Abstract
Perfluoroalkyl substances (PFASs) are highly persistent chemicals that might be associated with asthma and allergy, but the associations remain unclear. Therefore, this study examined whether pre- and postnatal PFAS exposure was associated with childhood asthma and allergy. Measles, mumps, and rubella (MMR) vaccination in early life may have a protective effect against asthma and allergy, and MMR vaccination is therefore taken into account when evaluating these associations. In a cohort of Faroese children whose mothers were recruited during pregnancy, serum concentrations of five PFASs – Perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) – were measured at three timepoints (maternal serum in pregnancy week 34–36 and child serum at ages 5 and 13 years) and their association with immunoglobulin E (IgE) (cord blood and at age 7 years) and asthma/allergic diseases (questionnaires at ages 5 and 13 years and skin prick test at age 13 years) was determined. A total of 559 children were included in the analyses. Interactions with MMR vaccination were evaluated. Among 22 MMR-unvaccinated children, higher levels of the five PFASs at age 5 years were associated with increased odds of asthma at ages 5 and 13. The associations were reversed among MMR-vaccinated children. Prenatal PFAS exposure was not associated with childhood asthma or allergic diseases regardless of MMR vaccination status. In conclusion, PFAS exposure at age 5 was associated with increased risk of asthma among a small subgroup of MMR-unvaccinated children but not among MMR-vaccinated children. While PFAS exposure may impact immune system functions, this study suggests that MMR vaccination might be a potential effect-modifier.
Introduction
Perfluoroalkyl substances (PFAS) constitute a group of highly persistent chemicals used in a variety of products including furniture, carpets, clothing, food packaging, and firefighting foams (Fromme et al. Citation2009). PFAS are found in human serum worldwide (Houde et al. Citation2006), and children appear to have a greater exposure than teenagers and adults (Trudel et al. Citation2008; Kato et al. Citation2009). Recently, higher serum-PFAS concentrations in childhood have been associated with increased odds of concurrent asthma (Dong et al. Citation2013), perhaps due to a shift toward a T-helper type 2 cell (TH2) immune response (Dong et al. Citation2011) as seen in allergic asthma and allergic diseases. However, other studies could not replicate this association (Humblet et al. Citation2014; Stein et al. Citation2016). Reported associations between prenatal PFAS exposure and asthma, wheezing, and other allergic diseases in children also have not been consistent (Wang et al. Citation2011; Okada et al. Citation2012, Citation2014; Granum et al. Citation2013; Smit et al. Citation2015).
Furthermore, measles, mumps, and rubella (MMR) vaccination in early life may have a protective effect against asthma or asthma symptoms (Gruber et al. Citation2003; Roost et al. Citation2004), an association that was substantiated in our previous analyses (Timmermann et al. Citation2015). The protective effect is possibly due to the elicitation of a TH1-biased response (Pabst et al. Citation1997; Ovsyannikova et al. Citation2003), and it is possible that a TH1-biased response induced by MMR vaccination could suppress a PFAS-induced TH2 immune response thus minimizing the effect of PFAS on asthma among MMR-vaccinated children.
The objective of the present study was therefore to investigate whether pre- or postnatal PFAS exposure was associated with asthma and allergic diseases in a birth cohort while considering the potential effect modification by MMR vaccination.
Materials and methods
Sample population
Data were collected as part of the Children’s Health and Environment in the Faroe Islands (CHEF) study (Grandjean et al. Citation2010; Heilmann et al. Citation2010; Timmermann et al. Citation2015). The birth cohort was formed in 1997–2000, and a blood sample for measuring environmental exposures was obtained when the women were included in gestational week 34–36. Obstetric information was recorded, and a cord blood sample was obtained at birth. At ages 5, 7, and 13 years, children were invited for physical examinations (including blood sampling), and parents filled out a questionnaire together with a research nurse.
MMR vaccinations were routinely administered at 15 months-of-age; vaccination dates were obtained from the child’s vaccination card at age 5. There were no specific contraindications against the vaccination. Children were considered eligible for this study if they attended the age-5 examination. They were excluded if they had had measles or if no information was provided about this disease (Timmermann et al. Citation2015).
Written informed consent was obtained from the parents of all children included in the study. The study was carried out in accordance with the Helsinki Declaration and was approved by the Ethical Review Committee serving the Faroe Islands and the U.S. (Harvard) Institutional Review Board (IRB).
Asthma and allergy outcomes
Total Immunoglobulin E (IgE) and A (IgA) concentrations were quantified in cord blood by ImmuniCAP and in-house ELISA. Analyses have been described in more detail elsewhere (Timmermann et al. Citation2015). IgE measures from samples with IgA concentrations >50 μg/ml were disregarded due to possible contamination with maternal blood (Bonnelykke et al. Citation2010). At age 5, parents were asked whether the child had been diagnosed with or was suspected to suffer from asthma and whether the child had been diagnosed with hypersensitivity or allergy. At age 7, a blood sample was drawn from which total IgE was quantified. At age 13, parents were asked whether the child had ever suffered from asthma. Rhinoconjunctivitis symptoms (in past 12 months) and eczema (ever) were also identified through the parental questionnaire using questions from the International Study of Asthma and Allergies in Childhood (ISAAC) as previously described (Timmermann et al. Citation2015). The children underwent a skin prick test (SPT) with extracts (Soluprick, ALK, Hørsholm, Denmark) of five common allergens (birch/grass pollen, dog/cat dander, and house dust mite [Dermatophagoides pteronyssinus]) at age 13. A wheal size ≥3 mm in diameter resulting from any of the five allergens was considered positive.
PFAS analysis
Perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluoro-octanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) were measured in maternal pregnancy serum and child serum at ages 5 and 13 (Grandjean et al. Citation2012). Analyses were carried out by isotope dilution and online solid-phase extraction followed by high performance liquid chromatography with triple quadropole mass-spectrometric analysis according to the methods of Haug et al. (Citation2009). PFOS was quantified by integration of two adjacent peaks, which represent the branched isomers and the linear isomer. The between batch-imprecision was < 7.7%, and the limit of detection (LOD) for the five PFAS was 0.03 ng/ml. All five PFAS were found at concentrations above the LOD in all maternal and child serum samples.
Potential confounders
Information about variables known to be associated with asthma and/or allergic diseases (Bodner et al. Citation1998; Reichman and Nepomnyaschy Citation2008; Just et al. Citation2010; Nagel et al. Citation2010; Civelek et al. Citation2011; Herr et al. Citation2012; Chang et al. Citation2013; de Vries et al. Citation2014; Dogaru et al. Citation2014; Mu et al. Citation2014; Netting et al. Citation2014) was obtained from the recruiting midwife’s chart (maternal pre-pregnancy BMI (weight/height2), maternal smoking during pregnancy (none/any), premature birth (< 37 weeks/≥ 37 weeks), birth season (spring: March–May; summer: June–August; fall: September–November; winter: December–February), sex, birth weight, and parity (zero/one/more than one previous birth), the parental questionnaires at age 5 (total duration of breastfeeding = exclusive and partial breastfeeding in months, number of siblings, parental smoking at home [yes/no], weekly fish dinners, and daycare attendance [yes/no]) and age 13 (fish dinners, domestic pets, and family history of asthma and allergic diseases [no/from one parent’s side/from both parents’ sides]).
Statistics
Among children included in this study, all missing values were imputed using multiple imputation by chained equations with 40 imputations based on all exposures, outcomes, and potential confounders, as well as three auxiliary variables (Azur et al. Citation2011), i.e., information about the father’s primary education (7–8th Grade/9–10th Grade), whether the child had lived abroad between ages 7 and 13 (yes/no), and whether the child is allergic to anything (yes/no or do not know). IgE and PFAS concentrations were right skewed and therefore were log10–transformed to avoid violating model assumptions when performing imputations and conducting association analyses.
Each interaction between MMR vaccination and PFAS concentration measures was tested in relation to all asthma and allergic disease measures (except cord blood IgE, which could not have been affected by subsequent MMR vaccination) in marginal analyses using the unimputed data. Interactions considered to be consistent (interactions with p < .2 in the same direction for at least three out of five PFAS measures) were included in the imputation of the asthma and allergic disease measures on which they were found to interact.
All imputations were performed using the mi impute chained command in Stata version 14.0 (StataCorp, College Station, TX). The imputation models are described in further detail in Appendix A. Using the imputed data, associations between serum concentrations of each PFAS and asthma and allergic diseases at ages 5 and 13 were determined in logistic regression models, and associations between each PFAS and total IgE in cord blood and at age 7 were determined in linear regression models. If interactions were identified in the marginal analyses using the unimputed data, an interaction term for PFAS exposure and MMR vaccination was included in the model, and potential confounders were included if associated with the PFAS measures (Appendix B). When investigating interactions, information about birth weight and family history of chronic bronchitis/asthma was also included in the models because these factors are associated with MMR vaccination uptake in the Faroese cohort and so might confound the association between MMR vaccination and asthma/allergic diseases (Timmermann et al. Citation2015). Since both PFAS concentrations and IgE measures were log-transformed, the estimates of association were converted to express the percent change in IgE associated with a doubled serum-PFAS concentration in the linear regression models and the odds ratio with a doubling of the PFAS exposure in the logistic regression models.
Sensitivity analyses were also performed in which analyses were conducted using the unimputed data, and information about maternal education (none/any education above primary school), maternal pregnancy serum dichlorodiphenyldichloroethylene (DDE), and the sum of maternal pregnancy serum polychlorinated biphenyl (PCB) concentrations was included one at a time. A simplified sumPCB concentration was calculated as the sum of congeners CB-138, CB-153, and CB-180 – multiplied by 2. Last, subgroup analyses were performed separately comparing children with atopic and nonatopic asthma to children with no asthma. At age 5, atopic asthma was classified as having both asthma and allergy (41% of asthma cases), and at age 13, atopic asthma was classified as having both asthma and positive SPT (59% of asthma cases). In these analyses, only children with complete information about both asthma and allergy/SPT were included. All analyses were performed in Stata version 14.0.
Results
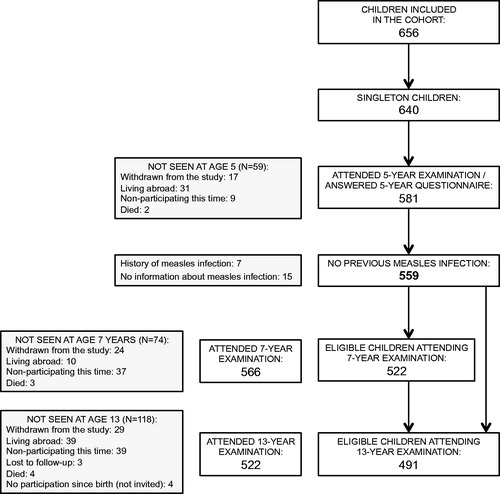
Informed consent was obtained from 648 mothers of whom 8 had twins, thus leaving 640 singleton children. Among these, 59 children were not seen at age 5, and 22 were excluded due to having a history of measles infection (n = 7) or not having provided information about measles infection (n = 15) (Timmermann et al. Citation2015), leaving 559 children (87%) eligible for the study (). The 81 (59 + 22) ineligible children had mothers with lower parity. Furthermore, among the ineligible children, mothers of 76 children had provided a blood sample in which PFAS was measured. These 76 mothers had lower serum concentrations of PFDA and PFNA, and higher levels of PFOA. Finally, 31 of the ineligible children attended the 13-year examination. These 31 children more often had asthma at age 13 compared to the 491 (522–31) children who attended the 13-year examination and were eligible for the study. No other significant (p < .05) differences between the eligible and ineligible children were found with regard to outcomes, exposures, or potential confounders.
The distributions of exposures, outcomes, and potential confounders are shown in . Among the 559 mothers included in this study, 30 did not provide a blood sample for PFAS analysis during pregnancy. Furthermore, 35 children did not provide a blood sample for PFAS analysis at age 5, and 68 children did not participate in the examination at age 13. An additional six children did not provide a blood sample for PFAS analysis at age 13. These missing values were imputed along with missing information about the outcomes and covariates. In the unimputed data, the median PFHxS and PFOS concentrations were highest (4.5 and 27.4 ng/ml) in maternal pregnancy serum and lowest in child serum at age 13, while the medians for PFOA and PFNA were highest in age-5 child serum (4.0 and 1.0 ng/ml); PFDA concentrations were stable across the three timepoints (0.3 ng/ml). At age 5, 14.2% and 13.5% were reported to have asthma and allergy, respectively; at age 13, 16.9% had had asthma, 38.7% had a positive SPT, 12.0% had allergic rhinoconjuctivitis, and 20.8% had had eczema. Only 22 out of 555 children had not received the MMR vaccination scheduled for age 15 months before the 5-year examination (). PFAS distributions including minimum and maximum values for children with and without asthma at ages 5 and 13 years by MMR vaccination status are shown in Appendix C. None of the small subgroups contain extreme values. As previously shown, children without MMR vaccination had higher risk of asthma and allergic diseases (Timmermann et al. Citation2015).
Table 1. Distribution of exposures, outcomes, and potential confounders (pre-imputation).
In the marginal analyses on the unimputed data, no MMR-vaccination interaction with maternal PFAS concentrations was found in relation to any of the outcome measures, but interactions between MMR vaccination and age-5 or age-13 PFAS in relation to some of the asthma and allergic disease measures were observed. Thus, increased serum-PFAS concentrations at age 5 tended to be associated with higher odds of a history of asthma at ages 5 and 13 and allergy at age 5 among the MMR-unvaccinated children, and among MMR-vaccinated children, an increase in serum-PFAS concentrations at age 5 tended to be associated with reduced odds of history of asthma at ages 5 and 13 and allergy at age 5. Few of these associations were, however, significant; the interaction between MMR vaccination and PFASs at age 5 was significant for PFOA in relation to asthma at ages 5 and 13 and for PFNA and PFDA in relation to asthma at age 5. The same pattern was found in the association between PFAS concentrations at age 13 and atopic eczema at age 13 (data not shown).
When using the data with imputations and taking potential confounding factors into account, the pattern described above persisted. As shown in , a doubling of the serum-PFOA concentration at age 5 was significantly associated with 10-fold increased odds of asthma at ages 5 and 13 among the MMR-unvaccinated children. A doubling of the age 5 serum-PFNA concentration was likewise significantly associated with similarly increased odds of asthma at both ages among the MMR-unvaccinated children, and a doubling of the age 5 serum-PFDA concentration showed a significant, though slightly weaker, association with asthma at age 5 among the MMR-unvaccinated children (). The interaction between MMR vaccination and age-5 PFAS was significant for PFOA, PFNA, and PFDA in relation to asthma at ages 5 and 13 and for PFHxS in relation to asthma at age 5 (). Results were similar when maternal education, pregnancy serum-DDE, or pregnancy serum-PCB concentrations were included in an analysis using the unimputed data (results not shown).
Table 2. Differences in IgE (%) and OR for history of asthma, history of allergic diseases, and SPT associated with a doubling of serum-PFAS concentrations at age 5.
In the adjusted analyses, in which interactions with MMR vaccination were not included (because no interaction was found in the marginal analyses), no clear patterns of association were found. Specifically, no associations were found between PFAS exposure at age 5 and total IgE at age 7, positive SPT at age 13, allergic rhinoconjunctivitis at age 13, or atopic eczema at age 13 (). Increased maternal serum-PFHxS concentrations were associated with increased odds of atopic eczema at age 13 (), but given the large number of analyses conducted, the risk of Type I errors was increased and this one significant association was likely a chance finding. No associations were found between maternal PFAS exposure and the other outcome measures (). Additionally, increased serum-PFHxS and serum-PFNA concentrations at age 13 were associated with reduced odds of asthma and allergic rhinoconjunctivitis, respsectively (). However, since no other significant associations were found with PFAS exposure at age 13, these associations could also have been chance findings.
Table 3. Differences in IgE (%) and OR for history of asthma, history of allergic diseases, and SPT associated with a doubling of maternal serum-PFAS concentrations.
Table 4. OR for history of asthma, history of allergic diseases, and SPT associated with a doubling of serum-PFAS concentrations at age 13.
When dividing asthma into atopic and nonatopic asthma, the interactions between MMR vaccination and PFAS at age 5 persisted for atopic asthma, whereas no significant interactions or associations were seen for nonatopic asthma (). Among MMR-unvaccinated children, a doubling in the concentration of the PFASs at age 5 was significantly associated with 9–75-fold higher odds of atopic asthma at age 5, and a doubling of the PFNA concentration was likewise significantly associated with 7-fold higher odds of atopic asthma at age 13 ().
Table 5. OR for history of allergic and nonallergic asthma associated with a doubling of serum PFAS concentrations at age 5.
Discussion
Serum-PFAS concentrations at age 5 were associated with increased odds of asthma history at ages 5 and 13 in children who had not been MMR vaccinated, although the numbers were small. The associations were reversed among MMR-vaccinated children. The same tendencies, though not significant, were seen in the association between serum-PFAS at age 5 and history of allergy at age 5 and between serum-PFAS at age 13 and history of atopic eczema at age 13.
A Taiwanese case-control study reported that increased PFAS exposures were associated with concurrent asthma among 10–15-year-olds (Dong et al. Citation2013), while a cross-sectional study using the U.S. National Health and Nutrition Examination Survey (NHANES) data (1999–2000, 2003–2004, 2005–2006, 2007–2008) of 12–19-year-olds found that serum-PFOA was associated with increased odds of asthma while PFOS showed a negative association (Humblet et al. Citation2014), and finally a recent study using NHANES data (2005–2006) of 12–19-year-olds did not find any association between PFAS and asthma but found that higher serum-PFOS concentrations were associated with reduced odds of sensitization to any allergen, while increased PFOA and PFNA were associated with increased serum IgE (Stein et al. Citation2016). Neither of these studies examined MMR vaccinations or other factors that might contribute to the different findings.
In regard to prenatal PFAS exposure, cord blood PFOS and PFOA concentrations have been found to correlate positively with cord blood IgE levels in boys (Wang et al. Citation2011), while high maternal PFOA concentrations were associated with decreased cord blood IgE levels in girls (Okada et al. Citation2012) and reduced risk of allergic diseases during the first 24 months (Okada et al. Citation2014). Recent studies found that maternal PFOA concentrations were inversely associated with current wheezing among Ukrainian but not in Greenlandic children aged 5–9 years (Smit et al. Citation2015), and other types of maternal PFAS were inversely associated with allergic diseases among 4-year-old children (Goudarzi et al. Citation2016). Several other analyses, however, showed no associations between prenatal exposure to PFAS and cord blood IgE (Ashley-Martin et al. Citation2015) or asthma and allergy in childhood (Wang et al. Citation2011; Okada et al. Citation2012; Granum et al. Citation2013), which is in accordance with the current findings.
The mechanisms underlying immunomodulating effects of various PFAS are far from fully understood, but these chemicals have been shown to affect activation of human immune cells and reduce both pro- and antiinflammatory cytokine production in vitro (Corsini et al. Citation2014). In mice, PFOS exposure has been shown to lead to an increase in interleukin (IL)-4 and IL-10 and to decrease IL-2 and interferon (IFN)-γ, thereby indicating a shift toward a TH2 immune response (Dong et al. Citation2011). If such a shift occurs, it could perhaps explain the positive association between serum-PFAS concentrations and asthma among the MMR-unvaccinated children in the current analyses, while the MMR vaccination may rather shift the immune response toward a more TH1-prone state (Pabst et al. Citation1997; Ovsyannikova et al. Citation2003), thereby preventing or counteracting a PFAS-induced TH2-shift. If PFAS exposure affects the TH1/TH2 balance, an interaction between MMR vaccination and serum-PFAS concentrations thus seems plausible.
If MMR vaccination modifies the effect of PFAS on asthma as suggested by the current findings, differences in vaccination schedules might explain why the association between PFAS exposure and asthma differs between studies from different parts of the world. However, the range of serum-PFAS concentrations is also important. Both the age 5- and 13-year serum-PFAS concentrations of children in this study were lower than among Taiwanese children, except for PFOA, which was higher (Dong et al. Citation2013), and the age 5 serum-PFAS concentrations were similar to those of American children whereas the age 13 serum-PFAS concentrations were lower in the present study (Humblet et al. Citation2014). It is possible MMR vaccination can protect against adverse effects of PFAS on asthma only when PFAS concentrations are relatively low.
It is important to note that a main limitation of this study is that very few cohort children were not MMR vaccinated, which limited the power to detect interactions between MMR vaccination and PFAS concentrations. Therefore, the present hypothesis about MMR vaccination modifying the association between PFAS and asthma needs to be reexamined in populations with a larger number of MMR-unvaccinated children. In addition, this study conducted a large number of analyses, which increased the risk of chance findings. Therefore, it did not look for single significant associations but rather patterns in the associations.
As common in cohort studies, follow-up was not complete, and this study compensated for missing data by multiple imputations. Assuming that data were missing at random, this approach would be expected to produce more correct estimates of the true associations (Azur et al. Citation2011). However, those cohort members who did not provide information on history of measles infection had to be excluded. They differed from those included with regard to maternal serum-PFAS concentrations and asthma at age 13, and one cannot rule out the possibility of some selection bias. However, with 87% of the original cohort included in the present study, it is believed that any potential bias was minimal. These analyses showed that some confounding did occur, but only marginally affected the results. However, in observational studies, the possibility of residual confounding can never be excluded.
Imprecision of the asthma measures must also be considered. At age 13, questionnaire data about asthma have been shown to have a high sensitivity and specificity in relation to clinical asthma (Fuso et al. Citation2000), but this measure is probably less precise at age 5 due to difficulties in distinguishing asthma from bronchitis. Any misclassification is, however, unlikely to be associated with the PFAS exposure or misclassification of PFAS exposure; therefore any bias likely would have been toward null (Kristensen Citation1992).
Overall, prenatal PFAS exposure did not seem to affect the risk of childhood asthma or allergy. Much previous research has focused on prenatal exposure, but both the pre- and postnatal periods are known to be important in establishing an immune deviation from TH2 to TH1-skewed immunity (Romagnani Citation2014), and in this study, associations were found only with postnatal exposure. In addition, it has been established that children are highly exposed to PFAS in early life through breastfeeding (Mogensen et al. Citation2015), emphasizing the need to further study the effects of postnatal PFAS exposure.
Among the MMR-unvaccinated children, stronger associations were found between PFAS concentrations and asthma than between PFAS and other allergic diseases, and stronger associations were observed for atopic than for nonatopic asthma. This indicated that PFAS exposure may affect development of allergy. However, given the small number of unvaccinated children, these findings should be interpreted with caution.
Conclusions
Serum PFAS concentrations at age 5 years were associated with increased odds of asthma at ages 5 and 13 years among MMR-unvaccinated children. The associations were reversed among MMR-vaccinated children. Similar analyses failed to identify any association with prenatal PFAS exposure. These findings suggest that MMR vaccination should be considered as a potential effect-modifier in studies of adverse effects of PFAS on immune functions, but larger studies are needed to support the current findings.
Disclosure statement
The authors report no conflict of interests. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Ashley-Martin J, Dodds L, Levy A, Platt R, Marshall J, Arbuckle T. 2015. Pre-natal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL-33. Environ Res. 140:360–368.
- Azur M, Stuart E, Frangakis C, Leaf PJ. 2011. Multiple imputation by chained equations: What is it and how does it work? Int J Methods Psychiatr Res. 20:40–49.
- Bodner C, Godden D, Seaton A. 1998. Family size, childhood infections and atopic diseases. The Aberdeen WHEASE Group. Thorax. 53:28–32.
- Bonnelykke K, Pipper C, Bisgaard H. 2010. Transfer of maternal IgE can be a common cause of increased IgE levels in cord blood. J Allergy Clin Immunol. 126:657–663.
- Chang W, Yang K, Wu M, Wen Y, Hsi E, Chang J, Lin Y, Kuo H, Chang W. 2013. Close correlation between season of birth and the prevalence of bronchial asthma in a Taiwanese population. PLoS One. 8:e80285.
- Civelek E, Cakir B, Orhan F, Yuksel H, Boz A, Uner A, Sekerel B. 2011. Risk factors for current wheezing and its phenotypes among elementary school children. Pediatr Pulmonol. 46:166–174.
- Corsini E, Luebke R, Germolec D, DeWitt J. 2014. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol Lett. 230:263–270.
- de Vries A, Reynolds R, Seckl J, van der Wal M, Bonsel G, Vrijkotte T. 2014. Increased maternal BMI is associated with infant wheezing in early life: a prospective cohort study. J Devel Orig Health Dis. 25:1–10.
- Dogaru C, Nyffenegger D, Pescatore A, Spycher B, Kuehni C. 2014. Breastfeeding and childhood asthma: Systematic review and meta-analysis. Am J Epidemiol. 179:1153–1167.
- Dong G, Liu M, Wang D, Zheng L, Liang Z, Jin Y. 2011. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch Toxicol. 85:1235–1244.
- Dong G, Tung K, Tsai C, Liu M, Wang D, Liu W, Jin Y, Hsieh W, Lee Y, Chen P. 2013. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ Health Perspect. 121:507–513.
- Fromme H, Tittlemier S, Volkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds – Exposure assessment for the general population in Western countries. Intl J Hyg Environ Health. 212:239–270.
- Fuso L, de Rosa M, Corbo G, Valente S, Forastiere F, Agabiti N, Pistelli R. 2000. Repeatability of the ISAAC video questionnaire and its accuracy against a clinical diagnosis of asthma. Respir Med. 94:397–403.
- Goudarzi H, Miyashita C, Okada E, Kashino I, Kobayashi S, Chen CJ, Ito S, Araki A, Matsuura H, Ito Y, et al. 2016. Effects of pre-natal exposure to perfluoroalkyl acids on prevalence of allergic diseases among 4-year-old children. Environ Intl. 94:124–132.
- Grandjean P, Andersen E, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 307:391–397.
- Grandjean P, Poulsen L, Heilmann C, Steuerwald U, Weihe P. 2010. Allergy and sensitization during childhood associated with pre-natal and lactational exposure to marine pollutants. Environ Health Perspect. 118:1429–1433.
- Granum B, Haug L, Namork E, Stolevik S, Thomsen C, Aaberge I, van Loveren H, Lovik M, Nygaard U. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 10:373–379.
- Gruber C, Illi S, Lau S, Nickel R, Forster J, Kamin W, Bauer CP, Wahn V, Wahn U; MAS-90 Study Group. 2003. Transient suppression of atopy in early childhood is associated with high vaccination coverage. Pediatrics. 111:282–288.
- Haug L, Thomsen C, Becher G. 2009. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatog. 1216:385–393.
- Heilmann C, Budtz-Jorgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. 2010. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect. 118:1434–1438.
- Herr M, Just J, Nikasinovic L, Foucault C, Le Marec A, Giordanella J, Momas I. 2012. Risk factors and characteristics of respiratory and allergic phenotypes in early childhood. J Allergy Clin Immunol. 130:389–396.
- Houde M, Martin J, Letcher R, Solomon K, Muir D. 2006. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol. 40:3463–3473.
- Humblet O, Diaz-Ramirez L, Balmes J, Pinney S, Hiatt R. 2014. Perfluoroalkyl chemicals and asthma among children 12-19-years-of-age: NHANES (1999-2008). Environ Health Perspect. 122:1129–1133.
- Just J, Belfar S, Wanin S, Pribil C, Grimfeld A, Duru G. 2010. Impact of innate and environmental factors on wheezing persistence during childhood. J Asthma. 47:412–416.
- Kato K, Calafat A, Wong L, Wanigatunga A, Caudill S, Needham L. 2009. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001-2002. Environ Sci Tech. 43:2641–2647.
- Kristensen P. 1992. Bias from nondifferential but dependent misclassification of exposure and outcome. Epidemiology. 3:210–215.
- Mogensen U, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol. 49:10466–10473.
- Mu M, Ye S, Bai M, Liu G, Tong Y, Wang S, Sheng J. 2014. Birth weight and subsequent risk of asthma: A systematic review and meta-analysis. Heart Lung Circ. 23:511–519.
- Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP; ISAAC Phase Two Study Group. 2010. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 65:516–522.
- Netting M, Middleton P, Makrides M. 2014. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition. 30:1225–1241.
- Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, Itoh K, Ikeno T, Tamakoshi A, Kishi R. 2014. Pre-natal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ Intl. 65:127–134.
- Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, Konishi K, Ito Y, Ito R, Nakata A, et al. 2012. Pre-natal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res. 112:118–125.
- Ovsyannikova I, Reid K, Jacobson R, Oberg A, Klee G, Poland G. 2003. Cytokine production patterns and antibody response to measles vaccine. Vaccine. 21:3946–3953.
- Pabst H, Spady D, Carson M, Stelfox H, Beeler J, Krezolek M. 1997. Kinetics of immunologic responses after primary MMR vaccination. Vaccine. 15:10–14.
- Reichman N, Nepomnyaschy L. 2008. Maternal pre-pregnancy obesity and diagnosis of asthma in offspring at age 3 years. Matern. Matern Child Health J. 12:725–733.
- Romagnani S. 2014. T-cell sub-populations. Chem Immunol Allergy. 100:155–164.
- Roost H, Gassner M, Grize L, Wuthrich B, Sennhauser F, Varonier H, Zimmermann H, Braun-Fahrlander C, Team S. 2004. Influence of MMR vaccinations and diseases on atopic sensiti-zation and allergic symptom in Swiss schoolchildren. Pediatr Allergy Immunol. 15:401–407.
- Smit L, Lenters V, Hoyer B, Lindh C, Pedersen H, Liermontova I, Jonsson B, Piersma A, Bonde J, Toft G, et al. 2015. Pre-natal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy. 70:653–660.
- Stein C, McGovern K, Pajak A, Maglione P, Wolff M. 2016. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12-19 yr: National Health and Nutrition Examination Survey. Pediatr Res. 79:348–357.
- Timmermann C, Osuna C, Steuerwald U, Weihe P, Poulsen L, Grandjean P. 2015. Asthma and allergy in children with and without prior measles, mumps, and rubella vaccination. Pediatr Allergy Immunol. 26:742–749.
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. 2008. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 28:251–269.
- Wang I, Hsieh W, Chen C, Fletcher T, Lien G, Chiang H, Chiang C, Wu T, Chen P. 2011. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ Res. 111:785–791.
Appendix A
Imputation models.
Appendix B
Associations between parental/child characteristics and PFAS tested in linear regression models, N = 559.
Appendix C
Distributions of PFASs by MMR vaccination status at age 5 and asthma status at ages 5 and 13 years (pre-imputation).