Abstract
Chronic beryllium disease (CBD), an irreversible, debilitating granulomatous lung disease is caused by exposure to beryllium. This occupational hazard occurs in primary production and machining of Be-metal, BeO, beryllium - containing alloys, and other beryllium products. CBD begins as an MHC Class II-restricted, TH1 hypersensitivity, and the Human Leukocyte Antigen, HLA-DPB1E69, is associated with risk of developing CBD. Because inbred strains of mice have not provided good models of CBD to date, three strains of HLA-DPB1 transgenic mice in an FVB/N background were developed; each contains a single allele of HLA-DPB1 that confers a different magnitude of risk for chronic beryllium disease: HLA-DPB1*0401 (OR ≈ 0.2), HLA-DPB1*0201 (OR ≈ 3), and HLA-DPB1*1701 (OR ≈ 46). The mouse ear swelling test (MEST) was employed to determine if these different alleles would support a hypersensitivity response to beryllium. Mice were first sensitized on the back and subsequently challenged on the ear. In separate experiments, mice were placed into one of three groups (sensitization/challenge): C/C, C/Be, and Be/Be. In the HLA-DPB1*1701 mice, the strain with the highest risk transgene, the Be/Be group was the only group that displayed significant maximum increased ear thickness of 19.6% ± 3.0% over the baseline measurement (p < 0.05). No significant changes were observed in the other transgenic strains for any treatment condition. In addition, inter-strain differences in response to beryllium in seven inbred strains were investigated through use of the MEST, these included: FVB/N, AKR, Balb/c, C3H/HeJ, C57/BL6, DBA/2, and SJL/J. The FVB/N strain was least responsive, while the SJL/J and C57/BL6 strains were the highest responders. Our results suggest that the HLA-DPB1*1701 transgene product is an important risk factor for induction of the beryllium-sensitive phenotype. This model should be a useful tool for investigating beryllium sensitization.
Introduction
Beryllium, an alkaline earth metal, is located in the 4th position on the periodic table of elements with an atomic weight of 9.012 and occurs naturally in the environment. It is lightweight, but is stiffer than steel. It is non-sparking, non-corrosive, a neutron moderator, and anti-galling, and has a high melting point. Owing to its unique physicochemical properties, beryllium is a strategic metal, indispensable for national defense programs and weaponry, and has applications in telecommunications, electronics, aerospace, and biomedicine. Workers in these industries can be exposed to beryllium dust or fumes during manufacturing and machining processes. Exposure to beryllium has been highly documented in epidemiological studies and has been shown to cause an irreversible, debilitating granulomatous lung disease, chronic beryllium disease (CBD), in as many as 3–5% of exposed workers (Kriess et al., 1996, 1997; Henneberger et al., Citation2001). While epidemiological studies have pointed to particle inhalation as the chief route of exposure, Tinkle et al. (Citation2003) also demonstrated, in an ex vivo model, the ability of beryllium particles to penetrate intact human skin and, in an in vivo model, for dermally-applied particles to produce sensitization in the mouse ear swelling test (MEST).
Due to the difficulty in developing a CBD animal model that duplicates the human pathobiology, mechanistic research on beryllium exposure-disease relationships has been limited. Epidemiological studies have shown a genetic susceptibility component to CBD; those with the Human Leukocyte Antigen (HLA) – DPB1 Glu69 variant are at an increased risk to developing the disorder (Richeldi et al., Citation1993, Citation1997; Wang et al., Citation1999; Saltini et al., Citation2001; Rossman et al., Citation2002; Maier et al. Citation2003; McCanlies et al. Citation2004; Snyder et al. Citation2008). We hypothesized that insertion of a Human Leukocyte Antigen (HLA) – DPB1 E69 gene would result in mice that display the phenotype of increased sensitivity to beryllium. Previously, Chapoval et al. (Citation1998) have shown that when mouse lines were developed with human MHC Class II DQ6 and DQ8 genes, the sensitivity to ragweed allergens phenotypes that developed, were similar to those found in humans with those genes.
In the present study, three transgenic strains of mice were created incorporating the human antigen presenting moiety, HLA – DPB1 E69, hypothesizing that this would provide correct beryllium presentation to T-lymphocytes sufficient for subsequent pulmonary granuloma formation. We also aimed to develop a mouse that did not have the glutamic acid in the 69th position, but a lysine, which does not correlate with such an increased risk. Each of the three strains contains a different allele of the gene which confers a different magnitude of risk in developing chronic beryllium disease: HLA-DPB1*0401 (OR ≈ 0.2), HLA-DPB1*0201 (OR ≈ 3), and HLA-DPB1*1701 (OR ≈ 46) (Weston et al., Citation2005). The HLA-DPB1*0401 gene carries the lysine in the 69th position, while the HLA-DPB1*0201 and HLA-DPB1*1701 each have the glutamic acid at this site, (E69) which, in epidemiological studies, correlates with an increased risk of development of CBD. The difference in the magnitude of risk between HLA-DPB1*0201 and HLA-DPB1*1701 is explained by characteristics of the molecules for which they code other than E69, and evidence has been found that the more negatively charged HLA-DPB1E69 proteins confer greatest risk of CBD (Snyder et al., Citation2008).
To determine whether these genetically modified mice would have functional expression of the transgene, a MEST was employed to measure hypersensitivity responses to beryllium. Mice were first sensitized with either the control vehicle or beryllium, followed by challenge with vehicle or beryllium, and the thickness of the mouse’s right ear was measured pre, during, and post-challenge. We also examined the hypersensitivity response to beryllium in six inbred strains of mice in addition to the FVB/N mice, the background strain for the transgenic mice. By determining whether inbred strains (FVB/N, AKR, Balb/c, C3H/HeJ, C57/BL6, DBA/2, and SJL/J) produced different responses in the MEST, we could begin to establish whether host factors can modulate the sensitization response to beryllium.
Materials and methods
Animals
Female and male FVB/N, AKR, Balb/c, C3H/HeJ, C57/BL6, DBA/2, and SJL/J mice (7–9-wk-of-age) were purchased from The Jackson Laboratory (Bar Harbor, ME). The FVB/N transgenic mouse strains containing different alleles HLA-DPB1*0401, HLA-DPB1*0201, and HLA-DPB1*1701, were developed using previously described methods. Three human BAC libraries were screened for DPA1 and DPB1 genes. BAC clones containing DPA1 and/or DPB1 were genotyped, but none were found to contain the HLA-DPB1*1701 allele. Among the 24 human DNA samples, the sequence of one allele (HLA-DPB1*1401) was very close to HLA-DPB1*1701 allele, with only four nucleotides different within a 36 bp region, thus reflecting a three amino acid difference in hypervariable regions D and E. Therefore, the HLA-DPB1*1701 allele was prepared by site-directed mutagenesis of the HLA-DPB1*1401 allele. A 456 bp fragment containing exon 2 (264 bp) of HLA-DPB1*1401 was cloned into a TOPO TA vector and 48 bp primers were synthesized according to the HLA-DPB1*1701 sequence. Exon 2 of HLA-DPB1*1401 was mutagenized into exon 2 of HLA-DPB1*1701 using QuickChange XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA).
Exon 2 of HLA-DPB1*1701 generated by mutagenesis was used to substitute for HLA-DPB1*1401 in subclones using the unique restriction sites NarI and SacII surrounding exon 2. Next, for each transgene, a 32 kb (approximate) EagI-FspI fragment from pWEB subclones or a 32 kb EagI fragment from pBe1BAC11 subclones was gel-purified and then injected into mouse fertilized eggs to generate transgenic mice in the FVB/N background. The expression levels: (ie, mRNA abundance levels) of HLA-DPB1 genes in each founder mouse were measured by TaqMan RT-PCR using total leukocyte RNA purified from 200-300 μl of tail blood. Expression data were normalized to endogenous 18S (QuantumRNA 18S Internal Standards, Ambion, Austin, TX) expression levels, and for each transgene, the three highest expression lines were bred with FVB/N mice to generate F1 transgenic mice for studying and line maintenance.
All mice were acclimated for at least 1 wk before exposure and housed in a positive pressure environment with a 12-hr light/dark cycle starting at 6:00 A.M. All mice were provided with water and standard rodent chow (Purina, Indianapolis, IN) ad libitum under an IACUC-approved protocol.
Experimental design
A 1.5 × 3. 0 cm area was shaved on the backs of all mice (Stylique Designer/Liner pet trimmer, Wahl, Shelton, CT) on Day 0. On Days 1, 2, and 3, mice were treated with either 50 μl dibutyl pthalate (DBPT) and 50 μl MilliQ H2O [control/control (sensitization/challenge) and control/Be groups] or 50 μl DBPT and 50 μl 1 M BeSO4 (Sigma-Aldrich, St. Louis, MO). On Day 8, a baseline ear thickness measurement was taken with a digital micrometer (Model #700-118, Mitymuto, Japan) as previously described (Tinkle et al., Citation2003). Following the baseline measurement on Day 8, control/control animals were challenged on the right ear with 25 μl DBPT and 25 μl MilliQ H2O; control/Be and Be/Be animals were challenged on the right ear with 25 μl DBPT and 25 μl 1 M BeSO4. On Day 9, the thickness of the right ear was measured and then again challenged with either 25 μl DBPT and 25 μl MilliQ H2O (control/control) or 25 μl DBPT and 25 μl 1 M BeSO4 (control/Be and Be/Be groups). Right ear measurements were repeated on Days 10 and 11. After observing that the control/Be group did not produce significantly different results than the control/control group, for the mouse ear swelling test for the inbred mouse strains, only two groups were used: control/control and Be/Be.
Data analysis
To evaluate changes in the ear thickness of mice of different strains, all values are presented as the mean thickness ± SE. For statistical purposes, we analyzed the data using the maximum increase in swelling without regard to the day on which it occurred. Differences between means were assessed using the SAS statistical package (Cary, NC) incorporating individual Welch modified two-sample t-tests, which does not make the assumption that the variances are the same. The Welch modified two-sample t-tests were used to assess the inbred mouse MEST. When there were three groups (C/C, C/Be, and Be/Be), a one-way ANOVA was followed by a post-hoc Dunnett’s test. Statistical significance was defined at p ≤ 0.05.
Results
MEST in transgenic mice
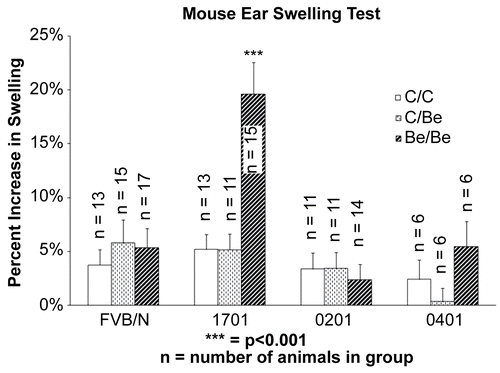
In the first study, the MEST was performed on the three different transgenic strains and their background FVB/N strain. The hypersensitivity response was significantly increased (p < 0.001) over control values in the HLA-DPB1*1701 transgenic strain only. Using the maximum increase in ear thickness, regardless of the measurement day, the transgenic HLA-DPB1*1701 mice experienced an increased ear swelling of 19.6% (± 3.0%; mean ± SE) above baseline (). Taking time of response into account, the mean increased thickness peaked on Day 11 ( 48 hr post-challenge) at 14.3% (± 3.4%) above baseline ear measurement. However, a greater number of animals had their peak response 24 hr after the final challenge (Day 10) as opposed to 48 hr post-exposure (Day 11). On Day 12, 72 hr post-challenge, the increased thickness in the HLA-DPB1*1701 strain had subsided somewhat from its highest level to 12.8% (± 3.1%) above baseline. The other transgenic strains (HLA-DPB1*0201 and HLA-DPB1*0401) were not significantly different than the FVB/N control animals when taking the maximum increase into account ().
Strain-dependent differences in the MEST
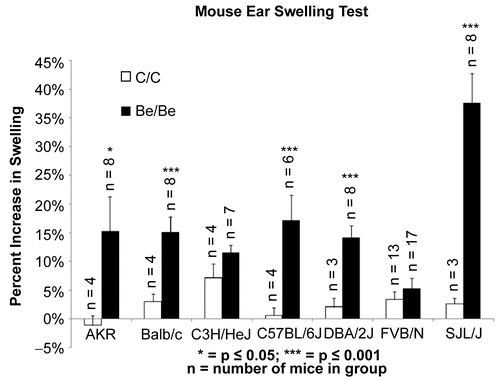
A total of seven informative inbred mouse strains (AKR, Balb/c, C3H/HeJ, C57/BL6, DBA/2, FVB/N, and SJL/J) were also tested in the MEST to examine differences in response amongst the seven strains. Mice were treated with either control/control or Be/Be as described above. Most of the mice had their greatest response 48 hr post-treatment (Day 11). While the SJL/J had the highest maximum increase (without regard to day which this occurred) of 38.7% (± 6.9%) () over baseline, some of the SJL/J mice also had accompanying irritation (redness) at the treatment site on the ear. Beryllium sensitized and challenged Balb/c, C57/BL6 AKR, and DBA/2 strains also had significant (p < 0.001) swelling over their control counterparts. The FVB/N and C3H strains had the smallest changes in ear thickness (not statistically significant).
Figure 2. Beryllium-induced ear swelling in inbred mouse strains. The greatest statistically significant difference from control was seen in the SJL/J, Balb/C, C57BL/6J, and DBA/2J. The strains that did not have a statistically significant increase in treatment over control were C3H/HeJ and FVB/N.
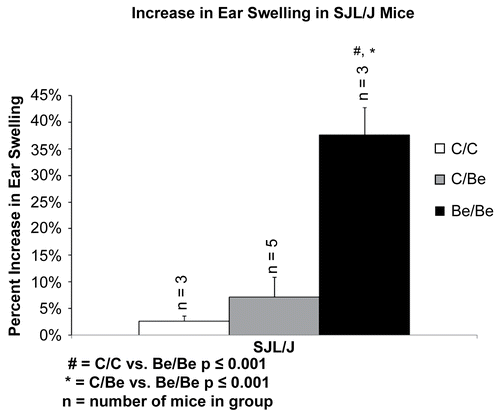
To ensure that the observed increase in ear swelling was due to an actual beryllium hypersensitivity response and not simply skin irritation to the application site of beryllium on the ear, a subsequent experiment was performed. Using SJL/J mice, chosen because they were amongst the highest responders, a MEST was repeated where mice were exposed to sensitization by vehicle on the back followed by a challenge of beryllium on the ear. shows there was not a significant difference between the SJL/J control/control group and the SJL/J control/beryllium mice.
Figure 3. Beryllium-induced ear swelling in SJL/J mice. The SJL/J mice were the highest responding strain in the inbred mouse ear swelling test. To ensure that the response was due to beryllium and not irritation, a second control group, (C/Be) was tested. In the C/Be group mice were sensitized on the back with vehicle (DBPT and water) and then challenged on the ear with beryllium (DPBT and BeSO4). Both controls were statistically significant from the treatment group, but there was no statistical difference between the control groups.
Discussion
The etiology of CBD has been difficult to determine because, like many lung diseases such as asthma and chronic obstructive pulmonary disease, it is likely that CBD has a polygenic basis. Yet, genes alone do not determine the outcome of an individual; there is a complex interaction between multiple environmental stimuli with the predetermined genetic (host) background. Understanding the relationship of these gene-environment interactions is challenging but will provide greater understanding into the disease process. Based upon our experiments in inbred and transgenic strains of mice, it is likely that there are unknown genes that modify the beryllium hypersensitivity response in different inbred mouse strains. Currently, a beryllium-specific lymphocyte proliferation test (BeLPT) developed by Williams and Williams in 1982 is performed on employees exposed to beryllium.
After the development of the BeLPT, it was shown that anti-MHC II antibodies had the ability to block lymphocyte proliferation in vitro, suggesting that there is an immunogenetic basis to this disease (Saltini et al., Citation1989). More recently, epidemiological case-control studies have linked the HLA-DPB1 allele to the development of chronic beryllium disease (Richeldi et al., Citation1993, Citation1997; Wang et al., Citation1999; Saltini et. al., Citation2001; Rossman et al., Citation2002; Maier et al., Citation2003; McCanlies et al., Citation2004). However, until now, an animal study using mice containing an inserted human transgene to support the epidemiological studies had not been conducted. Our work with the HLA-DPB1*1701 mice in the FVB/N background now provides a compelling animal model to examine sensitization. Previous observations that were based solely on epidemiological findings can now be tested in this transgenic mouse strain.
Wang et al. (Citation1999) noted that although over 95% of CBD patients had the glutamic acid encoded in the 69th position in their HLA-DBP1 protein, 30–45% of the control group which was exposed to beryllium but not affected, also carried this same glutamic acid. This observation suggests that it is not merely the presence of the glutamic acid encoded in the 69th position that is responsible for the hypersensitive phenotype, but it is a subset of alleles that contain the Glu69 which may play a larger role in determining susceptibility for disease development. Our findings support those of Wang et al. (Citation1999, Citation2001) that the rare HLA-DPB1E69 alleles are more important for sensitization than the HLA-DPB1*02 family of alleles. In the epidemiological study, the HLA-DPB1*1701 transgene is rarely observed in people who are exposed to beryllium but not sensitized, and much more prevalent in those who are in the beryllium sensitized group. These findings may explain the epidemiological studies and may describe why we saw a significant increase in ear swelling in the HLA-DPB1*1701 mice but not in the HLA-DPB1*0201 mice. We did not see a difference in the HLA-DPB1*0401 strain when compared to the control mice; this was expected since HLA-DPB1*0401 codes for the positively-charged lysine at the 69th position instead of the negatively-charged glutamic acid.
Our studies indicate that a functional transgene has been inserted into the HLA-DPB1*1701 transgenic mice. This transgene results in the phenotypic response of a greater ear swelling in the transgenic mice over that of their parental control FVB/N strain. While this phenotypic response was seen in the transgenic strain with the highest reported odds ratio in epidemiology studies (HLA-DPB1*1701, OR ≈ 46), it was not seen in the other transgenic strains with lower odds ratios (HLA-DPB1*0401, OR ≈ 0.2; HLA-DPB1*0201, OR ≈ 3). It should be noted that these odds ratios were determined from allele frequencies from relatively small case-control studies (Weston et al., Citation2005). Thus, they provide a ranking, and the actual ORs should be considered estimates. A more solid understanding of the ORs associated with specific groups of HLA-DPB1E69 alleles was determined recently by (Snyder et al., Citation2008).
It has been shown that HLA-B1DPB1E69 is capable of binding beryllium with a 40–100-fold higher affinity than when lysine is in the 69th position (Amicosante et al., Citation2001). Because HLA-DPB1*0401 codes for lysine at the 69th position, and not glutamic acid, it is understandable that the risk associated with the HLA-DPB1*0401 transgene was not sufficient to produce an observable increase in the ear swelling phenotype in beryllium-treated mice. In addition, it is possible that despite the successful demonstration of HLA-DPB1*0201 transgene insertion by PCR analysis, the gene product of this HLA-DPB1 transgene was not functionally expressed:, thus resulting in a lack of heightened response in HLA-DPB1*0201 allele, as stated previously, is not as potent in determining beryllium sensitivity. Future studies are necessary to delineate the functional expression of the protein product of this human transgene in antigen presenting cells in the mouse.
It is interesting to note that the FVB/N strain, the background strain of the transgenic mice, had the lowest increase in ear swelling among the seven tested inbred strains. This suggests that the effect of the Be/Be treatments in these mice is solely due to the transgene and not the genetic background of the FVB/N strain. Future work could include placement of the transgene in an inbred strain background that had greater ear swelling than that of the FVB/N strain. However, it is reasonable to believe that the current FVB/N strain is the optimal strain for placement of the transgene as the effects can be attributed to the transgene alone and not other contributing host factors for the ear swelling phenotype. The AKR, Balb/c, C57/BL6, DBA/2, and SJL/J inbred strains all had significantly increased ear swelling over their control counterparts, thus suggesting that in these strains there exist host genetic factors that modify the immune response to beryllium. Different genetic backgrounds may augment the effect of the HLA-DPB1*1701 transgene. This finding would correlate with human epidemiologic studies where individuals with the same HLA-DPB1 allele have varying sensitivity to beryllium even with similar exposure levels indicating there are other modifier genes or host factors involved in the beryllium sensitization process.
The major objective of our research is to determine the genetic basis for beryllium hypersensitivity using a mouse model with many inbred mouse strains. Inter-strain interspecies differences in the pulmonary response to specific particle exposures have been noted before (Ichinose et al., Citation1997; Miyabara et al., Citation1998; Ohtsuka et al., Citation2000; Wesselkamper et al., Citation2005). To address the beryllium susceptibility question, we examined the mouse ear swelling response as a hypersensitivity proxy in inbred and transgenic mouse strains. Inbred strains are almost genetically homogeneous within a strain (99.9%) resulting from inbreeding mice, using brother-sister pairs, over many generations (Silver, Citation1995). Strains with a greater evolutionary divergence will have a greater degree of polymorphisms than two strains that are closer together on a phylogenetic tree (Tsang et al., Citation2005). The inbred strain-dependent differences we observed in beryllium sensitivity in the mouse ear swelling test suggest that one or more loci contribute to the genetic variance observed among the strains. Furthermore, since we saw differences in responses in inbred mouse strains which lack, of course, the human HLA-DPB1*1701 transgene, it may be concluded that there are modifier genes, yet to be uncovered, which contribute to the observed beryllium hypersensitivity response.
Surprisingly, although FVB/N mice had no response to beryllium in the ear swelling assay, SJL/J mice, a genetically- related strain on a mouse phylogenetic tree (Tsang et al., Citation2005), had the greatest response. Examining where these two strains are genetically divergent may elucidate parts of the mouse genome that are responsible for phenotypic differences in the beryllium hypersensitivity response.
One appropriate extension of this project would be to examine the ear swelling response of a greater number of strains with known phylogeny and genetic data. CBD is a multigenic disease; assessing the hypersensitivity response to beryllium in many strains of mice could lend itself to examining the data through an in silico approach aiming at arriving at other genes responsible in the beryllium sensitization process. In addition, to complement the skin sensitization study, ongoing oropharangeal aspiration studies with many different inbred strains of mice are being conducted to look at the granuloma formation process, which is the central disease feature of chronic beryllium disease. As in the skin sensitization study, significant interstrain differences in beryllium-induced lung granulomas are observed (unpublished data).
Funding
Department of Energy (DE-FG02-04ER63919); National Institute of Environmental Health Sciences (ES011473).
Acknowledgments
We would like to thank Karen Galdanes for her expertise in the lab.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of NIOSH.
References
- Amicosante, M., Sanarico, N., Berretta, F., Arroyo, J., Lombardi, G., Lechler, R., Colizzi, V., Saltini, C. 2001. Beryllium binding to HLA-DP molecule carrying the marker of susceptibility to berylliosis glutamate β69. Human Immunol. 7:686–693.
- Chapoval, S. P., Neeno, T., Krco, C. J., Marietta, E. V., Harders, J., and David, C. S. 1998. HLA-DQ6 and HLA-DQ8 transgenic mice respond to ragweed allergens and recognize a distinct set of epitopes on short and giant ragweed Group 5 antigens. J. Immunol. 161:2032–2037.
- Henneberger, P.K., Cumro, D., Deubner, D.D., Kent, M.S., McCawley, M., and Kreiss, K. 2001. Beryllium sensitization and disease among long-term and short-term workers in beryllium ceramics. Int. Arch. Occup. Environ. Health 74:167–176.
- Ichinose, T., Takano, H., Miyabara, Y., Yanagisawa, R., and Sagai, M. 1997. Murine strain differences in allergic airway inflammation and immunoglobulin production by a combination of antigen and diesel exhaust particles. Toxicology 122:183–192.
- Kreiss, K., Mroz, M. M., Newman, L. S., Martyny, J. W., and Zhen, B. 1996. Machining risk of beryllium disease and sensitization with median exposures below 2 μg/m3. Am. J. Ind. Med. 30:16–25.
- Kreiss, K., Mroz, M. M., Zhen, B., Wiedemann, H., and Barna, B. 1997. Risks of beryllium disease related to work processes at a metal, alloy, and oxide production plant. Occup. Environ. Med. 54:605–612.
- Maier, L. A., McGrath, D., Sato, H., Lympany, P., Welsh, K., du Bois, R., Silveira, L., Fontenot, A. P., Sawyer, R. T., Wilcox, E., and Newman, L. S. 2003. Influence of MHC Class II in susceptibility to beryllium sensitization and Chronic Beryllium Disease. J. Immunol. 171:6910–6918.
- McCanlies, E.C., Ensey, J.S., Schuler C.R., Kreiss, K., Weston, A. 2004. The association between HLA-DPB1Glu69 and chronic beryllium disease and beryllium sensitization. American Journal of Industrial Medicine. 46: 95–103.
- McCanlies, E., Ensey, J., Schuler, C., Kreiss, K., and Weston, A. 2004. The association between HLA-DPB1Glu69 and chronic beryllium disease and beryllium sensitization. Am. J. Ind. Med. 2:95–103.
- Miyabara, Y., Yanagisawa, R., Shimojo, N., Takano, H., Lim, H. B., Ichinose, T., and Sagai, M. 1998. Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur. Resp. J. 11:291–298.
- Ohtsuka, Y., Clarke, R. W., Mitzner, W., Brunson, K., Jakab, G. J., and Kleeberger, S. R. 2000. Inter-strain variation in murine susceptibility to inhaled acid-coated particles. Am. J. Physiol. 278:L469–L476.
- Richeldi, L., Sorrentino, R., and Saltini, C. 1993. HLA-DPB1 glutamate 69: A genetic marker of beryllium disease. Science 262:242–244.
- Richeldi, L., Kreiss, K., Mroz, M.M., Zhen, B., Tartoni, P., Saltini, C. 1997. Interaction of genetic and exposure factors in the prevalence of berylliosis. American Journal of Industrial Medicine. 32:337–340.
- Richeldi, L., Kreiss, K., Mroz, M., Zhen, B., Tartoni, P., and Saltini, C. 1997. Interaction of genetic and exposure factors in the prevalence of berylliosis. Am. J. Ind. Med.4:337–340.
- Rossman, M. D., Stubbs, J., Lee, C. W., Argyris, E., Magira, E., and Monos, D. 2002. Human leukocyte antigen Class II amino acid epitopes: Susceptibility and progression markers for beryllium hypersensitivity. Am. J. Resp. Crit. Care Med. 165:788–794. http://www.jimmunol.org/cgi/ ijlink?linkType=ABST&journalCode=ajrccm&resid=165/6/788
- Saltini, C., Winestock, K., Kirby, M., Pinkston, P., and Crystal, R.G., 1989. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T-cells. New Engl. J. Med. 320:1103–1109.
- Saltini, C., Richeldi, L., Losi, M., Amicosante, M., Voorter, C., van den Berg-Loonen E., Dweik, R. A., Wiedemann, H. P., Deubner, D. C., and Tinelli, C. 2001. Major histocompati-bility locus genetic markers of beryllium sensitization and disease. Eur. Resp. J. 18:677–684. http://www.jimmunol.org/cgi/ijlink?linkType=ABST&journalCode=erj&resid=18/4/677
- Silver, L. M. (Ed.) 1995. Mouse Genetics: Concepts and Applications, 1st Edition. New York: Oxford University Press, pp. 159–253.
- Snyder, J. A., Demchuck, E., McCanlies, E., Schuler, C., Kreiss, K., Andrew, M., Frye, B., Ensey, J., Stanton, M., and Weston, A. 2008. Impact of negatively charged patches on the surface of MHC Class II antigen-presenting proteins on risk of chronic beryllium disease. J. Royal Soc. 24:749–758.
- Snyder, J.A., Demchuk, E., McCanlies, E.C., Schuler, C.R., Kreiss, K., Andrew, M.E., Frye, B.L., Ensey, J.S., Stanton, M.L., Weston, A. 2008. Impact of negatively charged patches on the surface of MHC class II antigen-presenting proteins on risk of chronic beryllium disease. Journal of the Royal Society Interface. 5:749–758.
- Saltini, C., Winestock, K., Kirby, M., Pinkston, P., Crystal, R.G. 1989. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. New England Journal of Medicine. 320: 1103–1109.
- Tinkle, S. S., Antonini, J. M., Rich, B. A., Roberts, J. A., Salmen, R., DePree, K., and Adkins, E. 2003. Skin as a route of exposure and sensitization in Chronic Beryllium Disease. Environ. Health Perspect. 111:1202–1208.
- Tsang, S., Sun, Z., Luke, B., Stewart, C., Lum, N., Gregory, M., Wu, X., Subleski, M., Jenkins, N., Copeland, N. G., and Munroe, D. J. 2005. A Comprehensive SNP-based genetic analysis of inbred mouse strains. Mammalian Genome 16:476–480.
- Wang, Z., White, P. S., Petrovic, M., Tatum, O. L., Newman, L. S., Maier, L. A., and Marrone, B. L. 1999. Differential susceptibilities to chronic beryllium disease contributed by different Glu69 HLA-DPB1 and -DPA1 alleles. J. Immunol. 163:1647–1653.
- Wang, Z., Farris, G. M., Newman, L. S., Shou, Y., Maier, L. A., Smith, H. N., and Marrone, B. L. 2001. Beryllium sensitivity is linked to HLA-DP genotype. Toxicology 165:27–38.
- Wesselkamper, S. C., Chen, L. C., and Gordon, T. 2005. Quantitative trait analysis of the development of pulmonary tolerance to inhaled zinc particles in mice. Resp. Res. 6:73–84.
- Weston, A., Snyder, J., McCanlies, E., Schuler, C., Andrew, M., Kreiss, K., and Demchuk, E. 2005. Immunogenetic factors in beryllium sensitization and chronic beryllium disease. Mutat. Res./Fundam. Molec. Mech. Mutagen. 592:68–78.
- Williams, WR, Williams, WJ. 1982. Development of beryllium lymphocyte transformation tests in chronic beryllium disease. Int Arch Allergy Appl Immunol. 67:175–80.