responses in humans, guinea pigs, and mice. Additionally, they have been strongly associated with asthma morbidity. Molds are an important indoor environmental contaminant. The Institute of Medicine’s (IOM, Citation2004) report on dampness and health found there was sufficient scientific evidence linking molds and damp environments with the exacerbation of asthma symptoms. However, the report found insufficient evidence linking molds with asthma induction and recommended more research to clarify this relationship. Furthermore, very few of the mold allergens have been characterized, despite their widespread distribution and potential importance in the induction and exacerbation of asthma.
Metarhizium anisopliae is a fungal biopesticide that has been used for agricultural pest control for over a century because of its pathogenicity for a broad range of susceptible insect hosts. Additionally, M. anisopliae is licensed in the United States for indoor use in cockroach control. Recent reports by Blanford et al. (Citation2005) and Scholte et al. (Citation2005) have identified M. anisopliae as a potentially useful pesticide to combat malaria. To date, M. anisopliae has not been demonstrated to be either infectious or toxic to mammalian species. Studies in our laboratory (Ward et al., Citation1998, Citation2000a, Citationand b) have demonstrated that mice exposed via the respiratory tract to extracts of M. anisopliae developed responses similar to those of human allergic asthmatics. Anecdotal information as well as limited clinical data has suggested that some individuals exposed occupationally to M. anisopliae have become sensitized (Kaufman and Bellas, Citation1996).
In order to assess M. anisopliae’s allergic potential, crude antigen extracts of the fungal components (mycelium, conidia and inducible enzymes) were combined in equal protein amounts (MACA) for animal exposures in previous studies. MACA has elicited inflammatory and respiratory physiological responses characteristic of allergic lung disease following multiple intratracheal aspiration (IA) exposures in a BALB/c mouse model (Ward et al., Citation2000b). Additionally, Instanes et al. (2005) demonstrated that M. anisopliae mycelia extract contains substances that have adjuvant activity in a mouse ovalbumin allergy model.
We hypothesized that one component extract of MACA would be dominant in the induction of allergic responses. Therefore, the objective of this study was to evaluate the allergic potential of each MACA component extract in BALB/c mice.
Materials and methods
Immune serum
Mice were immunized by intraperitoneal (IP) injection of 25 μg MACA in a total volume of 0.2 ml of 1.3% alhydrogel (aluminum hydroxide adjuvant) (Superfos Biosector a/s distributed by Accurate Chemical & Scientific Corp., Westbury, NY). Two booster immunizations of 15 μg MACA in 0.2 ml Hank’s balanced salt solution (HBSS; Gibco, Invitrogen, Carlsbad, CA) were given at 3-wk intervals. Five days following the final immunization, the mice were anesthetized with an IP injection of 0.5 ml of sodium pentobarbital (at 5 mg/ml) and exsanguinated. Blood was collected by cardiac puncture, transferred to serum separator tubes, and allowed to stand for at least 30 min at room temperature. Following centrifugation at 1000 × g for 30 min at 4°C, the serum was pooled, aliquoted, and stored at − 80°C.
Animals
Seven-week-old, female BALB/c mice (Charles River, Raleigh, NC) were group-housed in polycarbonate cages with bedding made of pine shavings in an environmentally-controlled, Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited animal facility. Sentinel mice were monitored serologically and were found to be free of Sendai, mouse pneumonia, mouse hepatitis, other murine viruses, and mycoplasma. Mice also were monitored for, and found to be free of, ectoparasites and endoparasites. The animal holding room temperature (22.2°C ± 1.1°C) and relative humidity (50% ± 10%) were maintained at the recommended levels. Mice were maintained on a standard diet (Purina Rodent Lab Chow, St. Louis, MO) and water ad libitum. Mice were acclimated one week at this facility prior to the start of the experiment.
Fungal antigen preparation
Metarhizium anisopliae strain 1080 was obtained from USDA-ARS Entomopathogenic Fungus Collection in Ithaca, NY. Extracts of Metarhizium anisopliae mycelium (MYC) and spores/conidia (CON), as well as inducible enzymes filtrate (IND), were produced as previously described (Ward et al., Citation1998). Proteins greater than 3000 daltons molecular weight were concentrated using a stirred-cell concentrator with an YM3 (MWCO 3000) filter (Amicon, Beverly, MA). Concentrated extracts were assayed for total protein concentration as described below. These component extracts/filtrates were combined in equal protein amounts to form the Metarhizium anisopliae crude antigen (MACA).
Experimental design
Five groups of six female BALB/c mice were exposed four times over a 4-wk period (experimental day (D)-28, D-14, D-7, D0) with 10 μg of MACA (positive control), CON, MYC, or IND extract, or bovine serum albumin (BSA; negative control) (Sigma-Aldrich Chemical Co, Milwaukee, WI) in 0.05 ml of HBSS by intratracheal aspiration (IA), as previously described in Ward et al. (Citation1998). Additionally, one group of six mice was exposed to HBSS (vehicle control) and four groups of six mice were exposed to HBSS three times plus a final IA of MYC (HB/MY) or IND (HB/IN) extracts (inflammatory control). Airway immediate responses to extracts (after each IA exposure) and responsiveness to doubling doses of a non-specific cholinergic challenge (methacholine) (Day 1 (D1) and Day 3 (D3) after the fourth IA exposure) were determined by whole-body plethysmography (see later). Prior to the collection of serum and BALF, the mice were rested for at least 1 hr after the D3 methacholine challenge. Additionally, the lungs were fixed in 10% formalin for pathology.
Measurements of airway responsiveness
Antigen-specific immediate responses
Antigen-specific airway responsiveness was measured immediately after each exposure (Days -28, -14, -7, 0) in unrestrained mice using whole, body plethysmography (Buxco Electronics, Troy, NY) as previously described in Viana et al. (Citation2002). Briefly, BioSystem XA software (SFT3812, version 2.0.2.48, Buxco Electronics) was used to calculate enhanced pause (PenH), a unitless index of airway hyperreactivity, which strongly correlates with lung resistance (Hamelmann et al., 1997). PenH is derived from the expiratory side of the respiratory waveform in a flow whole body plethysmograph flow parameters [respiratory rate, tidal volume, inspiratory and expiratory times (Ti, Te), peak inspiratory and expiratory flows (PIF, PEF), and relaxation time (RT)]. PenH reflects changes in pulmonary resistance during bronchoconstriction according to the following equation: PenH = (Te-RT) ÷ RT) × (PEF ÷ PIF). Baseline PenH measurements were recorded for 10 min and averaged for each animal. Mice were then IA-exposed to fungal extract or control agents, and placed back in the chambers within 5 min of dosing. PenH readings were then monitored and averaged over a 1 hr post-instillation period.
Airway responsiveness to methacholine aerosol
Airway responsiveness to methacholine (Mch) aerosol was determined by measurement of time-integrated changes in PenH in response to Mch on Days 1 and 3 after the final exposure. After measurement of baseline PenH for 5 min, either saline or Mch in increasing concentrations (4, 8, 16, 32, and 64 mg/ml) was nebulized and delivered through an inlet of the chamber for 1 min, and measurements of PenH were made for 4, 5, 6, 7, and 11 min, respectively, after each dose. After subtracting baseline values, time-integrated changes in PenH were calculated and expressed as area under the curve (AUC) for each concentration of Mch (Penh units × sec). Immediate responses and methacholine responsiveness were analyzed across treatment groups and for individual mice on different days of the protocol. The interaction between the treatment and day effects was also examined.
The statistical analyses for immediate (IR) and hyperresponsiveness (AUC) airway responses data were performed using SAS version 9.1.3 software (SAS Institute Inc, Cary, NC). The IR data was analyzed using an analysis of variance (ANOVA) procedure examining the main effects of each model as well as the interactive effects. A significant interaction resulted in pair-wise comparisons performed as a subtest of the ANOVA model adjusting the significance level for multiple comparisons using Tukey’s post hoc test. p < 0.05 was considered as statistically significant. PROC MIXED and PROC GLIMMIX procedures were used to analyze all the repeated measures AUC generated data. A linear mixed model with restricted maximum-likelihood estimation analysis and Tukey’s post hoc test was used to determine statistical differences for the AUC data. Reported values represent means ± standard error (SE).
Bronchoalveolar lavage (BAL) and blood collection
Blood and bronchoalveolar lavage fluid (BALF) samples were collected as previously described (Ward et al., Citation1998). Briefly, blood samples were collected by cardiac puncture and stored at room temperature for 30 min prior to centrifugation. Serum was stored at −80°C. The lungs were lavaged twice with 1ml aliquots of HBSS. BALF aliquots for each animal were pooled and stored on ice. Total cell counts were performed using a hemocytometer and viability assessed by trypan blue exclusion. The BALF was centrifuged at 800 rpm (100 × g) for 15 min at 4°C to pellet the remaining cells. Aliquots of BALF supernatant were assayed for total protein and lactate dehydrogenase (LDH) activity (as described later), and the remainder was stored at − 20°C for IgE and cytokine assays. The cell pellet was resuspended in 500 μl HBSS and cytospin preparations of 75–150 μl BALF were made by centrifugation onto glass slides (Shandon Cytospin: 300 rpm, 10 min) (Shandon Inc, Pittsburg, PA). Following Wright-Giemsa (Fisher Scientific Fair Lawn, NJ) staining, cells were differentially counted at 200 cells per slide (one slide per animal).
Total IgE ELISA
The BALF and serum total IgE ELISAs were performed as previously described (Ward et al., 2000). Briefly, microtiter plates were coated with rat anti-mouse IgE (Pharmingen, San Diego, CA) in phosphate-buffered saline (PBS; pH 7.3, Pharmingen) and incubated overnight at 4°C. Following blocking with blocking buffer (PBS plus 1% BSA), serum samples and the mouse IgE standard monoclonal anti-trinitrophenol (TNP); Sigma-Aldrich, ranging from 800 ng/ml to 1.5625 ng/ml diluted in blocking buffer were applied. The biotinylated detection antibody (rat anti-mouse IgE; Pharmingen) was diluted in blocking buffer. Streptavidin alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) and phosphatase substrate (p- nitrophenyl phosphate disodium; Sigma 104, Sigma) dissolved in substrate buffer 768.7 mM Tris (hydroxymethyl) aminomethane, 2 mM MgCl2.6H2O, and 0.5 mM ZnCl2, pH 8.2) were applied. Optical density was read on a ThermomaxR Plate Reader (Molecular Devices Corp., Menlo Park, CA) at a wavelength of 405 nm. Softmax ProR version 2.6.1 (Molecular Devices Corp.) software was used for data collection and conversion from optical density to protein concentrations. The limit of detection for this assay was 12.5 ng/ml.
Total IgA ELISA
The IgA ELISA was performed using the same protocol as described for the IgE ELISA. Briefly, microtiter plates were coated with anti-mouse IgA (Pharmingen) at 3.0 μg/μl in PBS (pH 7.3). BALF samples were diluted 1:100, and the IgA standard (purified mouse IgA, κ isotype, Pharmingen) was added in 2-fold dilutions, providing a range from 500 ng/ml to 0.49 ng/ml. The biotinylated anti-mouse IgA (Pharmingen) was added at 2.5 μg/μl. Streptavidin-horseradish peroxidase (Zymed Labs, San Francisco, CA.) and TMB substrate (Dako Corp, Carpinteria, CA.) were applied and optical density determined as described here. Softmax Pro software was used for data collection and conversion from optical density to protein concentrations. The limit of detection for this assay was 3.906 ng/ml.
Antigen-Specific IgE Assay
A rat basophilic leukemia (RBL) cell β-hexosaminidase release assay was performed as an indirect measure of antigen-specific IgE in serum. The procedure is based on the method by Hoffmann et al. (Citation1997), with modifications by D. Leadbeater and D. Basketter (personal communication, Unilever Safety and Environmental Assurance Center, UK). The procedures have been previously described in Chung et al. (Citation2005). Briefly, 96-well flat-bottom tissue culture plates were seeded with 105 RBL-2H3 cells (ATCC, Rockville, MD) and incubated for 18 hr at 37°C in a 5% CO2 humidified incubator. The cells were passively sensitized with a 1:5 dilution of individual mouse serum or with normal sera (spontaneous and total release controls) for 2 hr. Cells were then washed with Tyrode’s buffer followed by the addition of fungal extracts (10 μg) and incubated for 1 hr at 37°C in a 5% CO2 humidified incubator.
For spontaneous release, cells were incubated with extract or buffer alone. For total release, cells were incubated with 1% (v/v) Triton X-100 (Sigma) in Tyrode’s buffer. Subsequently, the β-hexosaminidase activity was measured by combining cell culture supernatant and substrate (p-nitrophenyl-N-acetyl β-d-glucosaminide) in a sterile 96-well plate and incubating the plate for 1 hr at room temperature. The enzyme reaction was stopped by the addition of 0.2 M glycine. Absorbance was measured 30 min later using SpectraMax 340 PC Plate Reader (Molecular Devices Corp.) at a wavelength of 405 nm and data collected by Softmax Pro® software (version 2.6.1, Molecular Devices Corp.). Data are presented as percent total release following subtraction of spontaneous release. Additionally, the extract dose resulting in 10% of total release was calculated by interpolation.
Total Protein and Lactate Dehydrogenase (LDH) Assays
BALF samples were assayed for total protein using Pierce Coomassie Plus Protein Assay Reagent (Pierce, Rockford, IL). Concentrations were determined from a standard curve using BSA standards (Sigma Chemical Co.). Additionally, the BALF samples were assayed for LDH using a commercially-prepared kit and controls from (Sigma). The assay reagent proportions remained the same, but volumes were reduced for use on the Cobas Fara II Centrifugal Spectrophotometer (Hoffman-LaRoche, Branchburg, NJ).
Histopathology
Following BAL, the lungs were fixed in 10% buffered formalin acetate (Fisher Scientific), embedded in paraffin, and sectioned. The lung lobe was sectioned along the main stem bronchus and stained with hematoxylin–eosin solution prior to histological examination (one slide per mouse). The entire lung was examined at several magnifications. The relative degree of severity of inflammatory, degenerative, and proliferative changes was graded on a scale of 1 to 5: 1 = minimal, 2 = slight/mild, 3 = moderate, 4 = moderately severe, 5 = severe/high. The pathology scores for lung sections were summarized as follows: incidence is the number of mice in a group exhibiting a particular pathology; severity is the numeric average of the pathology scores for a particular lesion/treatment group (Experimental Pathology Laboratories, Inc., Research Triangle Park, NC). Diffuse alveolar macrophage accumulation consisted of activated macrophages present in alveolar air spaces in a large area of the lung parenchyma. Alveolitis consisted of focal thickening of alveolar septae, often with inflammatory cells in the affected alveolar spaces. Edema was diagnosed when there was a distinct distended area surrounding vessels that contained proteinaceous fluid.
Neurotrophin ELISAs
All reagents and incubation periods were at room temperature and all volumes added were 100 μl unless otherwise noted. NGF, NT-3 and NT-4 ELISAs were performed using NGF, NT-3, and NT-4 Emax ImmunoAssay Kits, respectively, according to manufacturer’s instructions (Promega Corp. Madison, WI). Briefly, coating antibody (anti-NGF, anti-NT-3, or anti-NT-4) in carbonate coating buffer (pH 9.7) was placed in flat-bottom microtiter plates (Nunc International, Rochester, NY), and then the plates were sealed and incubated overnight at 4°C. Following blocking with 200 μl of Block & Sample buffer (BSB) per well, BALF samples, and purified NGF, NT-3, or NT-4 standards were applied and allowed to incubate for 6 hr. Biotinylated anti-NGF, anti-NT-3, or anti-NT-4 antibodies in BSB were then added and allowed to incubate overnight at 4°C.
Streptavidin-horseradish peroxidase conjugate was applied and the plates were then incubated for 2.5 hr at room temperature. One-Step Substrate Solution was added and incubated for at least 20 min. Reaction was stopped by adding 1N hydrochloric acid. Optical density was read on a SpectraMax 340PC Plate Reader (Molecular Devices Corp., Menlo Park, CA) at a wavelength of 450 nm. Softmax Pro® software (version 2.6.1, Molecular Devices Corp.) was used for data collection and conversion from optical density to neurotrophin concentrations. The limit of detection was 15.6 pg/ml for NGF and 4.7 pg/ml for both NT-3 and NT-4.
Protease Assay
Using the EnzChek7Protease Assay Kit (red fluorescence) (Molecular Probes, Eugene, OR), MACA and its component extracts were assayed for protease activity. Ten concentrations (0–100 μg/ml) of the extracts as well as the trypsin enzyme control were added to the digestion buffer (200 mM Tris-HCl [pH 7.8] containing 2 mM sodium azide). Following the addition of BODIPY7 TR-X labeled casein substrate in 0.1 M sodium bicarbonate (pH 8.3), the samples were protected from the light and incubated for 1 hr. Protease-catalyzed hydrolysis of the substrate and subsequent release of BODIPY7 TR-X dye-labeled peptides results in increased fluorescence that is proportional to protease activity. Fluorescence of the BODIPY7 TR-X dye (dye excitation/emission maxima of approximately 589/617 nm) was read in a fluorometer (Spectra Max, Gemini XS, Molecular Devices) using a filter with excitation = 590 ± 10 nm, emission = 645 ± 20 nm. Data is reported as percent of trypsin control.
Chitinase assay
Chitinase activity was determined for each of the mold component extracts using the Chitinase Assay Kit (Sigma). The enzymatic hydrolysis of chitinase substrates is performed in an acidic environment (pH ∼4.8) at 37°C, resulting in the liberation of p- nitrophenol. Duplicate assays were performed for each mold component extract. In order to quantitate the total chitinolytic activity, the reactions were run with three substrates (4-nitrophenyl-N-acetyl-β-d-glucosaminide at 1 mg/ml assay buffer, 4-nitrophenyl β-d-N,N′,N′-triacetylchitotriose at 0.5 mg/ml, and 4-nitrophenyl-N,N9-diacetyl-β-d-chitobioside at 1 mg/ml). Briefly, the substrates and standard solution were equilibrated to 37°C. For the extract samples, 90 μl of substrate was added to a 96-well plate followed by the addition of 10 μl (1 μg) of MACA, MYC, CON, or IND extract. Assay controls consisted of a blank (substrate solution only) to quantify spontaneous hydrolysis, a standard solution (p-nitrophenol, the detection molecule) for activity calculations, and a positive enzyme control (Trichoderma viride chitinase, diluted 1:20). Following incubation for 30 min at 37°C, the basic stop solution was added and the plate was read at 405 nm. One unit of chitinase activity is defined as the release 1.0 mmole of p-nitrophenol from the substrate per minute at pH 4.8 at 37°C. It is calculated as follows:
Where; A405sample = absorbance of the sample at 405 nm; A405blank = absorbance of the blank at 405 nm; 0.05 = mmole/ml of p-nitrophenol in the Standard Solution; 0.3 = final volume of the 96 well plate reaction after addition of the Stop Solution (ml); DF = Dilution Factor - fold dilution of the original chitinase enzyme or biological solution to prepare sample for the test; A405standard = absorbance of the Standard Solution at 405 nm; time = minutes; and, Venz= volume of the sample (ml).
Western blots
Samples (10 μg total protein) were heated for 10 min at 70°C prior to loading and separated under reducing conditions on a 10% Bis-Tris gel (Invitrogen). Proteins resolved by SDS-PAGE were transferred to 0.2 μm nitrocellulose membranes and blocked with 5% (w/v) dry milk in TBS/0.1% (v/v) Tween 20 (TBST). All washes were performed with TBST. The proteins were immunodetected by incubating the blot with hyperimmune serum raised against MACA followed by HRP-labeled rat anti-mouse IgE (Southern Biotechnology, Birmingham, AL), visualized with chemiluminescence (Western Lightning, Perkin-Elmer Life Sciences, Boston, MA), then exposed to Hyperfilm (Amersham, Piscataway, NJ).
Statistics
The data were analyzed using analysis of variance (ANOVA) (SAS, SAS Institute Inc., Cary, NC). Pair-wise comparisons were performed as subtests of the overall model. In cases of comparing a control group to other groups, Dunnett’s Test was used. Adjustments in significance levels, for multiple comparisons, were made using a modified Bonferroni Correction. p < 0.05 was considered as statistically significant. Reported values represent means ± standard error (SE).
The analysis of antigen-specific IgE (RBL assay) data estimated the dose at which a 10% release of total mediator occurred. This was accomplished through linear interpolation between responses at consecutive doses bracketing the 10% value.
Results
BALF total and differential cell counts
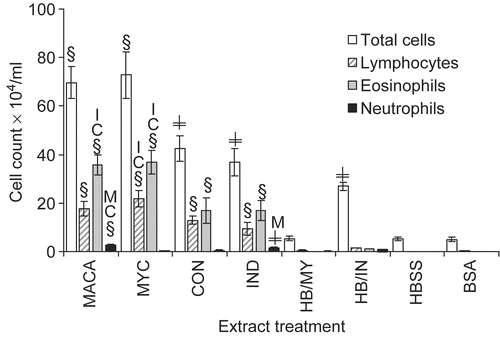
Mice that were exposed multiple times to any of the fungal extracts had significant increases in BALF total and differential cell counts () with the exception of neutrophilic influx when compared to controls. Additionally, MYC exposures induced significantly higher levels of lymphocytes and eosinophils compared to the other component extracts and were comparable to MACA exposures. Only IND and MACA exposures resulted in significant neutrophil influx. The inflammatory control HB/IN induced a significant increase in total cell influx compared to both HBSS- and BSA-treated mice. This total cell increase, though comparable in magnitude to that generated by multiple exposures to IND, was different in its composition. Macrophage influx induced upon first exposure to IND (HB/IN) was 88% of the total cell count while multiple exposures to IND resulted in 39% of the total cell count identified as macrophages (data not shown).
Figure 1. BALF total and differential cell counts. Data shown represent mean ± SE. (§) indicates significant differences compared to HBSS, BSA, HB/MY, HB/IN; or (≠) compared to HBSS, BSA, HB/MY only. Letter (A = MACA, C = CON, I = IND, M = MYC) identifies differences among treatments: at p < 0.05. n = 6.
Serum and BALF Total IgE and BALF IgA
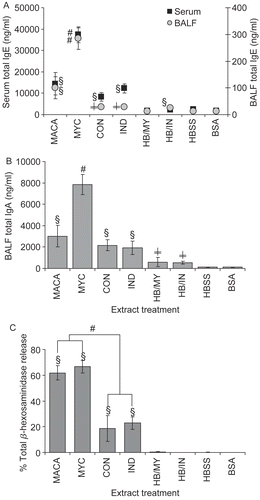
Multiple exposures to MACA and component extracts resulted in significantly elevated total IgE (serum and BALF) () and total IgA (BALF) (). However, MYC extract induced antibody levels significantly elevated above those of the other extracts, including MACA. Interestingly, the inflammatory controls HB/MY and HB/IN mice had significantly elevated levels of BALF total IgA compared to HBSS and BSA controls 3 d after their only exposure to fungal component extracts. Furthermore, HB/IN mice had significant levels of BALF IgE compared to HBSS and BSA controls and HB/MY.
Figure 2. (A) Serum and BALF total IgE. (B) BALF total IgA. (C) Antigen-specific IgE quantified in functional assay by measurement of mast cell mediator (β-hexosaminidase) release and presented as percent of total mediator release. Data shown represent mean ± SE. Significantly elevated compared to (§) all controls; (#) to other treatments; (≠) HBSS and BSA at p < 0.05. n = 6.
Extract-specific IgE
Antigen-specific IgE (), assessed as % of total β-hexosaminidase release upon antigen cross-linking of mouse serum IgE bound to RBL-2H3 cells, was significantly elevated following multiple exposures to the fungal extracts compared to the controls. Furthermore, serum from mice multiply exposed to MACA and MYC demonstrated significantly higher (≈3-fold) mediator release compared to serum from either CON- or IND-exposed mice. Serum from mice exposed to HBSS, BSA, or a single exposure to mold extract did not cause mediator release above background levels.
Airway responsiveness
Immediate response to antigen challenge
Early in the experimental protocol (D-28, D-14) mice exposed to the IND filtrate had significantly increased PenH values expressed as percent increase over baseline when compared to the control exposures (HBSS, BSA, HB/MY, HB/IN) (). By the third exposure (D-7), responses in mice exposed to MACA, MYC, and CON, in addition to those exposed to IND, were significantly elevated compared to controls. Furthermore, the responses induced by MACA, CON, and IND exposure were also significantly elevated compared to MYC treatment. All airway responses were significantly elevated following the fourth exposure, but differed significantly among the extracts: IND > MACA > CON > MYC.
Figure 3. (A) Immediate respiratory physiological responses averaged for 1 hr immediately following IA extract exposure. (B) Respiratory responses to increasing concentrations of methacholine aerosol at D1 and D3 after final IA exposure. Data shown represent mean ± SE. Significantly elevated compared to (#) HBSS and/or BSA; (§) all controls; (≠) all treatments; other treatments: C = CON, I = IND, M = MYC, A = MACA at p < 0.05. n = 6.
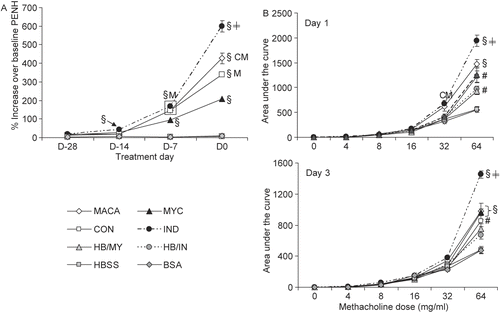
Airway hyperresponsiveness to non-specific challenge
At D1 () following the last IA antigen exposure, the IND mice challenged with the non-specific agonist methacholine (Mch) had significantly elevated responses compared to at least some controls at all challenge doses as well as MYC and CON at 8 or 32 mg/ml Mch doses. At the highest Mch dose (64 mg/ml), the IND mice responses were significantly elevated compared to all other treatments and controls. MACA-treated mice had significantly elevated responses compared to CON at 32 mg/ml and all or some controls at both 32 and 64 mg/ml. At the 64 mg/ml Mch dose, both MYC- and CON-treated mice had significantly higher responses than the vehicle and negative controls (HBSS, BSA).
At D3 () IND mice had significantly elevated responses compared to MACA and HBSS (4 mg/ml Mch), BSA and HBSS (8 mg/ml Mch). However, at 64 mg/ml Mch, IND mice responses were significantly elevated compared to all other treatments, although MACA, MYC, and CON mice responses were significantly elevated compared to vehicle and negative controls.
Histopathology
The mouse lungs were lavaged before excision and fixation of the lungs. Although even the gentlest lavage could result in some lung damage, the HBSS control mice had limited lesions, with the exception of peribronchial and perivascular mononuclear infiltrate and focal alveolitis. However, these lesions were not consistent within the group. This suggests that the lavage had minimal effect on the lung pathology identified in the fungal extract-treated mice. The most prominent histologic lesions for all treatment groups were the presence of perivascular and peribronchial mononuclear cell and neutrophilic infiltration, with the highest incidences and severities occurring in mice treated with MACA, CON, MYC, and IND (). However, CON treatment resulted in slightly lower incidence and severity compared to other treatments for perivascular mononuclear cell and neutrophilic infiltration, bronchial epithelial hypertrophy, and focal alveolitis. summarizes the incidence and group severity of lesions.
Table 1. Summary of incidence and severity of mouse lung pathology.
Neurotrophin assay
Neurotrophins are known to be elevated in the lungs of asthmatics (Virchow et al., Citation1998). Additionally, mice sensitized and challenged with Penicillium chrysogenum displayed significant dose-dependent increases in both BALF and serum levels of NGF, NT-3, and NT-4 (Chung et al., Citation2007). Therefore, mouse BALF from this study was assayed for NGF, NT-3, and NT-4 (). MYC extract not only induced significantly elevated levels of the neurotrophins compared to controls but also compared to other treatments. IND extract induced the lowest levels of neurotrophins. The IND induced NGF level was not significantly elevated compared to controls.
Figure 4. (A) The neurotrophins NGF, NT-3 and NT-4 in BALF were assayed by ELISA. The limit of detection was 15.6 pg/ml for NGF, 4.7 pg/ml for both NT-3, and NT-4. Data shown represent mean ± SE. Significantly elevated compared to (§) all controls; (≠) HBSS and/or BSA; other extracts: C = CON, I = IND, M = MYC, A = MACA at p < 0.05. n = 6. (B) Protease activity is shown as percent of trypsin control.
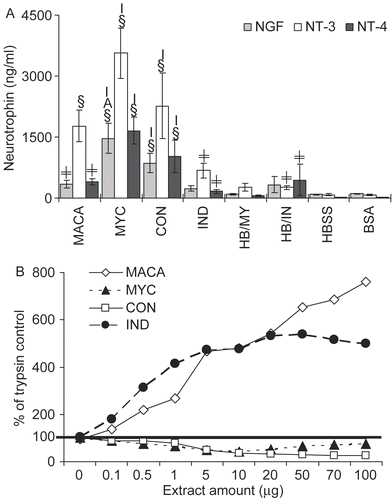
Protease activity
Compared to the trypsin control both IND extract and MACA demonstrated robust protease activity with 473% and 481% of trypsin control protease activity, respectively, at 10 μg of extract (our standard dosing amount) (). However, both the CON and MYC extracts demonstrated protease activity below the level of the trypsin control (37% and 43% of trypsin protease activity at 10 μg extract, respectively). This suggests that the primary source of MACA proteolytic activity is the IND extract.
Chitinase activity
The mediator β-hexosaminidase measured in the RBL assay is a chitinase. Therefore, it was important to determine if any portion of the mediator release measured in that assay was due to endogenous levels of chitinase activity. None of the extracts had a significant increase in either endochitinase or exochitinase activity compared to spontaneous chitinase substrate hydrolysis (data not shown).
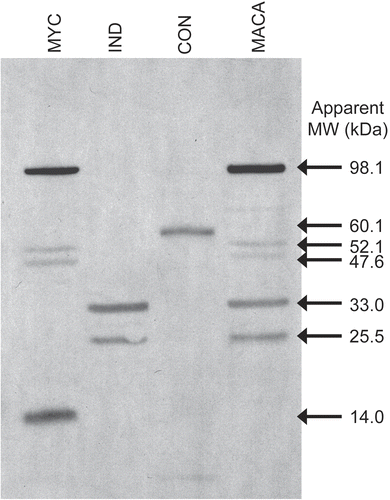
Western blot
Western blot analyses of MACA and the component extracts probed with serum from mice immunized with MACA revealed five clearly visible proteins in MACA that are reactive with mouse IgE (). These proteins appear to be contributed by the MYC (98.1, 52.1, 47.6 kD) components and IND (33.0, 25.5 kD) components. Although the CON extract displays one IgE-reactive protein band (60.1 kD), that band is not apparent in MACA. Additionally, there is one MYC protein (14 kD) that is also not apparent in the combined extract.
Discussion
This study was undertaken to elucidate the allergic potential of each M. anisopliae fungal component (MYC, CON, IND) extract thus providing insight into the source of the allergic asthma phenotype induced by MACA exposure in the mouse model. Although each of the fungal component extracts resulted in significantly elevated markers of allergic asthma, the magnitude and pattern of these responses are different for each (summarized in ). However, a single exposure to these component extracts did not induce increases in allergic endpoints (total serum and BALF IgE, antigen-specific/functional IgE, and BALF eosinophils) (data from current study or time course study described in Ward and Selgrade, Citation2007). MYC induced significantly elevated allergic endpoints compared to the other component extracts following multiple treatments. Furthermore, antigen-specific and functional IgE (as indicated by release of β-hexosaminidase from RBL-2H3 cells) induced by MYC-treated mouse serum was approximately three-fold higher than that of CON and IND.
Table 2. Response summary table.
MYC induced the most robust IgE and eosinophil influx responses of the three component extracts. Additionally, MYC induced significant increases in both immediate and hyperreactivity responses to non-specific challenge (Mch) compared to controls (HBSS, BSA). However, MYC airway responses were the lowest of the three component extracts. This suggests that factors in addition to IgE and airway eosinophil influx impact airway responsiveness. Although the source of this unexpected disparity in the MYC antibody/ cellular influx endpoints and airway responses is unclear, one notable possibility is the level of protease activity. MYC was demonstrated to have very little endogenous protease activity (less than the trypsin control). On the other hand, IND had the highest levels of protease activity (~10 more than MYC) and induced the most robust immediate airway responses to antigen challenge as well as hyperreactivity responses compared to the other component extracts.
It may be noted that BALF eosinophil counts were significantly elevated for all of the component extracts as well as the MACA-treated mice but were not identified in the lung histology. The percentage of BALF cell eosinophils ranged from ~36% for CON-treated mice to ~50% for MYC- and MACA-treated mice, which indicates a robust response to these extracts. The apparent discrepancy between the lavage and histology results could be due to a differential recruitment of eosinophils into the airway lumen, where they could be readily dislodged during bronchoalveolar lavage. In addition, degranulated eosinophils can be extremely difficult to differentiate from neutrophils in H&E-stained sections (as were evaluated in this study) and therefore may be underrepresented in the pathology analysis. These reasons suggest that the “discrepancies” between the lavage and pathology eosinophil count results may, in fact, be indicative of the robustness of the response to these extracts.
Additionally, extract or animal sample endpoints that have been suggested to play a role in allergic asthma were evaluated to further elucidate response differences among the M. anisopliae component extracts. One aspect contributing to these extract response differences may be varying proteolytic activity. Others have shown that proteolytic activity in the fungal biomass can damage epithelial cells (Kauffman, Citation2003a). Additionally, it has been suggested that this epithelial damage can facilitate the induction of allergy by allowing allergens through the epithelial surface and/or by inducing immune mediators such as cytokines and prostaglandin E2 through activation of cell surface receptors (Asokananthan et al., Citation2002; Kauffman, Citation2003b). Based on the protease activity assay, IND contains significantly higher levels of proteolytic activity than the other component extracts. IND also induced the highest airway responses (immediate and hyperreactivity responses) suggesting that proteolytic activity may correlate with airway responses.
Neurotrophins (NT) are a family of cell products involved in airway inflammation, airway hyperresponsiveness, and airway remodeling, which are clinical symptoms of allergic asthma (Nassentein et al., Citation2004). Unexpectedly, both MYC and CON induced neurotrophin responses that were significantly increased compared to IND exposure, which induced the more robust airway responses. Therefore, in this study, the neurotrophin levels appear to correlate with airway inflammation (BALF cell influx) but not the airway responses. In fact, Chung et al. (Citation2007) did find a correlation between BALF NT levels and differential cell counts for D1 (a timepoint not evaluated in this study). The lack of NT level correlation to airway response may be temporal in that BALF and serum samples were collected only on D3 following the last extract exposure.
Chitinases are widely distributed in living organisms and are thought to primarily play a role in pathogen defense. Recently, elevated levels of acid mammalian chitinase were observed in the lung tissue of asthmatic patients (Zhu et al., Citation2004) and exogenous chitinase (Streptomyces griseus) was shown to activate protease-activated receptor-2 in human airway epithelial cells (Hong et al., Citation2008). Although chitinases (optimal induction pH of 5) have been found in M. anisopliae (St. Leger et al., Citation1986, Citation1998), our extracts did not have significant chitinase activity above background levels. Therefore, even though exogenous chitinase may play a role in allergic asthma it does not appear to be a factor in this study.
How the differences among M. anisopliae component induced responses impact the severity of human allergic asthma is unclear. However, the response differences induced by the component extracts may in part be due to differential protein allergenic potentials. Kurup et al. (Citation2001) found that purified Aspergillus fumigatus recombinant allergens differentially induced allergic responses. It is noteworthy that each M. anisopliae component extract appears to contribute unique IgE-reactive proteins to the combined extract MACA. Differences in adjuvant effects by fungal components might also contribute to the differences in fungal extract allergic responses. Although this study was not designed to address adjuvant effects, M. anisopliae MYC has been shown to enhance ovalbumin-induced allergic responses that were only partly due to the fungal cell wall component (1→3)-β-d-glucan (Instanes et al., Citation2006).
Western blot analyses of the extracts detected two IgE-reactive protein bands (CON: 60.1 kD and MYC: 14 kD) that were not visualized in MACA. The sera used to probe the immunoblot were raised against MACA using an aggressive immunization protocol. Therefore, one explanation might be that the 10 μg of MACA run on the gel had insufficient amounts of the two proteins to visualize them. Another explanation might be that when the three components are mixed to form MACA, additional protein processing occurs resulting in an altered molecular weight-thus becoming “undetectable” in the MACA blot. It does appear that most if not all of the proteins identified in each component extract are unique to that component. However, further characterization of these IgE-reactive proteins is necessary to elucidate potential homology and the functional nature of the proteins.
Our data demonstrate that multiple exposures to any of the individual M. anisopliae component extracts can induce allergic/asthmatic-like responses in BALB/c mice when compared to control mice. The data suggest that the magnitude of neurotrophin induction, the level of IgE induction, and eosinophil influx may be correlated and that protease activity may play a role in airway responsiveness. Additionally, the most robust responses appear to be derived from different components of the mold extract. Much of the mold literature addresses the responses in an animal model to either spores (conidia) or mycelia extract exposure(s). However, the differences in magnitude and pattern of response indicate that allergenic risk does not reside in just one mold component. Although it is unclear whether or not the differences between the component extract responses are physiologically relevant to human allergic lung disease, further investigation into this area is clearly warranted.
Acknowledgements
The authors would like to thank Debora Andrews, Elizabeth Boykin, and Judy Richards of US EPA for technical assistance and Don Doerfler of US EPA for statistical analysis. Additionally, we would like to thank Drs. Christal Bowman, Timothy Dean, and Cherie Pucheu-Haston for their critical review of the manuscript. This research paper has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Declaration of interest: The authors report no conflicts of interest.
References
- Asokananthan, N., Graham, P. T., Fink, J., Knight, D. A., Bakker, A. J., McWilliam, A. S., Thompson, P. J., and Stewart, G. A. 2002. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J. Immunol. 168:3577–3585.
- Blanford, S., Chan, B. H., Jenkins, N., Sim, D., Turner, R. J., Read, A. F., and Thomas, M. B. 2005. Fungal pathogen reduces potential for malaria transmission. Science 308:1638–1641.
- Chung, Y. J., Haykal-Coates, N., Viana, M. E., Copeland, L. B., Vesper, S. J., Selgrade, M. K., and Ward, M. D. 2005. Dose-dependent allergic responses to an extract of Penicillium chrysogenum in BALB/c mice. Toxicology 209:77–89.
- Chung, Y. J., Farraj, A., Haykal-Coates, N., Gavett, S. H., and Ward, M. D. 2007. Increased neurotrophin production in a Penicillium chrysogenum-induced allergic asthma model in mice. J. Toxicol. Environ. Health 70:1020–1026.
- Centers for Disease Control and Prevention. 2002. Surveillance for asthma—United States, 1980-1999. MMWR Morb. Mortal. Wkly. Rep. 51:1–13.
- Hoffmann, A., Veiths, S., and Haustein, D. 1997. Biologic allergen assay for in vivo test allergens, with an in vitro model of the murine type 1 reaction. J. Allergy Clin. Immunol. 99:227–232.
- Holt, P. G., Macaubas, C., Stumbles, P. A., and Sly, P. D. 1999. The role of allergy in the development of asthma. Nature 402:B12–17.
- Hong, J. H., Hong, J. Y., Park, B., Lee, S. I., Seo, J. T., Kim, K. E., Sohn, M. H., and Shin, D. M. 2008. Chitinase activates protease-activated receptor-2 in human airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. May 12. [Epub ahead of print].
- Instanes, C., Ward, M. D., and Hetland, G. 2006. The fungal biopesticide Metarhizium anisopliae has an adjuvant effect on the allergic response to ovalbumin in mice. Toxicol. Lett. 161:219–225.
- Institute of Medicine, National Academies of Science. 2004. Damp Indoor Spaces and Health. Washington, DC: The National Academies Press, p. 355.
- Kauffman, H. F. 2003a. Immunopathogenesis of allergic bronchopulmonary aspergillosis and airway remodeling. Front. Biosci. 8:e190–e196.
- Kauffman, H. F. 2003b. Interaction of environmental allergens with airway epithelium as a key component of asthma. Curr. Allergy Asthma Rep. 3:101–108.
- Kaufman, G., and Bellas, T. 1996. Occupational allergy to Metarhizium. Transpacific Allergy and Immunology/Research Abstracts. Allergy Asthma Pro. 17:166.
- Kurup, V. P., Xia, J. Q., Crameri, R., Rickaby, D. A., Choi, H. Y., Fluckiger, S., Blaser, K., Dawson, C. A., and Kelly, K. J. 2001. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin. Immunol. 98:327–336.
- Leadbeater, D., Zhang, J. G., Crevel, R. W., Blaikie, L., and Basketter, D. A. 2001. Experience with a rat basophilic leukemia cell line assay for measurement of rat or mouse specific IgE as an alternative to passive cutaneous anaphylaxis. Toxicol. Sci. Suppl. 60:174.
- Nassenstein, C., Kerzel, S., and Braun, A. 2004. Neurotrophins and neurotrophin receptors in allergic asthma. Prog. Brain Res. 146:347–367.
- Pearce, N., Pekkanen, J., and Beasley, R. 1999. How much asthma is really attributable to atopy? Thorax 54:268–272.
- Platts-Mills, T. A. 1995. Is there a dose-response relationship between exposure to indoor allergens and symptoms of asthma? J. Allergy Clin. Immunol. 96:435–440.
- Pollart, S. M., Chapman, M. C., Fiocco, G. P., Rose, G., and Platts-Mills, T. A. E. 1988. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J. Allergy Clin. Immunol. 83:875–882.
- St. Leger, R. J., Charnley, A. K., and Cooper, R. M. 1986. Cuticle-degrading enzymes of entomopathogenic fungi: Synthesis in culture on cuticle. J. Invert. Pathol. 48:85–95.
- St Leger, R. J., Joshi, L., and Roberts, D. 1998. Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl Environ. Microbiol. 64:709–713.
- Scholte, E. J., Ng’habi, K., Kihonda, J., Takken, W., Paaijmans, K., Abdulla, S., Killeen, G. F., Bart, G., and Knols, G. J. 2005. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308:1641–1642.
- Stafford, R. S., Ma, J., Finkelstein, S. N., Haver, K., and Cockburn, I. 2003. National trends in asthma visits and asthma parmacotherapy, 1978–2002. J. Allergy Clin. Immunol. 111:729–735.
- Viana, M. E., Haykal-Coates, N., Gavett, S. H., Selgrade, M. K., Vesper, S. J., and Ward, M. D. 2002. An extract of Stachybotrys chartarum causes allergic asthma-like responses in a BALB/c mouse model. Toxicol. Sci. 70:98–109.
- Virchow, J. C., Julius, P., Lommatzsch, M., Luttmann, W., Renz, H., and Braun, A. 1998. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am. J. Respir. Crit. Care Med. 158:2002–2005.
- Ward, M. D., and Selgrade, M. K. 2007. Animal models for protein respiratory sensitizers. Methods 41:80–90.
- Ward, M. D., Madison, S. L., Andrews, D. L., Sailstad, D. M., Gavett, S. H., and Selgrade, M. K. 2000b. Comparison of respiratory responses to Metarhizium anisopliae extract using two different sensitization protocols. Toxicology 147:133–145.
- Ward, M. D., Madison, S. L., Andrews, D. L., Sailstad, D. M., Gavett, S. H., and Selgrade, M. K. 2000a. Allergen-triggered airway hyperresponsiveness and lung pathology in mice sensitized with the biopesticide Metarhizium anisopliae. Toxicology 143:141–154.
- Ward, M. D., Sailstad, D. M., and Selgrade, M. K. 1998. Allergic responses to the biopesticide Metarhizium anisopliae in Balb/c mice. Toxicol. Sci. 45:195–203.
- Weiss, K. B., and Sullivan, S. D. 2001. The health economics of asthma and rhinitis. I. Assessing the economic impact. J. Allergy Clin. Immunol. 107:3–8.
- Zhu, Z., Zheng, T., Homer, R. J., Kim, Y. K., Chen, N. Y., Cohn, L., Hamid, Q., and Elias, J. A. 2004. Acidic mammalian chitinase in asthmatic TH2 inflammation and IL-13 pathway activation. Science 304:1678–1682.