Abstract
Epidemiologic studies indicate that women who smoke cigarettes are more likely to experience adverse reproductive and immunological health effects. Despite these facts, 20–30% of American women still smoke during their reproductive years. As little is known of the relationship between smoking and the immune response during pregnancy, an investigation was conducted using parous and non-parous (virgin) B6C3F1 mice to investigate what role (if any) parity status had on cigarette smoke (CS) induced effects on immune functions important in surveillance against developing tumors. Pregnant mice were exposed to CS for 5 d/wk ( 4 hr/d) from gestational day 4 to parturition; virgin mice were exposed for an equivalent amount of time. Smoke- and parity-associated alterations in pulmonary histology and lung inflammation, along with tumor cell host resistance, and cytotoxic T-lymphocyte (CTL) activity were examined either 24- 48 hr or 5 wk post-exposure/parturition; in the parous mice, gestational parameters were also evaluated. Exposure to CS significantly increased tumor susceptibility in virgin mice first injected with EL4 lymphoma cells at the 5 wk post-exposure timepoint; tumor incidence began to increase in smoke-exposed virgin mice as early as 24– 48 hr post-exposure. Pregnancy itself increased tumor incidence in mice injected with EL4 cells 24– 48 hr after birth, but this effect then dissipated over 5 wk to levels seen in virgin mice. When EL4 injections were first performed at either timepoint in CS-exposed parous mice, the tumor incidence was not significantly different from that in the air-exposed parity-matched controls. CTL activity in CS-exposed parous mice was significantly increased from both nulliparous groups as well as from the parous air control mice examined 5 wk post-exposure. Results suggest that exposure to CS throughout gestation could act in combination with pregnancy-associated changes to up-regulate immune responses, potentially compromising fetal tolerance.
Introduction
Of the 6 million American women who are pregnant each year, 20-35% smoke cigarettes (Albrecht et al., Citation2004). Data from the Center for Disease Control (CDC) have demonstrated that although the number of adult female smokers has declined in the past 5 years (22% in 1998 to 19.2% in 2003), the percentage of women smoking in their child-bearing years has remained unchanged with 18–24- and 24–35-year-old females making up 21.5% and 21.3% of the total, respectively. Moreover, between 13 and 20% of these women continue to smoke during pregnancy. These numbers are daunting considering that smoking women may experience conception failure, premature menopause, and spontaneous miscarriage in both natural and assisted conception cycles (Hughes and Brennan, Citation1996; Windham et al., Citation2005), not to mention the well-established adverse health effects of maternal smoking on the fetus/newborn/child.
In addition to the reproductive and peri-/post-natal health consequences associated with cigarette smoking by women, active smoking is also associated with immune alterations. Cigarette smoke (CS) is linked to neutrophilia and lung inflammation, macrophage (Mø) activation and release of chemokines (i.e., interleukin [IL]-8) and cytokines (i.e., tumor necrosis factor-α [TNFα]), and production of reactive oxygen species (ROS) both in vitro and in exposed human subjects (Barnes et al., Citation2003; Oltmanns et al., Citation2005). Toxicological studies demonstrate that CS acts as an immunosuppressant, leading to alveolar Mø apoptosis both in vitro and in vivo (Aoshiba et al., Citation2001), reduced dendritic cell number, and decreased numbers of T-helper (CD4+) and suppressor (CD8+) lymphocytes in the mouse lung (Robbins et al., Citation2004). Smoke-related alterations in particular immune system functions may also lead to heightened sensitivity to insult by other toxic, immunomodulatory, or carcinogenic substances.
Cigarette smoke and/or its individual constituents (> 4600) have been shown to suppress immune surveillance mechanisms (defined as those specific immune functions used to recognize and remove tumor cells) suggesting a relationship between CS, immune suppression, and increased cancer risk. For example, exposure to smoke impairs lymphocyte proliferation (McCue et al., Citation2000) and antigen-mediated signaling in rat splenocyte-derived T-lymphocytes (Kalra et al., Citation2000), both key mechanisms in anti-tumor defense. Different studies demonstrated an inhibition of natural killer (NK) cell activity, as well as a reduction in IL-10 production in rats and in a human Mø cell line following exposure to nicotine-derived N-nitrosamine (Rioux and Castonguay, Citation1997, Citation2001). Furthermore, toxicological studies in this laboratory have revealed that CS-induced suppression of cytotoxic T-lymphocyte (CTL) activity (an important immune defense mechanism against developing tumors) can also manifest in progeny of mothers exposed to low doses of CS during pregnancy (Ng et al., Citation2006a, Citation2008).
An important and still unanswered question is whether and how parity status influences CS-induced immune tumor surveillance mechanisms. The success of pregnancy actually defies the precepts of immunology. Ordinarily, the mother would be expected to generate graft-attacking antibodies and CTL to combat the foreign (paternal) human leukocyte antigens (HLA) or other antigens expressed by fetal cells (Hunt et al., Citation2005). A major shift in immune response from adaptive to innate immunity occurs upon blastocyst implantation, and pregnant females develop fetal tolerance. Studies examining the effects of smoking during pregnancy on maternal immunity are extremely limited, and only one study was identified regarding CS-induced effects on maternal immunocompetence during this vulnerable time period. Cheng-Smart et al. (Citation1986) reported that circulating T-lymphocytes were significantly increased in pregnant smoking women compared to their non-smoking counterparts or to non-smoking, non-pregnant women. This finding not only contrasts the immunosuppressive effects described earlier in CS-exposed adults, but also conflicts with the innate immune shift that arises in pregnant women (Sopori, Citation2002).
Given that CS is a potent immune modulator, smoking while pregnant could adversely alter the maternal immune response, placing both mother and child at increased risk for immune-related adverse health outcomes. Thus, this study sought to determine the effects of inhaled CS on adaptive immune responses associated with immune surveillance against developing tumors, and to evaluate what role, if any, pregnancy plays in the effect.
Materials and methods
Animals
Male and female B6C3F1 mice, purchased from The Jackson Laboratory (Bar Harbor, ME), were between 8–10 wk-of-age at the time of arrival. Mice remained in the NYU animal facility for 2 wk prior to mating to allow ample time for acclimation. Animals, housed in pairs (females) or individually (males) in polycarbonate cages (with corncob bedding) in temperature- (20-23°C) and humidity-controlled (∼55% RH) rooms, were maintained on a 16/ 8 hr light/dark cycle and provided food (Purina 5001 lab chow) and water ad libitum. All animal procedures were conducted under an animal protocol approved by the New York University Institutional Animal Care and Use Committee (IACUC).
Mating and gestation
Female mice (10–12 wk of age) were randomly separated into two groups. Mice from the first set were mated, while females in the second group remained unpaired (i.e., nulliparous/virgins). Mating pairs consisted of 1 male and 2 female mice per cage and the day of pairing was considered gestational day [GD] 0. After 4 d of pairing, both sets of female mice were exposed via whole-body inhalation to either mainstream cigarette smoke (MCS) or filtered air for 4 hr/d (5 d/wk) for about 3 wk or until the coupled females gave birth. At parturition, each mother-offspring set was maintained in clean filtered air and litter size and number of litters with viable offspring was determined.
Experimental design
The extent of parity-related effects on selected immune parameters was determined by examining immune surveillance mechanisms of CS-exposed parous and nulliparous mice at two different timepoints post-exposure/parturition.
Experimental groups and evaluated endpoints are shown in . Female mice were randomly assigned into four groups including: (1) air control nulliparous (ACNP); (2) CS-exposed nulliparous (CSNP); (3) air control parous (ACP); and, (4) CS-exposed parous (CSP). Immune parameters were examined in recently pregnant air- and smoke-exposed mice and their virgin counterparts at 24– 48 hr and 5 wk post-exposure: for parous mice, these timepoints coincided with lactation (i.e., 24– 48 hr post-parturition) and post-weaning (i.e., 5 wk post-parturition). The effect of CS exposure on reproductive health was examined in parous mice at both post-parturition timepoints by determining the incidence of pregnancy (% of coupled females that gave birth to live pups) and litter size; pup sex ratio for each dam was examined only for those litters born to mothers examined 5 wk post-exposure. At the time of sacrifice, eight animals/group were euthanized by intraperitoneal (IP) injection of ketamine hydrochloride ( 100 mg/kg; Vetalar, Fort Dodge Laboratories, Fort Dodge, IA) and pentobarbital sodium ( 175 mg/kg; Sleepaway, Fort Dodge Laboratories).
Table 1 Experimental groups of mice and biological parameters evaluated.
At both post-exposure timepoints: blood and broncho-alveolar lavage (BAL) fluid were recovered for differential cell counts; spleens were harvested to determine cytotoxic T-lymphocyte (CTL) activity; and, the lungs were formalin-fixed and used for histological analyses. Remaining mice from each group were housed three/cage and used for in vivo tumor challenge. Additional maternal endpoints examined at 24– 48 hr post-parturition included: whole body and lymphoid organ weight; pubic symphysis diameter in parous and nulliparous mice (for comparison); and, blastocyst implantation number.
Cigarettes and exposure atmosphere
Mainstream CS was generated from the burning of 1R3F cigarettes (Kentucky Tobacco Research & Development Center, Lexington, KY) using the same automated continuous cigarette smoking machine (Baumgartner-Jaeger CSM 2070, CH Technologies [USA] Inc., Westwood, NJ) used in previous studies (Ng et al., Citation2006a, Citation2006b; Ng and Zelikoff, Citation2008). Briefly, the machine was adjusted to load and light 4–5 cigarettes simultaneously, each of which produced one 2-sec puffs (at 35 ml of air/puff) under the control of an automatically-regulated piston pump that cycled once per minute. Mainstream CS was diluted 90% prior to entrance into the exposure chamber by introduction of filtered air into the bottom of the generation chamber with exhaust output at the top.
Mice were exposed in polycarbonate cages with wire-mesh tops. Cages were rotated on three racks within the chamber to assure even smoke distribution to all animals. Chamber levels of total suspended particles (TSP) and carbon monoxide (CO) were determined throughout the exposure; TSP concentrations were measured gravimetrically from Pallflex Emfab filters (Pall Corporation, East Hills, NY) weighed before and after sampling smoke-chamber air for 10 min on an hourly basis (4 samples/d). Carbon monoxide was measured continually over the 4 hr exposure period and recorded every 30 min (8 samples/d) using a 48C CO Analyzer (Thermo Environmental Instruments Inc, Franklin, MA). Airflow into the chamber was adjusted accordingly to maintain a CO concentration of approximately 25 ppm. Over a 4-hr exposure time period, chamber TSP and CO averaged 14. 4 mg/m3 and 24.2 ppm, respectively.
Blood and bronchoalveolar lavage fluid (BALF) analyses
Blood recovered from the posterior aorta was used to prepare smears in triplicate for differential cell counts. Slides were stained with Hematoxylin and Eosin (H&E; EM Science, Gibbstown, NJ), and analyzed using light microscopy (100X).
Immediately following blood collection, lungs were deflated, a cannula (0. 6 mm internal diameter) inserted into the trachea, and lungs were washed three times each with 1 ml of Dulbecco’s phosphate-buffered saline (DPBS; GIBCO BRL, Life Technologies, Grand Island, NY). Approximately 0. 8 ml of BALF was recovered from each of three washes, pooled (yielding a total of 2. 4 ml per mouse), and placed immediately on ice (4°C). Pooled washes from each mouse were centrifuged at 1500 rpm (at 4°C) for 5 min and recovered cells resuspended in PBS ( 1 ml). Three 100 μl aliquots of each re-suspension (per animal) were then cytocentrifuged (Cytospin, Shandon Southern Products, Cheshire, UK), cells stained with H&E, and three slides per animal analyzed using light microscopy (100X). Cell profiles were analyzed by counting at least 100 cells/slide and the total percentages of Mø, polymorphonuclear leukocytes (PMN), and lymphocytes were calculated.
Cytotoxic T-lymphocyte (CTL) assay
Cytotoxic T-lymphocyte activity was determined using a protocol described by Ng et al. (Citation2006a). Spleens were removed and maintained at 37°C in 3 ml of DPBS without Ca2+ or Mg2+ (pH 7.2–7.4; GIBCO). Organs were homogenized and treated with erythrocyte-lysing buffer (0.9% [w/v] ammonium chloride; 0.1% [w/v] potassium bicarbonate; 0.03% [w/v] sodium-EDTA) for 3 min at room temperature (RT). Homogenates were centrifuged and splenocytes resuspended to a concentration of 6 × 107 viable cells/ml in Minimal Essential Medium with Earle’s balanced salt (EMEM) (JRH Biosciences, Inc., Lenexa, KS). Cell numbers and viability were determined by hemocytometer counting and trypan blue exclusion, respectively (Burleson et al., Citation1992).
Cultured mouse mastocytoma cells (i.e., P815), used as the tumor target cells, were treated in the dark for 30 min with mitomycin C ( 50 mg/2-5 × 107 cells) and then resuspended in EMEM to a concentration of 1.2 × 106 viable cells/ml. Splenocytes and P815 cells (each at 0. 5 ml) were incubated together for 5 d (at 37°C in 5% CO2) at an effector to target (E:T) cell ratio of 50:1. After incubation, a second culture (1 × 107 cells) of P815 cells were incubated for about 1 hr (at 37°C) in DMEM (supplemented with 10% L-glutamine and 10% heat-inactivated fetal bovine serum [FBS]) and pulsed with 200 μCi 51Cr (as sodium [51Cr]-chromate in sterile saline; PerkimElmer Life and Analytical Sciences, Boston, MA) for 75 min (at 37°C). Target cells were then resuspended (in EMEM) to a final concentration of 2 × 105 viable cells/ml. Sensitized splenocytes and 51Cr-labeled P815 target cells (each a 0. 1 ml) were incubated together (at 37°C in 5% CO2) for 4 hr at an E:T cell ratio of 25:1 in individual wells of a round-bottom microtiter plate (Corning Inc., Corning, NY); separate wells containing 51Cr-labeled P815 cells were incubated with either EMEM or 1% Triton-X (Sigma-Aldrich, St. Louis, MO) to determine spontaneous (negative control) and total 51Cr release (positive control), respectively.
Following incubation, all cells were centrifuged (1500 rpm for 5 min), supernatants (100 μl) harvested, and 51Cr release was measured (in cpm) using a γ-scintillation counter (LKB-Wallac 1275 Minigamma counter, Perkin Elmer Inc., Wellesley, MA). The percentage of cytotoxicity was calculated as: ([ER-SR]/[TR-SR]) X 100, where ER, SR, and TR represented experimental release, spontaneous release, and total release counts, respectively.
In vivo tumor challenge
EL4 lymphoma cells (ATTC, Manassas, VA), used as the tumor model for these studies, were grown in 75 cm2 flasks containing Dulbecco’s modified Eagle’s medium (DMEM; supplemented with 10% horse serum and 1% L-glutamine [Invitrogen, Carlsbad, CA]). Tumor cells were passaged twice before each experiment as described previously (Ng et al., Citation2006a). Just prior to injection, cells were washed once and resuspended to 5 × 105 cells/ml in PBS. EL4 cells were injected subcutaneously (SC) at a concentration of 1 × 105 cells/mouse (a concentration previously shown to yield a 20–40% tumor incidence in 5-wk-old naïve male mice), into the right rear thigh of each animal from a single exposure group (n = 8–15 mice/exposure group). Time-to-tumor formation was determined by daily palpation of the injection site; tumor size was assessed daily by caliper measurements of the palpable tumor (Fisher Scientific International, Inc., Hampton, NH). Injected mice were sacrificed at either 60 d post-challenge or when the tumor diameter reached 20 mm. Tumor growth rate (calculated as the increase in tumor diameter (mm)/day) and tumor incidence (calculated as “Number of palpable tumors/Number of total mice injected”) were based on the actual number of tumors formed per individual/treatment group.
Histology
Following bronchopulmonary lavage, lungs from the adult female mice were intratracheally infused in situ with 10% formalin [at a constant pressure]. After fixation for at least 24 hr, the lungs were removed from the carcass and tissue blocks from the right lung lobes were paraffin embedded and sectioned at a thickness of 5 μm. Lung tissue sections were stained with H&E, and light microscopically examined by a board-certified veterinary pathologist who was unaware of individual animal exposure histories prior to the examination. Lung sections from four mice group were examined for histopathological changes (e.g., inflammatory and epithelial changes in conducting airways and alveolar parenchyma).
Data analyses
Data generated from differential counts, tumor growth, time to tumor formation, and CTL assay were analyzed by one-way analysis of variance (ANOVA) followed by Fisher’s post-hoc testing when necessary (Abacus Concepts, Inc., Berkeley, CA). The percentage of coupled females giving birth and tumor incidence were analyzed by survival curves using Prism software (Graph Pad Software Inc., San Diego, CA). Significant differences between and within control and experimental groups were accepted when probability (p) values were p < 0.05.
Results
Gestational parameters, body weight and lymphoid organ profiles
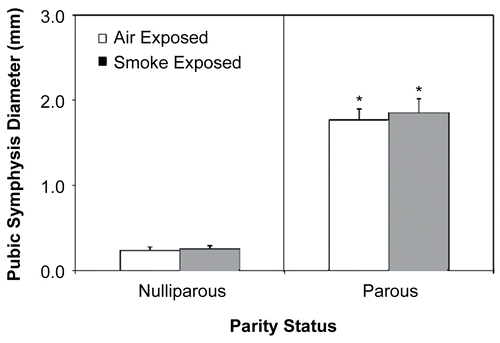
As shown in exposure to CS tended to decrease the incidence of pregnancy. Also, post-implantation pup loss was modestly (albeit, not significantly) increased in those CS-exposed parous females examined 24– 48 hr after giving birth (compared to the air-exposed, parity-matched controls). Pups born to parous females examined 5 wk post-parturition were separated from their mothers after nursing (3 wk), sexed, and the ratio of female to male offspring calculated; sex ratio was not calculated for litters born to mothers examined 24– 48 hr post-parturition, as pup gender was difficult to ascertain at this early age. No significant differences in litter size or offspring sex ratio were observed as a result of CS exposure. As expected, pubic symphysis diameter was significantly greater (p < 0.05) in parous mice compared to nulliparous mice measured 24- 48 hr after giving birth (); prenatal exposure to CS exposure had no effect on this parameter.
Table 2 Effect of cigarette smoke exposure on female fertility and gestation.
Figure 1. Effect of cigarette smoke (CS) exposure and pregnancy on pubic symphysis diameter. *Significantly increased (p < 0.05) from both virgin exposure groups. Values represent the mean (n = 6 dams/exposure group) ± SE, determined from the 24– 48 hr post-exposure timepoint.
Body, spleen and thymus weights were determined for all four treatment groups 24- 48 hr after the final CS exposure. While no significant CS-induced effects were observed on body or organ weights (compared to parity-matched, air-exposed controls), some of these same parameters were dramatically affected by parity status (). As expected, body weights of parous females were significantly greater (p < 0.01) than that of the age-matched, nulliparous females, regardless of exposure condition. Absolute spleen and thymus weights were significantly increased and decreased (p < 0.05), respectively, in parous females (compared to their nulliparous counterparts). While relative spleen weights of parous females were no different from those of nulliparous mice, relative thymus weights were dramatically decreased in parous females (p < 0.01) in comparison to treatment-matched, nulliparous groups. Body weights for the mice from each treatment group (at 5 wk post-exposure) were no different from each other (data not shown); organ weights were not evaluated at the later timepoint.
Table 3 Effects of cigarette smoke exposure and parity status on body and lymphoid organ weights determined 24– 48 hr post-parturition.
Bronchoalveolar lavage (BAL) and blood cell profile
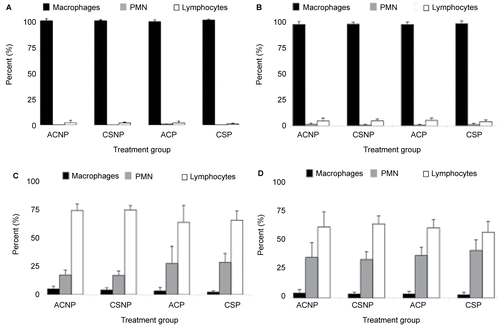
No significant differences in lavageable lung cell profiles were observed between any of the four experimental groups (i.e., ACNP, CSNP, ACP, and CSP) examined 24– 48 hr or 5 wk post-exposure. On average, Mø accounted for about 94% of the total BAL cell counts, while PMN and lymphocytes made up the remaining 1% and 5%, respectively ( and ). In addition, no significant differences in peripheral blood cell profiles were observed between treatment groups of either parity state for any post-exposure timepoint ( and ). Although a modest increase in circulating PMN was observed in both air- and CS-exposed parous females 24- 48 hr post-exposure compared to exposure-matched, nulliparous mice (), differences were not statistically significant; the same trend was not observed in parous mice examined 5 wk later ().
Figure 2. Effects of cigarette smoke (CS) exposure and parity status on lavageable lung cell profiles at (A) 24– 48 hr and (B) 5 wk post-exposure and, blood cell differential counts at (C) 24– 48 hr and (D) 5 wk post-exposure. A total of three slides were counted and 100 cells/slide. Values represent the mean (n = 6–8 mice/exposure group) ± SE. ACNP = Air control nulliparous; CSNP = Cigarette smoke nulliparous; ACP = Air control pregnant; and CSP = Cigarette smoke pregnant.
Pulmonary histopathology
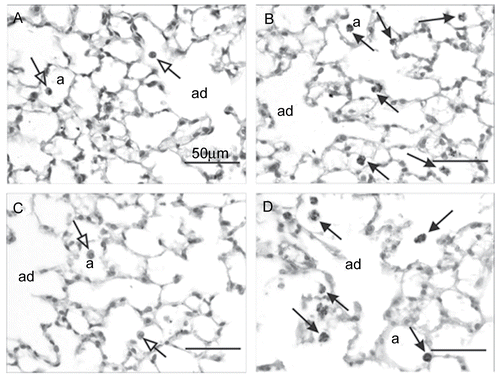
Particle-laden and mildly hypertrophic Mø were observed in all MCS-exposed nulliparous and parous females examined 24– 48 hr post-exposure ( and ), but were absent in similarly exposed mice sacrificed at 5 wk post-exposure. Severity and distribution of these changes in alveolar macrophages were similar in both MCS-exposed groups. No other MCS-exposure-related histologic changes were evident in the lungs of these animals.
Figure 3. Light photomicrographs of alveolar parenchyma from the lungs of filtered air alone- and cigarette smoke (CS)-exposed nulliparous and parous mice 24– 48 hr post-exposure. Alveolar parenchyma is shown from mice in experimental groups ACNP (A), CSNP (B), ACP (C), and CSP (D). A few, widely scattered, histologically normal alveolar macrophages (arrows with open arrowheads) can be seen in the air control mice (A and C). Increased numbers of slightly hypertrophic alveolar macrophages containing phagocytized dark brown/black particles (arrows with closed arrowheads) are apparent in the smoke-exposed groups (B and D). a = alveolar airspace; ad = alveolar duct airspace. No histological features of alveolar inflammation or epithelial proliferation were present in any of the lung sections. Tissues sections were stained with H & E and examined at 100 X magnification.
In vivo tumor challenge
Tumor incidence
Inhalation of MCS, at a concentration calculated to result in a mouse exposure equivalent to that of a human smoking < 1 pack of cigarettes/d and using an exposure regime that could reflect either a workplace or home environment, tended to increase tumor incidence in nulliparous mice injected with EL4 cells 24– 48 hr after the final smoke exposure (compared to parity-matched air control mice). The effect of MCS exposure was significant (p < 0.05) in nulliparous mice when they were first challenged with tumor cells 5 wk after exposure (). A similar effect of MCS on tumor incidence was not observed in the parous females examined at either post-exposure timepoint. Tumor incidence appeared dependent on parity status, particularly for the recently-pregnant air control mice whose tumor incidence increased dramatically (p < 0.01) from their nulliparous counterparts. By 5 wk post-exposure, tumor incidence of the air control “post-parous” females dropped to a level similar to that observed in their time-matched nulliparous air controls.
Figure 4. Effects of cigarette smoke (CS) and parity status on EL4 cell-induced tumor incidence. *Significantly increased (p < 0.05) from time- and parity-matched air controls. #Significantly increased (p < 0.01) from time- and treatment-matched virgin mice. Tumor incidence calculated as ([Number of mice with palpable tumors/Number of mice injected with EL4 cells]) × 100; (n = 8–15 mice injected with EL4 cells/exposure group).
![Figure 4. Effects of cigarette smoke (CS) and parity status on EL4 cell-induced tumor incidence. *Significantly increased (p < 0.05) from time- and parity-matched air controls. #Significantly increased (p < 0.01) from time- and treatment-matched virgin mice. Tumor incidence calculated as ([Number of mice with palpable tumors/Number of mice injected with EL4 cells]) × 100; (n = 8–15 mice injected with EL4 cells/exposure group).](/cms/asset/598b7681-8097-4c49-ab4e-b98e3ae6fd9f/iimt_a_395281_f0004_b.gif)
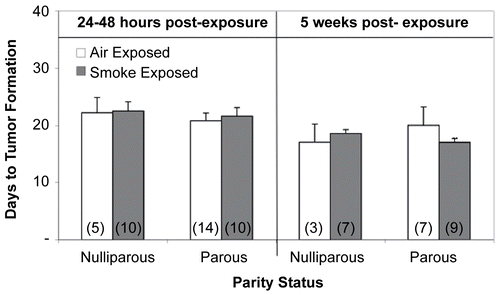
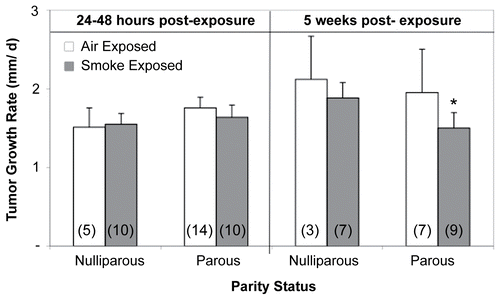
Tumor growth rate and time to tumor formation
Cigarette smoke exposure had no effect on tumor growth rate when nulliparous or parous mice were challenged with lymphoma cells 24- 48 hr after exposure. In contrast, tumors grew significantly slower (p < 0.05) in CS-exposed parous females injected with tumor cells 5 wk post-exposure than those in time-matched air-exposed parous mice or in both nulliparous treatment groups (). Neither parity status, treatment, or time post-exposure had any significant effects on time-to-tumor formation (). On average, tumors became palpable on or near Day 22 for those animals challenged with tumor cells 24– 48 hr post-exposure (ranged from Days 14–22) and at about Day 18 (ranged from Days 11–21) for mice injected at 5 wk post-exposure/parturition.
Figure 5. Effects of cigarette smoke (CS) and parity status on tumor growth rate. *Significantly decreased (p < 0.05) from time-matched air-exposed parous and nulliparous groups. Values represent the mean (n = 3–14 mice/exposure group) ± SE. Values determined from those mice demonstrating palpable tumors. (n) = Number of EL4-injected mice with palpable tumors.
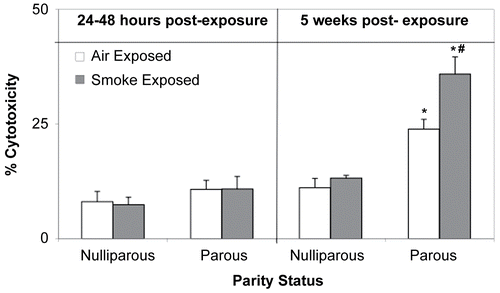
Cytotoxic T-lymphocyte activity
While CTL activity in mice examined 24– 48 hr post-exposure were unaffected by CS exposure or parity status, both factors had significant effects on CTL activity in mice examined 5 weeks after the final smoke exposure (). Cytotoxic activity in both air-and CS-exposed parous mice was significantly elevated (p < 0.01) compared to CTL activity in time- and exposure-matched nulliparous mice, and as compared to values associated with mice in all four treatment groups assessed at the 24– 48 hr timepoint. Moreover, cytotoxic activity measured in the CS-exposed parous females examined at 5 wk was significantly increased (p < 0.01) from that of its parity- and time-matched air control counterpart.
Figure 7. Effects of cigarette smoke (CS) and parity status on cytotoxic T-lymphocyte (CTL) activity. *Significantly increased (p < 0.01) from time- and exposure-matched nulliparous groups. #Significantly increased (p < 0.01) from time- and parity-matched air control mice. Values represent the mean (n = 6–8 mice/exposure group) ± SE.
Discussion
A number of studies exploring the effects of cigarette smoking on female health have revealed such adverse outcomes as early menopause and reduced fertility (http://www.smokefree.hk/cosh/ccs/detail.xml?lang=en&fldrid=197). Still, little is known about the effects on maternal immunocompetence from smoking during pregnancy. The current study examined whether and how pregnancy influences the impact of CS exposure on maternal innate and adaptive immune mechanisms important for tumor surveillance. While inhalation of CS by nulliparous mice increased their susceptibility to transplantable tumors 5 wk after exposure (compared to the air-exposed mice), a similar response was not observed in smoke-exposed parous mice.
Tumor susceptibility of air- and CS-exposed parous mice was dependent upon the length of time following parturition that the animals were injected with lymphoma cells. Air-exposed parous females challenged 24- 48 hr post- parturition demonstrated a greater tumor incidence than their nulliparous counterparts. As labor itself is a highly stressful process associated with large hormone fluctuations, it is possible that early post-partum may represent a time of increased susceptibility to tumor development, as is the case for infection (Lurie et al., Citation2007) and stress-induced changes in immune function (Brabin, Citation1985). Parturition-induced stress could (in part) explain increased tumor receptiveness among the air-exposed parous females immediately after birth and the return to control levels 5 wk later. Injection of tumor cells long after parturition may have allowed sufficient time for maternal hormone and immune stabilization to occur.
Interestingly, unlike the CS-induced increase in tumor susceptibility seen in nulliparous mice, exposure of parous mice (of the same age) to CS had no effect. In human studies, cancer associated with pregnancy is considered a rare occurrence and pregnant women who do develop some forms of cancer are often diagnosed at a later stage compared to their non-pregnant counterparts (Jacobs et al., Citation2004). In addition, it has also been shown that multi- and primiparous females have a lower risk of breast cancer development than nulliparous females due to full differentiation of breast lobules during pregnancy as a result of hormone (i.e., prolactin) stimulation (Russo et al., Citation2005; Ward et al., Citation2005). These studies suggest that the hormonal and immunological changes accompanying pregnancy may serve to protect the mother against tumor development long after parturition. Support for this notion comes from in vitro and in vivo studies, which demonstrate that estrogens can directly induce lysis of sarcoma cells (Boorman et al., Citation1980), and that the estrogen metabolite 2-methoxyesradiol inhibits angiogenesis and suppresses growth of malignant tumors (Lens et al., Citation2004). Likewise, in a study where non-pregnant rats received pregnant rat serum in twice-weekly injections, tumors grew to only 25–60% of those in rats receiving sera from non-pregnant animals (Loachim and Moroson, Citation1986).
Smoke-exposed parous females challenged with tumor cells 5 wk post-parturition demonstrated a slight reduction in tumor incidence compared to time- and/or exposure-matched nulliparous mice; further only, tumors grew at a significantly slower rate than those from exposure-matched nulliparous mice and parity-matched controls. As curious as it may at first appear, CS itself could have acted to “protect” pregnant females from developing tumors via smoke-induced alterations in various hormonal and immunological mechanisms. Specifically, CS has been shown to reduce breast-cancer risk by mediating mechanisms related to aromatase that could then act to alter production of endogenous estrogens (Band et al., Citation2002). Moreover, the CS-induced up-regulation of CTL activity observed in parous mice in this study lends support for these findings. Clearly more research needs to be performed to better understand the relationship between CS, hormone regulation associated with pregnancy, and immune tumor surveillance mechanisms that may act in combination to decrease tumor risk in pregnant females.
Cytotoxic T-lymphocyte activity, critical (among other innate and adaptive immune cell types) for in vivo identification and elimination of certain neoplastically-transformed and malignant cells (House and Thomas, Citation1995), was enhanced in parous control females examined 5 wk post-parturition; anti-tumor activity was even greater in those parous females exposed to CS. Elevated CTL activity in the air control parous mice were not unexpected, as immune and hormonal alterations associated with maintenance of pregnancy or parturition-induced stress may have returned to normal 5 wk after birth. During pregnancy, high concentrations of estrogens have been shown to suppress cell-mediated immunity, possibly through alterations in macrophage-derived pro-inflammatory cytokine expression (Cutolo et al., Citation1996; Zuckerman et al., Citation1996; Gregory, Citation2000).
A number of studies have shown similar up-regulation of certain T-lymphocyte subpopulations in parous humans and rodents. For example, CitationZimmer et al. (1998) demonstrated that CTL (CD3+CD8+) numbers were increased not only in multiparous women (compared to primiparous women), but were also directly correlated with maternal age. Similarly, Chakravarty and Sinha (Citation1992) reported that splenocytes isolated from parous rats two or more weeks post-parturition were significantly more cytotoxic to mammary adenocarcinoma cells than splenocytes from rats recovered 5–13 days after delivery. Other rodent studies demonstrating the direct effects of increased estrogen on T-lymphocyte activity and on numbers of estrogen receptors expressed on CD8+ T-lymphocytes (Cohen et al., Citation1983; Stimson, Citation1988; Amadori and Maltoni, Citation1995) also support the notion that hormones (particularly estrogens) are crucial regulators of T-lymphocyte-mediated tumor surveillance.
Although considered by many to be a potent immunosuppressant (Johnson et al., Citation1990; McAllister-Sistilli et al., Citation1998; Edwards et al., Citation1999), CS has also been shown to stimulate certain immune responses (Francus et al., Citation1992; Friedman et al., Citation2006). A study by Hughes et al. (Citation1985) revealed an increase in both T-helper (TH) and CTL cell numbers in cigarette smokers vs. non-smokers. Results here demonstrating increased CTL activity in CS-exposed parous mice examined 5 wk post-parturition aligned with the aforementioned studies in humans. Furthermore, the increase in CTL activity observed in smoke exposed parous mice taken together with the modest decrease seen in tumor incidence could suggest that CS-induced changes in CTL activity might underlie (at least in part) effects on tumor susceptibility. The fact that tumor incidence cannot be directly correlated with CTL activity in this study is not surprising given that a variety of immune cell types (e.g., NK cells, Mø) and cytokines (e.g., interferon-γ, IL-12) play a critical role in immune surveillance and CTL activity represents only one of several anti-tumor immune functions.
Be that as it may, these findings concerning CTL activity could have important implications for the fetus. As stimulation of cell-mediated immunity by CS could, potentially, shift the TH2 phenotype found normally in pregnant women back towards a TH1 response, and/or lead to the generation of graft-attacking antibodies and CTLs in the expectant mother (Hunt et al., Citation2005), these outcomes could have important implications for the unborn child.
In this study, absolute and relative thymus weight was significantly decreased in both treatment groups of parous mice examined 24- 48 hr post-parturition (in comparison to time- and exposure-matched, nulliparous mice). As the thymus is not only the site of T-lymphocyte development and differentiation, but is also considered to be the primary sex hormone-responsive immune organ (Sobhon and Jirasattham, Citation1974; Rijhsinghani et al., Citation1996; Shames, Citation2002), these effects may have resulted from hormonal fluctuations associated with pregnancy and parturition. Indeed, an early study by Sobhon and Jirasattham (Citation1974) demonstrated that estrogen and progesterone, two major pregnancy-related hormones, can act synergistically to induce thymic atrophy in rodents. Moreover, increased levels of corticosteroids associated with the stress of pregnancy can exert an even more dramatic effect by directly lysing thymic T-cells, ultimately resulting in atrophy (Cupps et al., Citation1982; Meuleman and Katz, Citation1985). Therefore, the decrease in relative thymus weight observed in parous females 24– 48 hr after giving birth is consistent with previous findings and with the notion that endocrine changes associated with pregnancy, such as increased estrogen levels, play a role in alterations of the immune response. In addition, as estrogens have also been reported to enhance B-lymphocyte proliferation and antibody formation (Harbour et al., 1991), the increase in absolute spleen weight in parous females of both treatment groups (compared to that of exposure-matched virgin mice) may have also been related to pregnancy-associated hormonal changes.
In contrast to a number of human and rodent studies showing that smoking increases neutrophil influx and retention in the lungs (Barnes et al., Citation2003; Seagrave et al., Citation2004), no significant pulmonary inflammation was observed here. The discrepancy between the investigations is most likely due to the low concentration of CS (∼ 14 mgTSP/m3; equivalent to smoking < 1 pack of cigarettes/d) used in the current exposures. For example, in a study also using B6C3F1 mice, extensive lung inflammation was observed in animals exposed for 6 wk to CS ( 6 hr/d; 5 d/wk) at a concentration of > 150 mg TSP/m3 (Seagrave et. al., Citation2004), which is a level > 10 times that of the total particulate concentration used here.
This study sought to evaluate what role, if any, pregnancy may have on CS-induced alterations of adaptive immune responses associated with the recognition and destruction of tumor cells. Taken together, results from this investigation indicate that parity status plays an important role in CS-induced immune modulation. Exposure to CS appears to stimulate maternal immune surveillance, possibly by way of cell-mediated pathways. However, such drastic alterations to the maternal immune system during pregnancy can lead to serious fetal consequences. More studies are needed to gain insight into the complex interactions between CS exposure, immune tumor surveillance, and pregnancy. By uncovering the underlying endocrine-immune links involved in pregnancy and parturition, a better understanding of the effects of CS exposure during this vulnerable time period on maternal and fetal health could emerge.
Acknowledgments
This research was supported by Philip Morris USA Inc. and Philip Morris International, and (in part) by New York University (NYU) National Institute of Environmental Health Sciences (NIEHS) Center Grant (ES00260). The authors would like to thank Shannon Doherty, Carol Hoffman, and Sally Lasano for their technical assistance, Dr. Maarten Bosland for some histological analyses, and Dr. Bernard Steinetz for his valuable input on gestational measurements.
Declaration of interest: None of the authors has any conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
References
- Albrecht, S. A., Maloni, J. A., Thomas, K. K., Jones, R., Halleran, J., and Osborne, J. 2004. Smoking cessation counseling for pregnant women who smoke: Scientific basis for practice for AWHONN’s SUCCESS project. J. Obstet. Gynecol. Neonatal Nursing: JOGNN/NAACOG 33:298–305.
- Amadori, D., and Maltoni, R. 1995. Role of biological indicators in the therapeutic decision in breast carcinoma. Chirurgia Ital. 47:15–22.
- Aoshiba, K., Tamaoki, J., and Nagai, A. 2001. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am. J. Physiol. Lung Cell. Molec. Physiol. 281:1392–1401.
- Band, P. R., Le, N. D., Fang, R., and Deschamps, M. 2002. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet 360:1044–1049.
- Barnes, P. J., Shapiro, S. D., and Pauwels, R. A. 2003. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Resp. J. 22:672–688.
- Boorman, G. A., Luster, M. I., Dean, J. H., and Wilson, R. E. 1980. The effect of adult exposure to diethylstilbestrol in the mouse on macrophage function and numbers. J. Reticulo endothel Soc. 28:547–560.
- Brabin, B. J. 1985. Epidemiology of infection in pregnancy. Rev. Infect. Dis. 7:579–603.
- Burleson, F. G., Chambers, T. M., and Wiedbrauk, D. L., Eds. 1992. Virology: A Laboratory Manual. Academic Press: London.
- Chakravarty, P. K., and Sinha, D. K. 1992. Pregnancy-induced anti-tumor cytotoxicity of T-cell-rich fraction against mammary adenocarcinoma cells in rats. Carcinogenesis 13:2449–2452.
- Cheng-Smart, Y. J., Cox, T. K., Roberts, M. W., Brinsmead, and Burton, R. C. 1986. Differential effect of cigarette smoking on recirculating T-lymphocyte subsets in pregnant women. J. Immunol. 137:1–8.
- Cohen, J. H., Danel, L., Cordier, G., Saez, S., and Revillard, J. P. 1983. Sex steroid receptors in peripheral T-cells: Absence of androgen receptors and restriction of estrogen receptors to OKT8-positive cells. J. Immunol. 131:2767–2771.
- Cupps, T. R., Edgar, L. C., and Fauci, A. S. 1982. Corticosteroid-induced modulation of immunoglobulin secretion by human B-lymphocytes: Potentiation of background mitogenic signals. J. Immunopharmacol. 4:255–263.
- Cutolo, M., Accardo, S., Villaggio, B., Barone, A., Sulli, A., Coviello, D. A., Carabbio, C., Felli, L., Miceli, D., Farruggio, R., Carruba, G., and Castagnetta, L. 1996. Androgen and estrogen receptors are present in primary cultures of human synovial macrophages. J. Clin. Endocrinol. Metabol. 81:820–827.
- Edwards, K., Braun, K. M., Evans, G., Sureka, A. O., and Fan, S. 1999. Mainstream and sidestream cigarette smoke condensates suppress macrophage responsiveness to interferon-gamma. Hum. Exper. Toxicol. 18:233–240.
- Francus, T., Romano, P. M., Manzo, G., Fonacier, L., Arango, N., and Szabo, P. 1992. IL-1, IL-6, and PDGF mRNA expression in alveolar cells following stimulation with a tobacco-derived antigen. Cell. Immunol. 145:156–174.
- Friedman, H., Pross, S., and Klein, T. W. 2006. Addictive drugs and their relationship with infectious diseases. FEMS Immunol. Med. Microbiol. 47:330–342.
- Gregory, K. D. 2000. Monitoring, risk adjustment and strategies to decrease cesarean rates. Curr. Opin. Obstet. Gynecol. 12:481–486.
- Harbour, D. V., Smith, E. M., and Blalock, J. E. 1987. Splenic lymphocyte production of an endorphin during endotoxic shock. Brain Behav. Immun. 2:123–133.
- House, R. V., and Thomas, P. T. 1995. In vitro induction of cytotoxic T-lymphocytes. In: Methods in Immunotoxicology (Burleson, G., Dean, J., and Munson, A., Eds.). Wiley-Liss: New York, pp. 159–171.
- Hughes, D. A., Haslam, P. L., Townsend, P. J., and Turner-Warwick, M. 1985. Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clin. Exper. Immunol. 61:459–466.
- Hughes, E. G., and Brennan, B. G. 1996. Does cigarette smoking impair natural or assisted fecundity? Fertil. Steril. 66:679–689.
- Hunt, J. S., Petroff, M. G., McIntire, R. H., and Ober, C. 2005. HLA-G and immune tolerance in pregnancy. FASEB J. 19:681–693.
- Loachim, H. L., and Moroson, H. 1986. Protective effect of pregnancy against transplantation of lymphoma in rats. J. Natl. Cancer Inst. 77:809–814.
- Jacobs, I. A., Chang, C. K., and Salti, G. I. 2004. Co-existence of pregnancy and cancer. Am. Surg. 70:1025–1029.
- Johnson, J. D., Houchens, D. P., Kluwe, W. M., Craig, D. K., and Fisher, G. L. 1990. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: A review. Crit. Rev. Toxicol. 20:369–395.
- Kalra, R., Singh, S. P., Savage, S. M., Finch, G. L., and Sopori, M. L. 2000. Effects of cigarette smoke on immune response: Chronic exposure to cigarette smoke impairs antigen-mediated signaling in T-cells and depletes IP3-sensitive Ca2+ stores. J. Pharmacol. Exp. Ther. 293:166–171.
- Lens, M. B., Rosdahl, I., Ahlbom, A., Farahmand, B. Y., Synnerstad, I., Boeryd, B., and Newton Bishop, J. A. 2004. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J. Clin. Oncol. 22:4369–4375.
- Lurie, G., Thompson, P., McDuffie, K. E., Carney, M. E., Terada, K. Y., and Goodman, M. T. 2007. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet. Gynecol. 109:597–607.
- McAllister-Sistilli, C. G., Caggiula, A. R., Knopf, S., Rose, C. A., Miller, A. L., and Donny, E. C. 1998. The effects of nicotine on the immune system. Psychoneuroendocrinology 23:175–187.
- McCue, J. M., Link, K. L., Eaton, S. S., and Freed, B. M. 2000. Exposure to cigarette tar inhibits ribonucleotide reductase and blocks lymphocyte proliferation. J. Immunol. 165:6771–6775.
- Meuleman, J., and Katz, P. 1985. The immunologic effects, kinetics, and use of glucocorticosteroids. Med. Clin. N. Amer. 69:805–816.
- Ng, S. P., Silverstone, A. E., Lai, Z. W., and Zelikoff, J. T. 2006a. Effects of prenatal exposure to cigarette smoke on offspring tumor susceptibility and associated immune mechanisms. Toxicol. Sci. 89:135–144.
- Ng, S. P., Steinetz, B. G., Lasano, S. G., and Zelikoff, J. T. 2006b. Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Exp. Biol. Med. 231:1403–1409.
- Ng, S. P., and Zelikoff, J. T. 2008. The effects of prenatal exposure of mice to cigarette smoke on offspring immune parameters. J. Toxicol. Environ. Health 71: 445–453.
- Oltmanns, U., Chung, K. F., Walters, M., John, M., and Mitchell, J. A. 2005. Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Resp. Res. 6:74–79.
- Rijhsinghani, A. G., Bhatia, S. K., Tygrett, L. T., and Waldschmidt, T. J. 1996. Effect of pregnancy on thymic T-cell development. Am. J. Reprod. Immunol. 35:523–528.
- Rioux, N., and Castonguay, A. 1997. Recovery from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced immunosuppression in A/J mice by treatment with non-steroidal anti-inflammatory drugs. J. Natl. Cancer Inst. 89:874–880.
- Rioux, N., and Castonguay, A. 2001. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone modulation of cytokine release in U937 human macrophages. Cancer Immunol. Immunother. 49:663–670.
- Robbins, C. S., Dawe, D. E., Goncharova, S. I., Pouladi, M. A., Drannik, A. G., Swirski, F. K., Cox, G., and Stampfli, M. R. 2004. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Amer. J. Resp. Cell and Mol. Biol. 30:202–211.
- Russo, J., Moral, R., Balogh, G. A., Mailo, D., and Russo, I. H. 2005. The protective role of pregnancy in breast cancer. Breast Cancer Res. 7:131–142.
- Seagrave, J., Barr, E. B., March, T. H., and Nikula, K. J. 2004 Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp. Lung Res. 30:1–15.
- Shames, R. S. 2002. Gender differences in the development and function of the immune system. J. Adolesc. Health 30:59–70.
- Sobhon, P., and Jirasattham, C. 1974. Effect of sex hormones on the thymus and lymphoid tissue of ovariectomized rats. Acta Anatomica 89:211–225.
- Sopori, M. 2002. Effects of cigarette smoke on the immune system. Nature Rev. Immunol. 2:372–377.
- Stimson, W. H. 1988. Oestrogen and human T-lymphocytes: Presence of specific receptors in the T-suppressor/cytotoxic subset. Scand. J. Immunol. 28:345–350.
- Ward, E., Jemal, A., and Thun, M. 2005. Regarding “Increase in breast cancer incidence in middle-aged women during the 1990s”. Ann. Epidemiol. 15:424–427.
- Windham, G. C., Mitchell, P., Anderson, M., and Lasley, B. L. 2005. Cigarette smoking and effects on hormone function in premenopausal women. Environ. Health Perspect. 113:1285–1290.
- Zimmer, J. P., Garza, C., Heller, M. E., Butte, N., and Goldman, A. S. (1998). Post-partum maternal blood helper T (CD3+CD4+) and cytotoxic T (CD3+CD8+)-cells: Correlations with iron status, parity, supplement use, and lactation status. Am. J. Clin. Nutr. 67:897–904.
- Zuckerman, S. H., Ahmari, S. E., Bryan-Poole, N., Evans, G. F., Short, L., and Glasebrook, A. L. 1996. Estriol: A potent regulator of TNF and IL-6 expression in a murine model of endotoxemia. Inflammation 20:581–597.