Abstract
Exposure to arsenic (As) is an ongoing, and in some places increasing, health problem. Still, however, the effects of As exposure on the immune system are not well understood. Dendritic cells (DC) are a critical immune cell that bridges the innate and adaptive immune systems. To determine the impact of inorganic (i)As exposure on DC, the effects of (geo)anthropogenically relevant levels of NaAsO2 on the function of porcine monocyte-derived DC (MoDC) were evaluated in an in vitro model. The results showed a low dose of iAs reduced the phagocytic capacity of MoDC. Furthermore, although surface expression of DC activation markers, such as major histocompatibility complex (MHC)-II, CD80/86, CD40 and CD25, were only slightly changed, MoDC T-cell proliferation-inducing capacity was remarkably diminished by iAs treatment. Additionally, iAs induced significant interleukin (IL)-6 secretion by MoDC after 12- or 24-h incubation, whereas IL-1β secretion was only significantly up-regulated after 12 h. The secretion patterns of IL-8, tumor necrosis factor α (TNFα and IL-10 by iAs-treated MoDC were almost similar to that by mock-treated MoDC. Considering the broad roles of DC in immunobiology, this finding deepens the understanding of molecular mechanisms/functional consequences underpinning the immunopathology, inflammation, and increases in infection arising from As exposure.
Introduction
As the 20th most-abundant element in the Earth’s crust, arsenic (As) is found in different chemical forms and oxidation states. Widespread As accumulation, mobility and distribution (dispersion) in soils, sediments, water and air (as component of dusts) is a critical environmental public health issue, worldwide. As a result of hydro-geochemical processes, high levels of As can be found in feed, food, water, soil and air in both organic and inorganic forms (Fillol et al. Citation2010), reaching toxic levels that can adversely affect biochemical processes of cells and organs. The origin of As in the environment is both geogenic and anthropogenic (Cohen Citation2004; Andrew et al. Citation2008; Taheri et al. Citation2016a). Inorganic As compounds are distributed in subsurface aquifers by natural processes, such as weathering, or by anthropogenic processes, such as industrial activities and the use of pesticides (Fillol et al. Citation2010; Li et al. Citation2012; Tisserand et al. Citation2014). These anthropogenic processes increase the bioavailability of As, particularly through As-contaminated drinking water and foodstuffs. Besides its known carcinogenicity (IARC 2004), As can modulate the immune system and thus increase host susceptibility to diseases (Kozul et al. Citation2009a, Citation2009b; Taheri et al. Citation2016a). Several immunotoxicology studies have shown that As exposure causes neutropenia and promotes recurrent infections. In addition, As exposure has been linked to many noninfectious diseases, including cancers and anemia (Argos et al. Citation2006; Ghosh et al. Citation2006; Andrew et al. Citation2008; Banerjee et al. Citation2009; Khan et al. Citation2012).
An underlying reason for the toxicity of As is its structural/chemical similarity to inorganic phosphate (Pi; PO43 - ), resulting in the replacement of phosphorous (P) in Pi-dependent enzymes and blocking of enzymatic activity (Biswas et al. Citation2008). For example, replacement by As in glucose-6-phosphate (initiator of glycolysis) and 6-phosphogluconate (pentose phosphate pathway) diminishes the cellular adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) production; in addition, As toxicity is also associated with an ability to disable several sulfhydryl-containing biomolecules (Gregus & Nemeti Citation2005). For example, microtubules that contain high numbers of thiol moieties are a target of As, resulting in breakdown of the tubules and cell dysfunction. Moreover, As also targets thiols in mitochondrial membranes, causing damage through activation of cytochrome C and several caspases (and so apoptosis).
Exposure of leukocytes to As at a concentrations of 0.5–5 μM led to apoptosis (Li & Broome Citation1999; Carré et al. Citation2002; Rojewski et al. Citation2004; Binet et al. Citation2005; Morzadec et al. Citation2012). As facilitates apoptosis by many other mechanisms including down-regulating expression of anti-apoptotic molecules like BCL2 (Li & Broome Citation1999; Carré et al. Citation2002; Rojewski et al. Citation2004; Binet et al. Citation2005, Citation2008; Khan et al. Citation2012; Li et al. Citation2014), interfering with mitogen-activated protein kinase activity, and increasing production of free radicals (Carpenter et al. Citation2011; Wang et al. Citation2013; Li et al. Citation2014). As also results in increased formation of tumor necrosis factor (TNF)-α (Peraza et al. Citation2003), impeded antibody production (Sankar et al. Citation2013) and reduced cell-mediated immunity (Banerjee et al. Citation2009; Kozul et al. Citation2009a, Citation2009b).
As key regulators of immune responses to antigens, dendritic cells (DC) are professional antigen-presenting cells (APC). Their antigen uptake and phagocytic capacity are vital to a host as these cells link innate and acquired immunity and so induction of immune responses to invade pathogens (Mellor & Munn Citation2004; Steinman & Banchereau Citation2007; Joffre et al. Citation2009; Devriendt et al. Citation2011). DC are localized at peripheral tissues where they continuously patrol and sample environmental antigens (Huang et al. Citation2001; Joffre et al. Citation2009). Upon antigen encounter, DC undergo a complex maturation process hallmarked by an up-regulated expression of MHC-II and several co-stimulatory molecules to efficiently present antigen to naïve T-cells. Moreover, their cytokine secretion patterns dictate polarization of naïve T-cells to effector T-cells that, in turn, drive the ensuing immune responses against pathogens (Steinman & Banchereau Citation2007; Joffre et al. Citation2009).
Little is known about the effect of As on DC functions. Accordingly, the present study was undertaken to examine this. Specifically, here, the impact of As on the function of porcine monocyte-derived DC (MoDC), in particular their phagocytic ability and phenotypical and functional maturation, was evaluated. Because swine represent an animal model whose immune system closely resembles that of humans (Fairbairn et al. Citation2011; Meurens et al. Citation2012; Dawson et al. Citation2013), these porcine cells would serve as an appropriate system to reflect potential effects on human DC in situ.
Materials and methods
Generation of porcine MoDC
Heparinized blood samples were obtained from the external jugular vein of four Belgian Landrace pigs (male, 8- to 20-week old) kept as blood donors under standard conditions at the Faculty of Veterinary Medicine, at Ghent University (Merelbeke, Belgium). All animal experiments were performed in accordance with the local animal welfare regulations and approved by the Ethical Committee of the Faculty of Veterinary Medicine at Ghent University. For all experiments outlined below, 3–4 blood samples were collected from individual piglets each time cells were needed to perform the experiments outlined herein.
Peripheral blood mononuclear cells (PBMCs) were isolated from each blood sample by lymphoprep density gradient centrifugation. Monocytes were further enriched to >97% purity (Supplementary material) by use of positive immunomagnetic bead selection (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) using kit-provided anti-CD172a monoclonal antibody (mAb, clone 74-12-15a) and goat anti-mouse microbeads together with LS separation columns (Miltenyi Biotec) (Devriendt et al. Citation2010; Mehrzad et al. Citation2015). Final isolates of CD172a+ monocytes were re-suspended in phenol-red-free Dulbecco’s modified Eagle’s Medium (DMEM; Gibco, Merelbeke), supplemented with 10% (v/v) fetal calf serum (FCS, Greiner Bio One, Wemmel, Belgium), 100 U penicillin/ml and 100 μg streptomycin/ml (Gibco, Grand Island, NY), ∼800 U recombinant porcine (rp) granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml and 5 ng rpIL-4/ml (R&D systems, Abingdon, UK) and counted. Thereafter, the cells were plated into wells of 24-well plates (Nunc, Thermo Fisher Scientific, Langenselbold, Germany) at 5.0 × 105 cells/ml and incubated at 37 °C in a humidified atmosphere at 5% CO2 to generate MoDC as previously described (Devriendt et al. Citation2010; Mehrzad et al. Citation2015). On Day 3 of culture, fresh medium supplemented with the same levels of rpGM-CSF and rpIL-4 was added. On Day 4 or 5, cells with long membrane protrusions – a typical feature of immature DC – dominated the cultures. Purity of the MoDC on Day 4 or 5 was always >97% (Supplementary material).
DC phagocytic activity
Stock solutions of As (as NaAsO2, Sigma, Taufkirchen, Germany) at 200 ng/ml were freshly prepared in Dulbecco’s phosphate-buffered saline (DPBS). All dilutions thereafter were also freshly prepared with DPBS. Harvested pure immature MoDC were seeded into 12-well plates (5.0 × 105/well), treated with As (final concentration of 20 ng/ml (0.27 μM) or vehicle only, and incubated for 1, 2, 12 and 24 h at 37 °C under 5% CO2. [Note: The selected dose was specifically based on results of previous experiments (Taheri et al. Citation2016a, Citation2016b) and were found to impart no remarkable side effects on DC necrosis/viability [data not shown]; indeed, at the end of exposure to As, in all cases (As-treated and non-treated) DC viability was >80–85%.] It is important to note also that this dose was far less than levels measured in human/animal hosts from regions known to have high environmental levels of As (Taheri et al. Citation2016a). The (geo)anthropogenic As levels in many of these countries (e.g. Bangladesh, India, Pakistan, Chili) have previously been shown to be associated with elevated incidences of immunotoxicity and (non)infectious diseases (Wu et al. Citation2001; Argos et al. Citation2006; Steinmaus et al. Citation2013; Syed et al. Citation2013).
At the end of each timepoint, 5.0 × 106 fluorescein isothiocyanate (FITC)-loaded polystyrene microbeads (1.0 μm diameter, Sigma) were added to the MoDC (10 beads/DC) and the cells incubated for 4 h at 37 °C under 5% CO2. Thereafter, the plates were placed on ice to stop phagocytic activity and the cells then dislodged by repeated pipetting/flushing of each well with 1 ml (metal-free) culture medium. The detached cells were immediately transferred to tubes held on ice and centrifuged (350 × g, 10 min, 4 °C), and then washed with ice-cold DPBS. Note: No trypsinization was used to detach cells; cell counts on harvested cells indicated that numbers isolated over time decreased as expected (due to stronger adherence); however, yields at each timepoint did not differ between the As-treated and control cells [data not shown].
Internalization of beads by the cells was then assessed by flow cytometry in a FACSCanto flow cytometer and analyzed with FACSDiva 6.1.3 software (BD biosciences, Erembodegem, Belgium). A minimum of 20 000 events/sample was acquired. Phagocytic index (PI) was calculated as 100% × (phagocytosis + As-treated MoDC/phagocytosis + control MoDC). Results were then presented as these percentages with the control MoDC PI% value being set at 100%. Based on findings from the phagocytosis assays (see Results), all subsequent experiments herein were performed only after incubations of 12 and 24 h.
Surface expression of DC activation markers
Surface expression of DC activation markers on MoDC treated with medium or As for 12 and 24 h was assessed by flow cytometry using mAb against MHC-II (MSA3, IgG2a), CD40 (G28-5, IgG1, anti-human [Bimczok et al. Citation2007]), CD25 (K231.3B2, IgG1) and a human CTLA4-μIgG2a fusion protein (all Ancell, Bayport, MN), respectively, followed by R-phycoerythrin- and AlexaFluor (AF)-647-conjugated isotype-specific anti-mouse secondary antibodies, as needed (Molecular Probes, Life Technologies, Merelbeke). In brief, MoDC were treated as above, harvested and washed in staining medium (DMEM +1% FCS), and then dedicated aliquots of the cells (2 × 105/sample) incubated with pre-titrated saturating concentrations of an individual primary Ab for 20 min at 4 °C. Cells stained with isotype-matched irrelevant mAb (Molecular Probes) were used to assess nonspecific binding. After washing, the cells were stained for 20 min at 4 °C in the dark with the appropriate secondary Ab (at manufacturer-recommended dilution) in staining medium. The cells were then washed and propidium iodide (5 μg/ml, Sigma) was added to help exclude dead DC from analyses. Samples were then analyzed as above to yield mean fluorescence intensity (MFI) for a given marker. Relative change in marker expression (%) among live cells was calculated as follows: % = 100 × [(MFIAs treatment − MFIcontrol)/MFIcontrol].
T-cell polarization capacity of DC
The T-cell stimulatory capacity of MoDC was analyzed in an allogeneic T-cell polarization assay as previously described (Devriendt et al. Citation2013; Mehrzad et al. Citation2015). T-cells were isolated from the original PBMC fractions by enriching CD6+ cells to a >95% purity by positive immunomagnetic selection with anti-CD6 mAb (IgG1, clone a38b2) and goat anti-mouse micro-beads, together with an LS column (Miltenyi Biotec).
For this assay, MoDC were treated with As or left untreated for 12 and 24 h, and then harvested, washed and counted. The MoDC were then co-cultured (in triplicate) in DMEM containing 10% FCS, penicillin/streptomycin and 2-mercaptoethanol (50 μM, Sigma) at titrated numbers with 2.0 × 105 allogenic CD6+ T-cells in round-bottomed 96-well microtiter plates (Nunc). To assure the assay system worked, CD6+ T-cells incubated only with 5 μg Concanavalin A (ConA)/ml (Sigma) served as a positive control. After 5 days of culture at 37 °C, the cells were pulse labeled with 1 μCi [3H]-methyl-thymidine/well (Amersham ICN, Bucks, UK) for another 18 h. Cells were then harvested onto glass fiber filters (Perkin-Elmer, Life Science, Brussels, Belgium) and thymidine incorporation measured using a β-scintillation counter (Perkin-Elmer). Data for effects from each length of exposure to As were presented as stimulation index (SI) calculated as follows: SI = cpm co-cultures DCAs treatment/cpm co-cultures DCcontrol wherein control values were those from cells that received 0 ng As/ml.
MoDC cytokine formation/secretion
Supernatants of As-treated MoDC cultures were collected at each timepoint noted above and levels of porcine TNFα, interleukin (IL)-1β, -6, -8 and -10 and then determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (TNFα, IL-1β, IL-6 and IL-8: R&D Systems, Minneapolis, MN; IL-10: Life Technologies, Merelbeke) according to the manufacturer instructions. All optical densities were measured in an ELISA plate reader (MTX Lab systems, Vienna, VA) at 450 nm. The cytokine concentrations were calculated using DeltaSOFT JV 2.1.2 software (BioMetallics, Princeton, NJ) with a 4-parameter curve-fitting algorithm applied for standard curve calculations. The level of sensitivity of the kits was 125 pg TNFα/ml, 62 pg IL-1β/ml, 4 pg IL-6/ml, 125 pg Il-8/ml and 3 pg IL-10/ml.
Statistical analyses
Data are reported as means ± standard error (SE). Effects of As on phagocytosis, marker expression, induced T-cell proliferation, and cytokine secretion were assessed using a non-parametric Mann–Whitney U test. All analyses were done with SPSS 20 software (IBM, Armonk, NY). Significance was accepted at p < .05.
Results
Effects on DC phagocytic activity
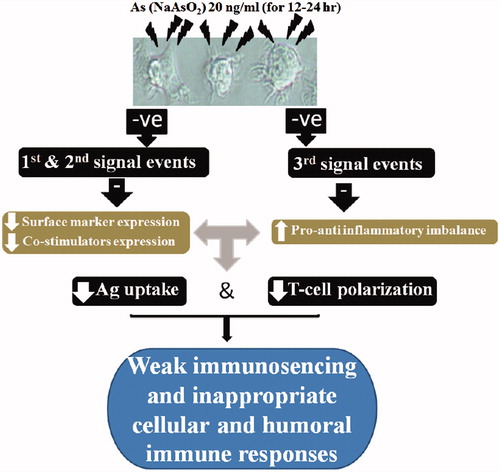
To assess the effects of As on MoDC-mediated phagocytosis, porcine MoDC were treated for different lengths of time with As (20 ng/ml) prior to the addition of fluorescent microbeads. At this low dose and as early as after just 2 h, As had caused a significant (p = 0.037) change in the cells that led to a subsequent initial increase in microbead internalization (vs. control cell levels normalized to 100%) (). However, as treatment length increased (to 12 and 24 h), the activity of the cells decreased significantly as compared to that of untreated MoDC (p = 0.037). Specifically, PI% values (control cell values set to 100%) of cells after 1 and 24 h As exposure were 137.46 [±21.74]% and 83.17 [±8.64]%, respectively. The data indicated that despite an initial increase in phagocytic activity, As impaired the MoDC activity as time progressed.
Figure 1. Phagocytic activity of porcine monocyte-derived dendritic cells (MoDC). MoDC were treated for 1, 2, 12 or 24 h with 20 ng As/ml or medium only. Fluorescent microbeads (1.0 μm) were then added to the MoDC to assess phagocytic activity. (a) Representative histograms of As-treated MoDC (a and b: without and with fluorescent microbeads, respectively). (b) Mean results from three experiments using separate samples/piglet/regimen. Dashed line = phagocytic index of control MoDC (set to 100%), solid line = index of As-treated MoDC. *p < .05 vs. control.
Effects on DC activation marker expression
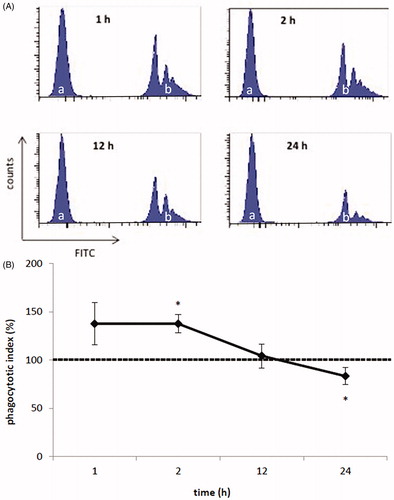
The effect of As on activation/phenotypical maturation of the porcine MoDC was assessed by measures of expression of cell surface MHC-II, CD40, CD80/86 and CD25 (=IL-2R). As shown in , As treatment had a clear time-related impact on expression of these markers, albeit in a non-significant manner. After 12 h of As treatment, relative expression of MHC-II and CD40 was decreased as compared to control MoDC, whereas relative expression of CD80/86 and CD25 was unaffected. After 24 h, relative expression of CD40, CD80/86 and CD25 was each up-regulated (by 15.1 [±9.1]%, 35.6 [±27.2], and 9.3 [±7.0]%, respectively), as compared to untreated MoDC, suggesting a phenotypical DC maturation or at least activation of the cells. However, all the observed differences were not statistically significant. Relative expression of MHC-II remained below that of the levels on control cells, but this too was a non-significant change.
Figure 2. Cell surface expression of DC activation markers. Cells were treated with 20 ng As/ml or 0 ng/ml (cntrl) for 12 and 24 h and subsequently analyzed for expression of CD40, CD80/86, CD25 and MHC-II by flow cytometry. Data shown are mean (±SE) relative changes as compared to expression on untreated MoDC (n = 4 separate samples/piglet/regimen).
Effects on T-cell polarization capacity
To assess whether As influenced antigen presentation capacity of the MoDC, the ability of the cells to induce proliferation among allogeneic T-cells was investigated. MoDC treated with As for 12 h did not significantly differ in their T-cell stimulatory ability (SI = 2.04 [±1.33]) as compared to that by control cells (i.e. 0 ng As/ml; normalized to SI = 1.0) (). In contrast, treatment for 24 h decreased their ability to induce T-cell proliferation (SI = 0.71 [±0.16]; p = 0.014).
Figure 3. T-Cell stimulatory capacity of MoDC. Cells were treated with 20 ng As/ml or 0 ng/ml (cntrl) for 12 and 24 h and subsequently co-cultured with allogeneic T-cells at a DC:T ratio of 1:30. T-cell proliferation was measured via [3H] thymidine incorporation. Data are presented as mean stimulation index (±SE) (n = 4 separate samples/piglet/regimen). *p < 0.05 vs. untreated cells.
![Figure 3. T-Cell stimulatory capacity of MoDC. Cells were treated with 20 ng As/ml or 0 ng/ml (cntrl) for 12 and 24 h and subsequently co-cultured with allogeneic T-cells at a DC:T ratio of 1:30. T-cell proliferation was measured via [3H] thymidine incorporation. Data are presented as mean stimulation index (±SE) (n = 4 separate samples/piglet/regimen). *p < 0.05 vs. untreated cells.](/cms/asset/8a5756d0-7e0e-4ddd-93b0-fa8314860149/iimt_a_1249985_f0003_c.jpg)
Effects on pro-inflammatory cytokine formation
Because cytokines play a crucial role in effector T-cell activation, this study also determined the effect of As on spontaneous MoDC cytokine secretion. Treatment with As induced significantly higher IL-6 secretion by the MoDC after 12 h (p = 0.046) and 24 h (p = 0.021), with values increasing to 18.7 [±6.3] and 16.3 [±2.4] pg/ml, respectively, from control levels of 6.1 [±1.8] and 6.1 [±2.1] pg/ml at corresponding timepoints (). IL-1β secretion was only significantly up-regulated after 12 h (p = 0.040), with levels reaching 194.0 [±91.9] pg/ml vs. control levels of 60.1 ± 15.5 pg/ml. Levels after 24 h of treatment were also quite elevated vs. control (177.5 [±91.0] vs. 67.13 [±17.8] pg/ml, respectively), but the difference was not statistically significant.
Figure 4. Cytokine expression by MoDC. Cells were treated with 20 ng As/ml or 0 ng/ml (cntrl) for 12 and 24 h and then culture supernatants were collected for analysis by ELISA. Data shown are mean [±SE] concentrations (n = 4 separate samples/piglet/regimen).
![Figure 4. Cytokine expression by MoDC. Cells were treated with 20 ng As/ml or 0 ng/ml (cntrl) for 12 and 24 h and then culture supernatants were collected for analysis by ELISA. Data shown are mean [±SE] concentrations (n = 4 separate samples/piglet/regimen).](/cms/asset/311ec2bf-6264-459a-81db-06e1beb87eb2/iimt_a_1249985_f0004_c.jpg)
Similarly, after each incubation period, As induced higher IL-8 and TNFα levels as compared to that by control MoDC, but in no case did these values reach statistical significance. IL-10 secretion by MoDC was uniformly unaffected by As.
Discussion
Worldwide occupational exposure to As is alarmingly increasing, and its toxic effects on immune systems are a threat to animal and public health (Kozul et al. Citation2009a, Citation2009b; Taheri et al. Citation2016a, Citation2016b). This study examined the effects of As on the function of porcine DC at levels that may be obtained by consumption of contaminated water, feed and foods. Indeed, the main rationale for choosing the dose of As for the in vitro tests here was the presence of As in drinking water in parts of Iran where some residents and animals suffer increased levels of (non)infectious diseases (Taheri et al. Citation2016a, Citation2016b). Indeed, levels of 20 ng As/ml or higher have been reported in biomarkers (i.e. blood, urine) of humans and food-producing animals exposed to As by inhalation, ingestion or skin contact (Taheri et al. Citation2016a). Furthermore, in countries where monitoring programs are absent, inhabitants may encounter far higher As levels than the dose used here. Although it is believed that exposure of food-producing animals and humans to As is relatively low in Europe, the spread of As due to industrialization and (geo)anthropogenic-related events is alarming. Therefore, to investigate the potential effects of As on DC function, the approaches used in this study closely mimicked in vivo conditions that reflect levels often encountered in real life.
Owing to the possible involvement of As in immunotoxicity and given the important role of DC in the induction of immune responses and the lack of data, we assessed the effect of As on DC function. The ability of DC to take up antigens is central to their function. Using flow cytometric assays, we assessed the phagocytic capacity of As-exposed DC. Here, As seemed to adversely affect the phagocytosis of microbeads by MoDC. This corroborated other studies indicating that As could decrease the phagocytic activity of neutrophils and macrophages (Kozul et al. Citation2009a; Taheri et al. Citation2016b). In contrast, the phagocytic capacity of DC was modestly increased at short lengths of exposure tested here (i.e. 1 h), possibly reflecting a response of the DC to As sensing. Interestingly, a 12 or 24 h treatment with As diminished microbead uptake by the MoDC. This probably implied an interference by As with membrane and/or cytoskeleton dynamics, two well-known key mechanisms necessary for efficient phagocytosis (Goodridge et al. Citation2012). Indeed, microtubule components possess abundant thiol groups and a strong affinity of As for these groups presumably results in As accumulation in the cytoskeleton (Li & Broome Citation1999; Binet et al. Citation2008). This might interfere with microtubule dynamics and so phagocytosis in the As-exposed DC. However, the entry of As into DC and induced changes in cytoskeleton structures remains to be fully elucidated.
Although the lower DC-induced T-cell activation observed here in the As-treated DC could result in part from the less efficient phagocytosis of foreign material, it might also be attributed to interference of As with the cell ability to interact with and activate T-cells. Ingestion of As-contaminated feed by mice resulted in a decreased T-cell proliferation, presumably due to a (direct) suppressive effect of As on DC, confirming that chronic exposure of animals to low As levels still could directly affect the immune system and prolong infection with pathogens (Kozul et al. Citation2009a, Citation2009b; Taheri et al. Citation2016b). Normal T-cell activation by DC requires three signals: (1) the interaction of the T-cell receptor with peptide–MHC complexes on the DC surface; (2) DC have to up-regulate expression of co-stimulatory molecules, such as CD40 and CD80/86, to fully activate naïve T-cells and (3) secretion of cytokines that influence polarization of activated T-cells. If one of those signals is absent, T-cell activation and consequently proliferation will be impaired (Huang et al. Citation2001; Mellor & Munn Citation2004; Steinman & Banchereau Citation2007; Devriendt et al. Citation2011). The observed effects of As on DC confirmed that As particularly interfered with the third signal in DC (i.e. inappropriate increase in pro-inflammatory cytokines). To the best of our knowledge, this study is the first to examine the specific effects of As on porcine DC function.
To address whether As impaired the ability of DC to activate T-cells, we assessed the effect of As exposure on the expression of activation markers and the secretion of pro-inflammatory cytokines. Changes in these parameters are often used to assess the immunotoxic effects of environmental toxins, such as mycotoxins (Devriendt et al. Citation2009; Hong et al. Citation2015). Exposure to As affected the cell surface marker expression by porcine DC. Indeed, the increased expression of CD25, CD40 and CD80/86 indicated an activation of the As-treated DC. Interestingly, As did not affect MHC-II surface levels, which might explain the reduced T-cell proliferation-inducing capacity of As-treated MoDC (Mehrzad et al. Citation2015). Immunotoxicologically though incomparable, nonetheless, this diminished T-cell proliferation-inducing activity is in line with a diminished cell-mediated immunity in piglets fed with other environmental toxicants (Meissonnier et al. Citation2008; Hong et al. Citation2015). Other DC surface molecules/markers (e.g. TLR, CD64 C5aR, CCR7, CD209, COX-2, LFA, etc.) that might be crucial remained to be examined in future studies.
Although As did not cause modulation of MoDC IL-10 formation/secretion, it did enhance their secretion of pro-inflammatory IL-6 and IL-1β. This outcome was not in line with previous data where other toxicants did not modulate IL-1β and TNFα expression in swine alveolar macrophages (Liu et al. Citation2002). However, as only a limited number of cytokines were evaluated here, a potential effect of the As on other cyto-/chemokines cannot be excluded. Still, the increased secretion of pro-inflammatory cytokines induced by environmentally relevant levels of As could result in a breakdown of immunological tolerance and provide a mechanism for the types of immunotoxicity that have been noted in As-exposed animals. Further functional assays are required to elucidate the molecular mechanisms behind this phenomenon.
Overall, the early enhancement and late reduction of MoDC function and a concomitant tendency for changes in expression of select cell surface molecules and increased production of IL-6 in As-exposed MoDC are intriguing. Although the present study focused on the initial evaluation of As effects on some aspects of porcine MoDC, it is clear that ongoing studies should also accommodate migration capacity of DC to a specific organ to better assess detailed of any effects observed here in MoDC. Lastly, since the overwhelming majority (i.e. >80%) of DC experiments have been performed with murine bone marrow (BM) DC as the gold standard, it would be worth performing side-by-side comparisons of murine BMDC vs. porcine primary DC – going forward – to gain a much better understanding of effects of As on DC overall.
Conclusion
The studies indicated that exposure of DC to environmentally relevant levels of iAs could result in changes in expression of some DC activation markers and so impact on inducible T-cell proliferation. Treatments also appeared to cause imbalances in pro-inflammatory cyto-/chemokine formation/secretion by the cells. These effects of As on DC phagocytosis, T-cell proliferation-inducing capacity and cytokine formation/secretion open new windows to understand the molecular mechanisms that might underpin the effects of As on inflammation, infection and cancer in animals and humans (). As the effects of As on DC functions were examined at limited timepoints and using only a single low dose (20 ng As/ml), and limited DC activation markers, further studies with an array of doses with varying lengths of As exposure on other DC activation markers would be valuable.
Supplementary Figure S1
Download TIFF Image (57.6 KB)Acknowledgements
The authors express their special thanks to Drs. Eric Cox, Bert Devriendt and Kim Baert (Faculty of Veterinary Medicine, Laboratory of Immunology, Ghent University) for their kind scientific and experimental supports. We also acknowledge Dr. S. Inumaru (Institute of Animal Health, Ibaraki, Japan) for kindly providing the rpGM-CSF, Dr. H. Rothkötter (Institute of Anatomy, Magdeburg, Germany) for the anti-CD40 hybridoma and Dr. A. Saalmüller (University of Veterinary Medicine, Vienna, Austria) for the MSA3 and a38b2 clones.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Andrew A, Jewell D, Mason R, Whitfield M, Moore J, Karagas M. 2008. Drinking water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 116:524–531.
- Argos M, Kibriya M, Parvez F, Jasmine F, Rakibuz-Zaman M, Ahsan H. 2006. Gene expression profiles in peripheral lymphocytes by arsenic exposure and skin lesion status in a Bangladeshi population. Cancer Epidemiol Biomarkers Prev. 15:1367–1375.
- Banerjee N, Banerjee S, Sen R, Bandyopadhyay A, Sarma N, Majumder P, Das J, Chatterjee M, Kabir S, Giri A. 2009. Chronic arsenic exposure impairs macrophage functions in the exposed individuals. J Clin Immunol. 29:582–594.
- Bimczok D, Rau H, Wundrack N, Naumann M, Rothkötter H, McCullough K, Summerfield A. 2007. Cholera toxin promotes the generation of semi-mature porcine monocyte-derived dendritic cells that are unable to stimulate T-cells. Vet Res. 38:597–612.
- Binet F, Cavalli H, Moisan E, Girard E. 2005. Arsenic trioxide (AT) is a novel human neutrophil pro-apoptotic agent: Effects of catalase on AT-induced apoptosis, degradation of cytoskeletal proteins and de novo protein synthesis. Br J Hematol. 132:349–358.
- Binet F, Chiasson S, Girard D. 2008. Arsenic trioxide induces de novo protein synthesis of annexin-1 in neutrophils: association with a heat shock-like response and not apoptosis. Br J Haematol. 140:454–463.
- Biswas D, Banerjee M, Sen G, Das J, Banerjee A, Su T, Pandit S, Giri A, Biswas T. 2008. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmacol. 230:57–66.
- Carpenter R, Jiang Y, Jing Y, He J, Rojanasakul Y, Liu L, Jiang B. 2011. Arsenite induces cell transformation by reactive oxygen species, AKT, ERK1/2, and p70S6K1. Biochem Biophys Res Commun. 414:533–538.
- Carré M, Carles G, Andre N, Douillard S, Ciccolini J, Briand C, Braguer D. 2002. Involvement of microtubules and mitochondria in the antagonism of arsenic trioxide on paclitaxel-induced apoptosis. Biochem Pharmacol. 63:1831–1842.
- Cohen M. 2004. Pulmonary Immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, copper, manganese, nickel, vanadium, and zinc. J Immunotoxicol. 1:39–69.
- Dawson H, Loveland J, Pascal G, Gilbert J, Uenishi H, Mann K. 2013. Structural and functional annotation of the porcine immunome. BMC Genomics. 14:332–347.
- Devriendt B, de Geest B, Cox E. 2011. Designing oral vaccines targeting intestinal dendritic cells. Expert Opin Drug Deliv. 8:467–483.
- Devriendt B, Gallois M, Verdonck F, Waché Y, Bimczok D, Oswald I, Goddeeris B, Cox E. 2009. The food contaminant fumonisin B(1) reduces the maturation of porcine CD11R1(+) intestinal antigen-presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet Res. 40:40–45.
- Devriendt B, Goddeeris B, Cox E. 2013. The Fcγ receptor expression profile on porcine dendritic cells depends on the nature of the stimulus. Vet Immunol Immunopathol. 152:43–49.
- Devriendt B, Verdonck F, Summerfield A, Goddeeris B, Cox E. 2010. Targeting of Escherichia coli F4 fimbriae to Fcgamma receptors enhances the maturation of porcine dendritic cells. Vet Immunol Immunopathol. 135:188–198.
- Fairbairn L, Kapetanovic R, Sester D, Hume D. 2011. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J Leukoc Biol. 89:855–871.
- Fillol C, Dor F, Labat L, Boltz L, Bouard JL, Mantey K, Mannschott C, Puskarszyk E, Viller F, Momas I, et al. 2010. Urinary arsenic concentration and speciation in residents living in an area with naturally contaminated soils. Sci Total Environ. 408:1190–1194.
- Ghosh D, Bhattacharya S, Mazumder S. 2006. Perturbations in the catfish immune responses by arsenic: Organ and cell specific effects. Comp Biochem Physiol C Toxicol Pharmacol. 143:455–463.
- Goodridge HS, Underhill DM, Touret N. 2012. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic. 13:1062–1071.
- Gregus Z, Nemeti B. 2005. The glycolytic enzyme glyceraldehyde-3-phosphate dehydro-genase works as arsenate reductase in human red blood cells and rat liver cytosol. Toxicol Sci. 85:859–869.
- Hong C, Lee C, Chen G, Chang K, Yu H. 2015. STAT3-dependent VEGF production from keratinocytes abrogates dendritic cell activation and migration by arsenic: A plausible regional mechanism of immunosuppression in arsenical cancers . Chem Biol Interact. 227:96–103.
- Huang Q, Liu D, Majewski P, Schulte L, Korn J, Young R, Lander E, Hacohen N. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science. 294:870–875.
- Joffre O, Nolte M, Sporri R, Reis e Sousa C. 2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 227:234–247.
- Khan S, Vala J, Nabi S, Gupta G, Kumar D, Telang A, Malik J. 2012. Protective effect of curcumin against arsenic-induced apoptosis in murine splenocytes in vitro. J Immunotoxicol. 9:148–159.
- Kozul C, Ely K, Enelow R, Hamilton J. 2009b. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect. 117:1141–1147.
- Kozul C, Hampton T, Davey J, Gosse J, Nomikos A, Eisenhauer P, Weiss D, Thorpe J, Ihnat M, Hamilton J. 2009a. Chronic exposure to arsenic in the drinking water alters the expression of immune response genes in mouse lung. Environ Health Perspect. 117:1108–1115.
- Li S, Xiao T, Zheng B. 2012. Medical geology of arsenic, selenium and thallium in China. Sci Total Environ. 421:31–40.
- Li Y, Broome J. 1999. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 59:776–780.
- Li Y, Xi M, Gue Y, Hai C, Yang W, Qin X. 2014. NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylase activity. Toxicol Lett. 224:165–174.
- Liu B, Yu F, Chan M, Yang Y. 2002. The effects of mycotoxins, fumonisin B1 and aflatoxin B1, on primary swine alveolar macrophages. Toxicol Appl Pharmacol. 180:197–204.
- Mehrzad J, Devriendt B, Baert K, Cox E. 2015. Aflatoxins of type B and G affect porcine dendritic cell maturation in vitro. J Immunotoxicol. 12:194–198.
- Meissonnier G, Pinton P, Laffitte J, Cossalter A, Gong Y, Wild C, Bertin G, Galtier P, Oswald I. 2008. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol Appl Pharmacol. 231:142–149.
- Mellor A, Munn D. 2004. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 4:762–774.
- Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. 2012. The pig: A model for human infectious diseases. Trends Microbiol. 20:50–57.
- Morzadec C, Bouezzedine F, Macoch M, Fardel O, Vernhet L. 2012. Inorganic arsenic impairs proliferation and cytokine expression in human primary T-lymphocytes. Toxicology. 300:46–56.
- Peraza M, Carter D, Gandolfi A. 2003. Toxicity and metabolism of sub-cytotoxic inorganic arsenic in human renal proximal tubule epithelial cell (HK-2). Cell Biol Toxicol. 19:253–264.
- Rojewski M, Korper S, Thiel E, Schrezenmeier H. 2004. Arsenic trioxide-induced apoptosis is independent of CD95 in lymphatic cell lines. Oncol Rep. 11:509–513.
- Sankar P, Telang A, Suresh S, Kesavan M, Kannan K, Kalaivanan R, Sarkar S. 2013. Immunomodulatory effects of nanocurcumin in arsenic-exposed rats. Int Immunopharmacol. 17:65–70.
- Steinman R, Banchereau J. 2007. Taking dendritic cells into medicine. Nature. 449:419–426.
- Steinmaus C, Ferreccio C, Romo J, Yuan Y, Cortes S, Marshall G, Moore L, Balmes J, Liaw J, Golden T, et al. 2013. Drinking water arsenic in northern Chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 22:623–630.
- Syed E, Melkonian S, Poudel K, Yasuoka J, Otsuka K, Ahmed A, Islam T, Parvez F, Slavkovich V, Graziano J, et al. 2013. Arsenic exposure and oral cavity lesions in Bangladesh. J Occup Environ Med. 55:59–66.
- Taheri M, Mehrzad J, Afshari R, Saleh-Moghaddam M, Mahmudy Gharaie M. 2016a. High soil and groundwater arsenic levels induce high body arsenic loads, health risk and potential anemia for inhabitants of northeastern Iran. Environ Geochem Health. 38:469–482.
- Taheri M, Mehrzad J, Afshari R, Saleh-Moghaddam M, Mahmudy Gharaie M. 2016b. Inorganic arsenic can be potent granulotoxin in mammalian neutrophils in vitro. J Immunotoxicol. 13:686–693.
- Tisserand D, Pili E, Hellmann R, Boullier AM, Charlet L. 2014. Geogenic arsenic in groundwaters in the western Alps. J Hydrol. 518:317–325.
- Wang F, Zhou X, Liu W, Sun X, Chen C, Hudson L, Jian Liu K. 2013. Arsenite-induced ROS/RNS generation causes zinc loss and inhibits the activity of poly(ADP-ribose) polymerase-1. Free Radic Biol Med. 61:249–256.
- Wu M, Chiou H, Wang T, Hsueh Y, Wang I, Chen C, Lee T. 2001. Association of blood arsenic levels with increased reactive oxidants/decreased anti-oxidant capacity in human population of northeastern Taiwan. Environ Health Perspect. 109:1011–1017.