Abstract
T-Helper 17 (TH17) cells are a CD4+ TH subset that plays a critical role in the pathophysiology of inflammatory disorders, especially chronic forms. It seems that the derivation of TH17 cells from their precursors take place in inflammatory microenvironment. The role of transforming growth factor (TGF)-β as an anti-inflammatory cytokine in TH17 cell differentiation is controversial. To address some of the discrepancies that exist among different studies, this study was undertaken to more clarify the TGFβ role in human TH17 cell differentiation. Here, CD4+ T-cells were isolated from peripheral blood samples and cultured in X-VIVO 15 serum-free medium. Purified cells were then treated with different combinations of polarizing cytokines (interleukin [IL]1-β, -6, and -23, with or without TGFβ), neutralizing anti-interferon (IFN)-γ and anti-IL-4 antibodies and polyclonal stimulators anti-CD3 and -CD28 antibodies, and then analyzed for IL-17, IFNγ, Foxp3, and CD25 expression by flow cytometry and for release of IL-17, -21, -22, and -10 into culture media by ELISA. The effects of selective inhibition of TGFβ signaling pathway on TH17 cell polarization were also determined by using small molecules SB-431542 and A83-01. The current study found that a combination of pro-inflammatory cytokines, including IL-1β, -6, and -23, but not TGFβ, could be used as a cytokine combination to induce development of human TH17 cells. It was also shown that TGFβ acted as a negative regulator in this regard and also led to reduced IL-17 and IL-22 production while inducing Foxp3 expression. Indeed, blocking of TGFβ signaling pathways by selective inhibitors up-regulated TH17 cell differentiation. From the data here, we concluded that TGFβ down-regulates human TH17 cell differentiation and that a presence of pro-inflammatory cytokines (along with IFNγ and IL-4 neutralizing antibodies) is sufficient for optimal differentiation of human TH17 cells.
Introduction
Traditionally, helper CD4+ T (TH) cells have been categorized into various subsets. The T-Helper 17 (TH17) subset is identified on the basis of an ability to produce interleukin-17 (IL-17) (also called IL-17A), IL-17F, -21, -22, -23, -6, and characterized by expression of retinoic acid-related orphan receptor (RORC) (the human ortholog of mouse ROR-γt) and signal transducer and activator of transcription-3 (STAT-3) (Annunziato et al. Citation2007; Korn et al. Citation2009). As all TH17 cell-derived cytokines have pro-inflammatory properties, it is not surprising TH17 cells are implicated in a wide range of inflammatory disorders including experimental autoimmune encephalomyelitis, multiple sclerosis, autoimmune arthritis, and inflammatory bowel disease (Furuzawa-Carballeda et al. Citation2007).
Several studies have shown an association between severity in inflammatory conditions and TH17 cell responses in humans and mice (Furuzawa-Carballeda et al. Citation2007; Maddur et al. Citation2012). Thus, identification of factors responsible for TH17 cell differentiation is of great interest due to the importance of these cells in health and disease. Since the discovery of TH17 cells, efforts have been made to identify factors that polarize TH17 cells. Some studies have showed that a combination of IL-6 and transforming growth factor (TGF)-β was crucial for optimal differentiation of CD4+ T-cells to TH17 cells in mice (Mangan et al. Citation2006; Veldhoen et al. Citation2006). Most of the information about differentiation of TH17 cells has been derived from experimental mouse models; comparably little is known about key polarizing factors needed for human TH17 cell development (Streeck et al. Citation2008). In the human scenario, it was shown that pro-inflammatory cytokines IL-1β, IL-6, and IL-23, as well as TGFβ were all essential for TH17 cell polarization (Manel et al. Citation2008; O’Garra et al. Citation2008; Volpe et al. Citation2008). However, some studies reported that pro-inflammatory cytokines but not TGFβ was needed for development of this cell type (Acosta-Rodriguez et al. Citation2007; Ghoreschi et al. Citation2010). Other studies have also reported dose-dependent effects of TGFβ on TH17 cell differentiation (Hakemi et al. Citation2011; Ghaedi et al. Citation2015). Clearly, studies are somewhat conflicting with regard to roles of cytokines in TH17 cell generation and the uncertainty as to whether or not TGFβ has any role to play, has not yet been fully resolved.
Cytokines are critical messengers that control the differentiation and directing of TH cells. TGFβ is believed to promote anti-inflammatory conditions by inducing the expansion of CD4+ Foxp3+ Treg (T-regulatory) cells that are essential for immunological tolerance (Shevach et al. Citation2008). TGFβ signaling is also essential for regulating the expansion, activation, and effector function of mature CD4+ T-cells (Letterio Citation2005). The mechanism of TGFβ signaling is now understood in better detail. Specifically, TGFβ signals through a receptor complex comprised of a Type I and Type II receptor, both serine/threonine kinases. The TGFβ dimers bind to a Type II receptor that recruits, phosphorylates, and activates the Type I receptor. This finally leads to SMAD (Sma- and Mad-related protein) complex accumulation in the nucleus that, in turn, act as transcription factors in the regulation of target gene expression (Shi & Massague Citation2003).
Small molecule inhibitors have proven extremely useful to investigate signal transduction pathways. The inhibitors SB-431542 and A83-01 are known potent specific inhibitors of TGFβ superfamily Type I receptors (Inman et al. Citation2002). There are some reports that SB-431542 can reduce Foxp3 expression and inhibit Treg cell-mediated suppression (Eddahri et al. Citation2006; Oida et al. Citation2006). Another study noted that T-cells treated with TGFβ RI kinase inhibitor SB-431542 retained a low amount of TH17 cell polarization (Hasan et al. Citation2015). Indeed, to our knowledge, there has been no report about effects of A83-01 in this regard, although A83-01 is known to be more potent in inhibiting TGFβ signaling than SB-431542 (Tojo et al. Citation2005). By blocking TGFβ signaling pathway (using SB-431542 and A83-01), the role of TGFβ in development of human TH17 cells can be determined more accurately.
As noted above, there is some controversy regarding the role of TGFβ in TH17 cell differentiation. It seems these cells are derived under the influence of inflammatory microenvironments and inflammatory cytokines. Because TGFβ is a known anti-inflammatory protein, its presence and participation in TH17 cell differentiation in inflammatory conditions is a subject of debate as well. Thus, the purpose of the current study was to evaluate the effects of TGFβ on human TH17 cell differentiation and to identify cytokine combination that could optimize human TH17 cell polarization. As part of these efforts, the selective inhibitors of TGFβ superfamily Type I receptors (SB-431542 and A83-01) were employed.
Material and methods
Cell isolation and differentiation of human TH17 cells
Peripheral blood mononuclear cells (PBMC) were obtained from the peripheral blood of healthy human volunteers following obtaining a written informed consent from each subject. The informed consent was prepared and approved by the Review Board at Royan Institute (Tehran, Iran). PMBC were isolated by density gradient centrifugation using Ficoll-Histopaque solution.
From each sample, CD4+ T-cells were isolated by magnetic activated cell sorting (MACS) using human CD4 MicroBeads (Miltenyi Biotec, London, UK), according to the manufacturer instructions. The purity of separated CD4+ T-cells was ≥96% as determined by flow cytometry. The cells were cultured in X-VIVO 15 Chemically-Defined Serum-free Hematopoietic Cell Medium (Lonza, Allendale, NJ) in 24-well plates at a density of 3 × 105 cells/well and were treated with different combinations of recombinant polarizing cytokines including human IL-1β (20 ng/ml), human IL-6 (40 ng/ml), human IL-23 (20 ng/ml) (all recombinant cytokines from Peprotech, London, UK). Neutralizing antibodies (purified NA/LE Mouse anti-human IFNγ [5 μg/ml; BD Pharmingen, Oxford, UK] and anti-IL-4 [5 μg/ml; R&D Systems, Abingdon, UK]) with T-cell expanders (anti-human CD3 and anti-CD28 Functional Grade [each at 2 μg/ml; eBioscience; Hatfield, UK]) were also used in the cultures. As controls, cells in some wells were only treated by the T-cell expanders (i.e. the cytokine-untreated cells).
For determining the role of TGFβ in human TH17 cell polarization, in some experiments different concentrations of TGFβ1 (10, 20, 40, 80, or 160 ng/ml) were added to cultured cells as well. In addition, the SB-431542 (10 μM; Sigma, St. Louis, MO) and A83-01 (3 μM, Sigma), were added to culture medium in some culture wells, as selective inhibitors of TGFβ signaling pathways, to more clarify the role of TGFβ in induction of TH17 cells. On Days 3 and 6 of culture, all above cytokines and antibodies were refurbished through replacement of culture medium. On Day 7 of culture, the cells were re-stimulated with anti-CD3 and anti-CD28 in the presence of the transport inhibitor Brefeldin A (BFA) (5 μg/ml; Sigma) for 12 hr. The cells were then harvested for flow cytometric analysis of intracellular IL-17, IFNγ, CD25, and Foxp3 and the culture supernatants were collected for cytokine analysis.
Flow cytometry and intracellular staining
For each control and test sample, single cell suspension of 0.5–1.0 × 106 cells were washed with BD Perm/Wash Buffer (BD, London, UK). After washing, 200 μl BD Cyto-fix/Cytoperm solution was added to each cell pellet and the cells were incubated for 20–30 min at 4 °C. The cells were then washed twice with BD Perm/Wash Buffer and incubated in phosphate-buffered saline (PBS, pH 7.4) containing 5% bovine serum albumin (BSA, Sigma) for 10–15 min at room temperature. After washing with BD Perm/Wash Buffer, the cells were aliquoted into dedicated tubes and then treated with conjugated antibody at manufacturer-recommended titers (i.e. incubated 30–45 min at 4 °C in the dark). PE (phycoerythrin)-labeled mouse anti-human antibodies against intracellular cytokines IL-17A (eBioscience), IFNγ (BD Bioscience), Foxp3 (Biolegend, San Diego, CA), and CD25 (BD Bioscience), as well as PE-conjugated mouse IgG1κ Isotype Control (BD Bioscience) were used in dedicated tubes for cell staining. All samples were analyzed with Flow cytometer (BD FACSCaliburTM) and Flow-Jo software. A minimum of 30 000 events/sample was acquired.
Analysis of cytokine production by ELISA
Levels of IL-17, IL-21, IL-22, and IL-10 in culture supernatants were measured using the appropriate commercial ELISA Ready-SET-Go kits (eBioscience), according to manufacturer instructions. The level of sensitivity of the kits was 4 pg IL-17/ml, 8 pg IL-21/ml, 8 pg IL-22/ml, and 2 pg IL-10/ml.
Statistical analysis
All results are expressed as means ± SEM. Statistical analysis was performed using a one-way analysis of variance (ANOVA). All calculations were performed using Prism Software (v.6.0, GraphPad, San Diego, CA). A p-value < 0.05 was considered significant. All experiments were repeated on at least five PBMC samples prepared from different healthy adult donors.
Results
IL-17-producing TH cell generation requires pro-inflammatory cytokines but not TGFβ
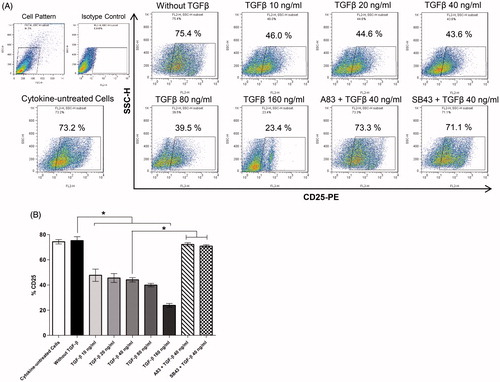
To define cytokine requirements for the induction of human TH17 cell development, differentiation of isolated CD4+ T-cells with pro-inflammatory cytokines (IL-1β, IL-6, IL-23), neutralizing anti-IFNγ and anti-IL-4 antibodies (for inhibition of TH1 and TH2 differentiation, respectively), and polyclonal stimulation of T-cells through anti-CD3 and -CD28 antibodies was performed. presents images of proliferated CD4+ T-cell colonies during any TH17 cell polarization. To assess the role of TGFβ in induction of human TH17 cell differentiation, this study evaluated TH17 cell polarization in the absence/presence of TGFβ (10–160 ng/ml in range). The results showed that TGFβ acted as a negative regulator for human TH17 cell development and that a presence of pro-inflammatory IL-1β, IL-6, and IL-23 was sufficient for induction of TH17 cells and increased production of IL-17. The percentages of cells expressing intracellular IL-17 decreased from 29.41 [±0.84]% to ≈15% in the presence of TGFβ. Nevertheless, TGFβ could not completely suppress IL-17 expression and TH17 differentiation ()).
Figure 1. Colonies of proliferating CD4+ T-cells during in vitro human TH17 cell differentiation. For stimulation and expansion of the T-cells, polyclonal stimulators (including anti-CD3 and anti-CD28 antibodies) were used (see “Methods” section). Representative micro-images are shown with different magnifications.
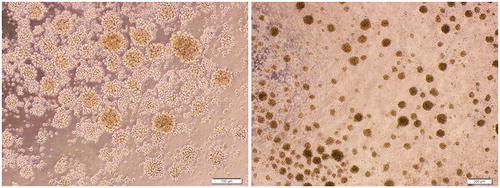
Figure 2. IL-1β, IL-6, and IL-23 – but not TGFβ – are required for optimal in vitro differentiation of human CD4+ TH17 cells. (A) Flow cytometric dot-plot analysis of intracellular IL-17 expression by CD4+ T-cells. Representative data of five independent experiments (i.e. PBMC from five different donors) are shown. (B) Columns represent mean percentage (±SEM) of T-cells expressing intracellular IL-17 in each culture condition. ns: Non-significant (*p < 0.05).
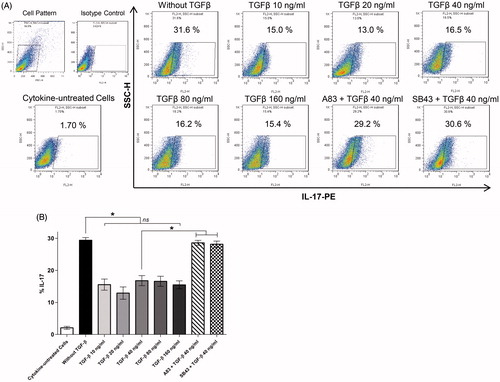
In a second set of experiments, to explore the role of TGFβ signaling on TH17 development in vitro, effects from inhibition of TGFβ receptor signaling pathways by addition of SB-431542 and A83-01 were evaluated. The results indicated that blocking TGFβ signaling could restore TH17 cell differentiation and production of IL-17 ()). Collectively, these data demonstrated that TGFβ and TGFβ signaling were not required for, and were down-regulators of, in vitro development of human TH17 cells.
Human TH17 cells produce IFNγ (TH17/1 cells) in a manner regulated by TGFβ
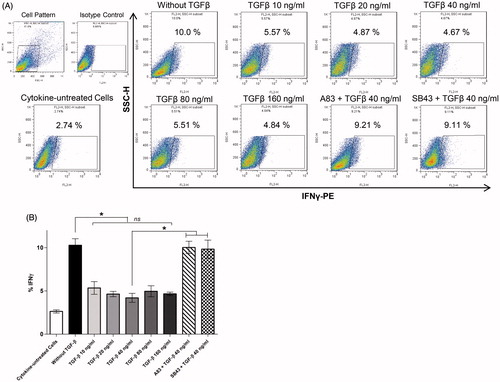
A majority of IL-17-secreting TH cells also produce IFNγ; these TH17 cells are known as TH17/1 cells and produce both IL-17 and IFNγ (Zielinski et al. Citation2012; Duhen & Campbell Citation2014). The current experiments revealed that a certain percentage (10.29 [±0.75]%) of the human TH17 cells differentiated under the influence of IL-1β, IL-6, and IL-23, and produced IFNγ along with IL-17 (IL-17 + IFNγ + TH cells). The results also indicated that TGFβ modulated secretion of IFNγ ()) and reduced TH17 cell differentiation. Addition of SB-431542 and A83-01 as TGFβ signal pathway inhibitors restored IFNγ production by the TH17 cells.
Figure 3. IFNγ production by human T-cells differentiated in presence of IL-1β, IL-6, and IL-23 – with or without TGFβ as well. (A) Flow cytometric analysis of intracellular expression of IFNγ by TH17 cells derived from CD4+ T-cells. (B) Mean expression levels (±SEM) from five independent experiments (i.e. PBMC from five different donors) using various culture conditions (i.e. different TGFβ concentration and inhibitors of TGFβ signaling pathways) are shown. ns: Non-significant (*p < 0.05).
TGFβ shifts human TH17 cell polarization to Foxp3+ cells
During differentiation of CD4+ TH cells to IL-17-producing cells, a presence of polarizing factors is essential. In the case of TGFβ, its presence up-regulates expression of Foxp3 transcription factor, a lineage specification factor for Treg cells. The present results showed that pro-inflammatory IL-1β, IL-6, and IL-23 were required for TH17 cell development but did not induce Foxp3 expression; in contrast, with an increasing presence of TGFβ (10–160 ng/ml), expression of Foxp3 increased – an outcome that indicated there was Treg cell differentiation instead of TH17 cell development ()). Both SB-431542 and A83-01 repressed Foxp3 induction.
Figure 4. TGFβ effects on Foxp3 expression by CD4+ T-cells differentiated under TH17 cell-promoting conditions. (A) Intracellular staining of Foxp3. (B) Columns are mean (±SEM) Foxp3 expression levels in five independent experiments (i.e. PBMC from five different donors) (*p < 0.05).
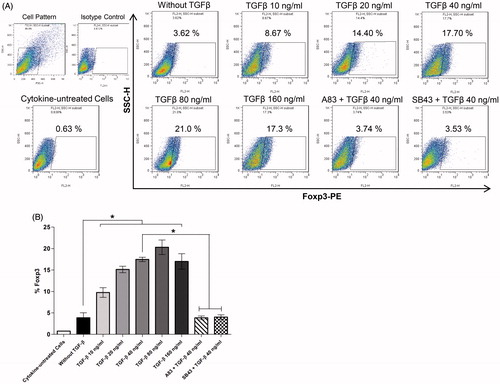
To further investigate TGFβ effects on TH cells during differentiation, CD25 expression on the cells was measured. As seen in , TGFβ suppressed CD25 expression and increasing TGFβ concentrations led to an overall decline in cell CD25 expression. These data showed TGFβ could suppress TH17 differentiation, in part, through reduced T-cell activation. By blocking TGFβ signaling using SB-431542 and A83-01, expression of CD25 was re-enhanced to >70% of its expression in the absence of TGFβ ()).
TH17 cell-derived cytokines modulated by TGFβ
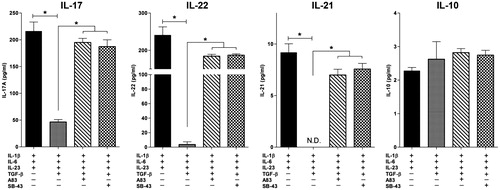
These experiments also measured production of cytokines associated with TH17 cell differentiation, e.g. IL-17, IL-21, IL-22, and IL-10, in the cultures. The results showed that a combination of IL-1β, IL-6 and IL-23 in the presence of anti-IFNγ and anti-IL-4 antibodies and polyclonal stimulators (anti-CD3 + anti-CD28) were necessary to induce human TH17 cells that produced >200 pg/ml IL-17 and 240 pg/ml IL-22 (TH17 cell hallmark cytokines) and low amounts of IL-21 and IL-10. TGFβ significantly suppressed production of IL-17, IL-21, and IL-22 (). Production of the same cytokines after blocking of TGFβ signaling pathways (via A83-01 and SB-431542) was also analyzed. Notably, all TH17 cell-associated cytokines were up-regulated; this outcome emphasized the fact TGFβ could inhibit optimal TH17 cell differentiation.
Figure 6. TH17 cell-derived cytokines; modulation by TGFβ and blocking of TGFβ signaling. Cytokine levels in supernatants of CD4+ T-cells differentiated toward TH17 states in the presence or absence of TGFβ and/or inhibitors of signaling pathways. Data showed means ± SEM from five independent experiments (i.e. PBMC from five different donors). ND: not detected (*p < 0.05).
Discussion
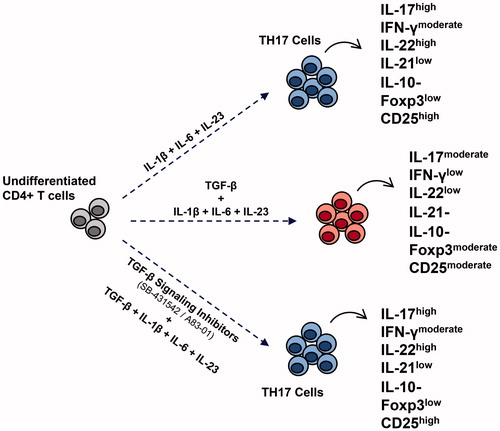
The current study was designed to examine the critical factors involved in regulation of human TH17 cell differentiation, especially the role of TGFβ and related signaling in derivation of TH17 cells in vitro. The present results indicated that pro-inflammatory cytokines (e.g. IL-1β, IL-6, IL-23) were crucial for this differentiation – independent of any TGFβ or associated signaling. Moreover, TGFβ treatments induced Foxp3 expression and reduced IL-17, IL-22 and IFNγ production – indicating a negative role for TGFβ in this differentiation process ().
Figure 7. Schematic of human TH17 cell differentiation by pro-inflammatory cytokines independently of TGFβ and its associated signaling. In presence of IL-1β, IL-6, and IL-23 as pro-inflammatory cytokines, TH17 cells that secrete IL-17, IL-22, and IFNγ developed, whereas TGFβ suppressed production of IL-17, IL-22, and IFNγ and enhanced expression of Foxp3 on the differentiated cells. Selective TGFβ signaling inhibitors restored TH17 cell differentiation.
Some studies have illustrated that TGFβ + IL-6 were critical for TH17 cell development in mice (Bettelli et al. Citation2006; Mangan et al. Citation2006; Veldhoen et al. Citation2006). In contrast, the role of TGFβ in human TH17 cell differentiation is still unclear and controversial (). The present findings were consistent with studies reporting TGFβ had a suppressive function on development of the TH17 lineage, through reduction of IL-17 production (Acosta Rodriguez et al. Citation2007; Evans et al. Citation2007; Wilson et al. Citation2007). The present study also found that in vitro production of IL-17 and IL-22 was optimal in the presence of IL-1β, IL-6, and IL-23 – but only in the absence of TGFβ, and that TGFβ-related signaling was deleterious for the induction of IL-17-producing TH cells.
Table 1. Reported role of TGFβ in human TH17 cell differentiation.
TGFβ suppresses the immune system by inhibiting the function of immune inflammatory cells and promoting the differentiation and function of Treg cells (Shevach et al. Citation2008). It also seems that TGFβ plays a role in the interplay between TH17 and Treg cells (Awasthi et al. Citation2008) and can control the plasticity of TH17/Treg cell lineages (Lee et al. Citation2009; Kleinewietfeld & Hafler, Citation2013). The present results indicated that TGFβ promoted expression of Foxp3 and may have induced Foxp3+ Treg cell populations even in the presence of TH17-promoting cytokines. Similar with these data, there are reports that TGFβ induced Treg cells and inhibited TH17 cell differentiation (Shevach et al. Citation2008; Zhou et al. Citation2008). The current study also showed that TGFβ decreased cell CD25 expression. Classen et al. (Citation2007) also reported that loss of TGFβ signaling was important for T-cell proliferation and suggested TGFβ was a prominent factor in keeping human CD4+ T-cells in a resting state.
Some studies have indicated there is a subtype of human TH17 lymphocytes that is able to produce simultaneously both IL-17 and IFNγ and co-express T-bet and RORC – a hybrid (TH17/TH1) phenotype, i.e. TH17/1 cells (Annunziato & Romagnani Citation2010; Boniface et al. Citation2010; Duhen et al. Citation2013). Peters et al. (Citation2011) indicated that TH17 populations contain cells ranging from ‘classical’ more regulated to ‘alternative’ more pathogenic TH17 cells. The latter types are major cells involved in the pathogenicity of autoimmunity and inflammatory reactions; these cells produce IL-17, IL-22, and IFNγ, along with low amounts of IL-21. On the other hand, the classic TH17 cell produces IL-17, IL-21, and IL-10, with a low amount of IL-22. In our experiences, we have obtained TH17 lineages more similar to the pathogenic phenotype. When TGFβ plus pro-inflammatory cytokines were used, expression of both IL-17 and IFNγ decreased. Consistent with the present results, Annunziato et al. (Citation2009) and Wilson et al. (Citation2007) reported that use of a combination of IL-1β with IL-23 in the absence of TGFβ promoted differentiation of TH17/1 cells that co-expressed IL-17, IFNγ, and IL-22.
There is also debate about TGFβ intracellular signaling pathways that are involved in this process (Hirahara et al. Citation2010). The current results demonstrated that both SB-431542 and A83-01, as selective inhibitors of TGFβ signaling pathways, restored the production of IL-17 and IL-22 from differentiating TH17 cells. Indeed in the presence of TGFβ, selective inhibition of its signaling pathways up-regulates TH17 cell differentiation. There is a study reported that T-cells treated with SB-431542 did not differentiate to TH17 cells (Hasan et al. Citation2015). In this regard, Mohammed et al. (Citation2010) also showed that SB-431542 caused a block in any increase in IL-17 secretion. To date, there have been no reports for an effect of A83-01 in changing potential TH17 cell polarization. Though suggestive of a role of TGFβ-related signaling in many of these outcomes, those experiments were done with mice where TGFβ is already reported to be a prominent factor in their TH17 cell development.
In the present study, expression of Foxp3 (demonstrating Treg cell polarization) was inhibited by SB-431542 or A83-01 molecules even when TGFβ was present. Some other studies have also shown that SB-431542 abolished Treg cell-mediated immune suppression (Eddahri et al. Citation2006; Oida et al. Citation2006). The present results were also consistent with those of Zaccone et al. (Citation2011) and Olkhanud et al. (Citation2011) who showed that inhibition of TGFβ signaling (using SB-431542) dramatically reduced Foxp3 expression – while expression of IFNγ that was reduced by TGFβ was now up-regulated after a blocking of TGFβ signaling. While some investigators have found A83-01 was more potent than SB-431542 in inhibiting TGFβ signaling (Tojo et al. Citation2005), the present study found no significant differences between the agents with regard to impact on TH17 cell development. Therefore, while the data here showed that inhibition of TGFβ activity by blocking TGFβ signaling pathways did not restrict human TH17 cell derivation, these results may pertain only to the set of conditions established in this in vitro experiment.
Conclusions
The present findings indicated to us that IL-1β, IL-6, and IL-23 – as pro-inflammatory cytokines – were likely required for human TH17 cell derivation and that these effects seemed to be independent of TGFβ and related signaling pathways. We also concluded that a presence of TGFβ and related signaling were moreover negative factors for in vitro human TH17 development and in fact, seemed to serve to promote Treg cell differentiation.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Funding
This study was done as one part of PhD research project and financially was supported by a research grant (Grant No. 1065782) from Tarbiat Modares University and a research grant (Grant No. 91000101) from Royan Institute.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007. Interleukins-1-β and -6 but not TGFβ are essential for differentiation of IL-17-producing human T-helper cells. Nat Immunol. 8:942–949.
- Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. 2009. Human TH17 cells: Are they different from murine TH17 cells? Eur J Immunol. 39:637–640.
- Annunziato F, Cosmi L, Santarlasci, V, Maggi L, Liotta F, Mazzinghi B, Parente, E, Filì L, Ferri S, Frosali F. 2007. Phenotypic and functional features of human TH17 cells. J Exp Med. 204:1849–1861.
- Annunziato F, Romagnani S. 2010. The transient nature of the TH17 phenotype. Eur J Immunol. 40:3312–3316.
- Awasthi A, Murugaiyan G, Kuchroo VK. 2008. Interplay between effector TH17 and regulatory T-cells. J Clin Immunol. 28:660–670.
- Bettelli E, Carrier Y, Gao W, Korn T, Stron TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T-cells. Nature 441:235–238.
- Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK., Sadekova S, de Waal Malefyt R. 2010. Human TH17 cells comprise heterogeneous subsets including IFNγ-producing cells with distinct properties from the TH1 lineage. J Immunol. 185:679–687.
- Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. 2007. Distinct regulation of IL‐17 in human T-helper lymphocytes. Arthritis Rheum. 56:2936–2946.
- Classen S, Zander T, Eggle D, Chemnitz JM, Brors B, Büchmann I, Popov A, Beyer M, Eils R, Debey S. 2007. Human resting CD4+ T-cells are constitutively inhibited by TGFβ under steady-state conditions. J Immunol. 178:6931–6940.
- Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. 2013. Cutting edge: the pathogenicity of IFNγ-producing TH17 cells is independent of T-bet. J Immunol. 190:4478–4482.
- Duhen T, Campbell DJ. 2014. IL-1β promotes differentiation of polyfunctional human CCR6+CXCR3+ TH1/17 cells specific for pathogenic and commensal microbes. J Immunol. 193:120–129.
- Eddahri F, Oldenhove G, Denanglaire S, Urbain J, Leo O, Andris F. 2006. CD4+CD25+ regulatory T-cells control magnitude of T‐dependent humoral immune responses to exogenous antigens. Eur J Immunol. 36:855–863.
- Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. 2007. Optimal induction of TH17 cells in humans requires T-cell receptor ligation in context of Toll-like receptor-activated mono-cytes. Proc Natl Acad Sci USA. 104:17034–17039.
- Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. 2007. Autoimmune inflammation from the TH17 perspective. Autoimmun Rev. 6:169–175.
- Ghaedi M, Namdari H, Rahimzadeh P, Morteza Gholi S, Azimi Mohamadabadi M, Salehi E. 2015. Different doses of TGFβ on in vitro differentiation of human naïve CD4+ T-cells to TH17. Iran J Allergy Asthma Immunol. 14:633–637.
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL Wei L, Davidson TS, Bouladoux N. 2010. Generation of pathogenic TH17 cells in the absence of TGFβ signalling. Nature. 467:967–971.
- Hakemi MG, Ghaedi K, Andalib A, Hosseini M, Rezaei A. 2011. Optimization of human TH17 cell differentiation in vitro: Evaluating different polarizing factors. In Vitro Cell Develop Biol. 47:581–592.
- Hasan M, Neumann B, Haupeltshofer S, Stahlke S, Fantini MC, Angstwurm K, Bogdahn U, Kleiter, I. 2015. Activation of TGFβ-induced non-Smad signaling pathways during TH17 differentiation. Immunol Cell Biol. 93:662–672.
- Hirahara K. Ghoreschi K, Laurence A, Yang XP, Kanno Y, O’Shea JJ. 2010. Signal transduction pathways and transcriptional regulation in TH17 cell differentiation. Cytokine Growth Factor Rev. 21:425–434.
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Kill CS. 2002. SB-431542 is a potent and specific inhibitor of TGFβ superfamily Type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 62:65–74.
- Kleinewietfeld M, Hafler DA. 2013. The plasticity of human Treg and TH17 cells and its role in autoimmunity. Semin Immunol. 25:305–312.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and TH17 Cells. Ann Rev Immunol. 27:485–517.
- Korn T, Oukka M, Kuchroo VK, Bettelli E. 2007. TH17 cells: Effector T-cells with inflammatory properties. Semin Immunol. 19:362–371.
- Laurence A, O’Shea JJ. 2007. TH17 differentiation: Of mice and men. Nat Immunol. 8:903–905.
- Lee YK, Mukasa R, Hatton RD, Weaver CT. 2009. Developmental plasticity of TH17 and Treg cells. Curr Opin Immunol. 21:274–280.
- Letterio JJ. 2005. TGFβ signaling in T-cells: Roles in lymphoid and epithelial neoplasia. Oncogene. 24:5701–5712.
- Letterio JJ, Roberts AB 1998. Regulation of immune responses by TGFβ. Ann Rev Immunol. 16:116–137.
- Maddur MS, Miossec P, Kaveri SV, Bayry J. 2012. TH17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 181:8–18.
- Manel N., Unutmaz D, Littman DR. 2008. The differentiation of human TH17 cells requires TGFβ and induction of the nuclear receptor RORγt. Nat Immunol. 9:641–649.
- Mangan, PR, Harrington, LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD., Wahl SM., Schoeb TR, Weaver CT. 2006. TGFβ induces development of the TH17 lineage. Nature. 441:231–234.
- Mohammed J, Ryscavage A, Perez-Lorenzo R, Gunderson AJ, Blazanin N, Glinck AB. 2010 TGFβ-induced inflammation in pre-malignant epidermal squamous lesions requires IL-17. J Invest Dermatol. 130:2295–2303.
- O’Garra A, Stockinger B, Veldhoen M. 2008. Differentiation of human TH17 cells does require TGFβ. Nat Immunol. 9:588–590.
- Oida T, Xu L, Weiner HL, Kitani A, Strober W. 2006. TGFβ-mediated suppression by CD4+CD25+ T-cells is facilitated by CTLA-4 signaling. J Immunol. 177:2331–2339.
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. 2011. Tumor-evoked regulatory B-cells promote breast cancer metastasis by converting resting CD4+ T-cells to T-regulatory cells. Cancer Res. 71:3505–3515.
- Park H, Li Z, Yang ZO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. 2005. A distinct lineage of CD4 T-cells regulates tissue inflammation by producing IL-17. Nat Immunol. 6:1133–1141.
- Peters A, Lee Y, Kuchroo VK. 2011. The many faces of TH17 cells. Curr Opin Immunol. 23:702–706.
- Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, Annunziato F. 2009. TGFβ indirectly favors the development of human TH17 cells by inhibiting Th1 cells. Eur J Immunol. 39:207–215.
- Shevach EM, Tran DQ, Davidson TS, Andersson J. 2008. The critical contribution of TGFβ to the induction of FoxP3 expression and Treg Function. Eur J Immunol. 38:915–917.
- Shi Y, Massagué J. 2003. Mechanisms of TGFβ signaling from cell membrane to the nucleus. Cell. 113:685–700.
- Streeck H, Cohen KW, Jolin JS, Brockman MA, Meier A, Power KA, Waring MT, Alter G, Altfeld, M. 2008. Rapid ex vivo isolation and long-term culture of human TH17 cells. J Immunol Meth. 333:115–125.
- Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M, Imamura T. 2005. The ALK‐5 inhibitor A‐83‐01 inhibits Smad signaling and epithelial‐to‐mesenchymal transition by TGFβ. Cancer Sci. 96:791–800.
- van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. 2007. Stimulation of intracellular bacterial sensor NOD2 programs dendritic cells to promote IL-17 production in human memory T-cells. Immunity. 27:660–669.
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T-cells. Immunity. 24:179–189.
- Volpe E, Servant N, Zollinger R, Bogiatzi SI., Hupe P, Barillot E, Soumelis V. 2008. A critical function for TGFβ, IL-23, and pro-inflammatory cytokines in driving and modulating human TH17 responses. Nat Immunol. 9:650–657.
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. 2007. Development, cytokine profile and function of human IL-17-producing helper T-cells. Nat Immunol. 8:950–957.
- Yang L, Anderson, DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. 2008. IL-21 and TGFβ are required for differentiation of human TH17 cells. Nature. 454:350–352.
- Zaccone P, Burton OT, Gibbs SE, Miller N, Jones FM, Schramm G, Haas H, Doenhoff DJ, Dunne DW, Cooke A. 2011. The S. mansoni glycoprotein ω‐1 induces FoxP3 expression in NOD mouse CD4+ T-cells. Eur J Immunol. 41:2709–2718.
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora G, Shen Y, Du J, Rubtsov YP, Rudensky AY. 2008. TGFβ-induced Foxp3 inhibits TH17 cell differentiation by antagonizing ROR-γt function. Nature. 453:236–240.
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. 2012. Pathogen-induced human TH17 cells produce IFNγ or IL-10 and are regulated by IL-1β. Nature. 484:514–518.