Abstract
The present study was undertaken to detect antibodies against etanercept (ETN) in a group of Colombian patients with rheumatoid arthritis (RA) and being treated with Enbrel® vs. Etanar®. From these patients with RA, clinical and laboratory data were collected and serum taken for anti-drug antibody (ADAb) analysis. Samples from 32 patients (16 who had been treated with Enbrel® and 16 with Etanar®) were analyzed. Positive sera for ADAb were found in six of the 32 subjects (18.7%); five (31.2%) in the Enbrel® group and one (6.25%) in the Etanar® group. Patients under treatment with Enbrel® registered a longer disease duration than patients being treated with Etanar® (15.4 years vs. 10.98 years, p = 0.175) as well as a longer average treatment with the drug (45.7 vs. 23.9 months, p = 0.052). The percentage of patients with disease activity defined as a disease activity score by C-reactive protein (DAS28-CRP) scores ≥2.3 was higher in those patients with positive sera in the enzyme-linked immunosorbent assay (ELISA) (66.7%) than in those with negative sera (34.6%). A logistic regression test revealed that the higher the DAS28-CRP value, the higher the ELISA absorbance value. The results showed evidence of greater frequency of ADAb in patients treated with ETN than has been reported to date. Greater disease activity was seen in those patients in whose serum ADAb had been detected. Significant differences were found between the positive ELISA for the group of patients treated with Enbrel® compared to those treated with Etanar®. Some of the factors that could explain this difference are the length of the treatment time with the drug, the commercial ELISA kit used to detect ADAb, or the immunogenicity itself of each product.
Introduction
Pharmaceutical biotechnology has developed proteins with therapeutic application which have positively impacted the quality of life and the course of various autoimmune diseases including rheumatoid arthritis (RA) (Kelley Citation2009). The safety and efficacy of these biotherapeutic products could be affected by unwanted immune responses which are more common with this type of drug given their proteic nature. Said immune activation could affect both the safety and effectiveness of the product. This immunogenic response may vary between irrelevant events and serious side effects such as autoimmune reactions or the creation of anti-drug antibodies (ADAb) that are linked to loss of effectiveness for some drugs (Descotes Citation2009).
The experimental models used during the research stages for these products cannot completely predict this immunotoxic potential since they are in vitro experiments, models in vivo with animal species or studies in small groups of human beings (Shukla & Thömmes Citation2010). Given the above, one of the most relevant objectives for pharmaco-vigilance of biotechnologic drugs must be the identification and active search for unknown immunogenic reactions to these pharmaceutical products. Bearing in mind the potential variations in immune responses to therapeutic proteins based on the genotype of each population group, it is not completely appropriate to extrapolate from the results of immunogenic studies of one population group to another or from one drug to another. The above is especially important in the midst of the current debate after the patent expiration for drugs of this nature which has led to the development and marketing of follow-on biological drugs also called biosimilars (van der Laan & van Loveren Citation2005; Roger Citation2010).
These monoclonal antibodies and fusion proteins are among the more highly developed products of “biotech” origin and currently hold an important place in therapy for immune-based diseases. One group of these molecules, those targeting tumor necrosis factor (TNF)-α, are of particular interest due to their frequency of use in different autoimmune diseases, i.e. RA (Singh et al. Citation2009; Geiler et al. Citation2011). However, host development of anti-bodies against anti-TNFα drugs, as well as the subsequent loss of the drug effectiveness is known to occur (Emi Aikawa et al. Citation2010). This presents a major problem in designing of therapies to treat these types of diseases.
Etanercept (ETN) was the first biotechnological product in Colombia that targeted TNFα to have health records for both the original product and the follow-on biologic, Etanar®, produced by the Shanghai CP Guojian Pharmaceutical Co (Shanghai, China). Both products are currently in use in the country and have reported similar results with regard to effectiveness (Rondon Citation2010; Santos-Moreno Citation2015, Citation2016). In spite of the frequency with which they are used, the number of antibodies produced for either of the two types of products among Colombians is unknown. The generation of ADAb for ETN in RA patients is described as having frequencies that vary between 0% and 5.6% on the global level. Among the anti-TNFα drugs, ETN has the lowest percentage of generating these types of antibodies in all cases of non-neutralizing antibodies (Moreland et al. Citation1999; Weinblatt et al. Citation1999; Keystone et al. Citation2004). No significant relationship has been seen between the presence of these antibodies with ETN serum concentrations, the patient’s clinical response, nor the frequency of adverse events in different clinical studies (Dore et al. Citation2007; Hoshino et al. Citation2012; Jamnitski et al. Citation2012).
Thus, the study reported here was undertaken to determine the presence of antibodies targeting ETN in a group of Colombian patients with a diagnosis of RA treated with either the original drug (Enbrel®) vs. a follow-on biologic (Etanar®) and to describe clinical variables that were possibly associated as part of an active process of pharmaco-vigilance. A new cutoff point for the enzyme-linked immunosorbent assay (ELISA) test was set lower than that recommended by the manufacturer of the product (Grifols®). The above was performed considering that the differences in absorbance values below the cutoff point recommended in the commercial kit were also of experimental interest. While the differences could correspond to low concentrations of ADAb, the latter would be important for making comparisons of immunogenicity of different biological products that would be expected to be highly similar. Any such differences in ADAb arising between these types of drugs would be suggestive of differences in their immunogenicity.
Materials and methods
Patients
Subjects were recruited from two medical centers that specialized in treating patients with rheumatic and autoimmune diseases in Bogota (Colombia). Only patients who were over 18 years-of-age and met the 1987 diagnostic criteria of the American College of Rheumatology for RA (Arnett et al. Citation1988) and who had been treated for a period of no less than 3 months with etanercept [whether it was Enbrel® or Etanar®] were included. Patients who had been treated with a different biotechnological drug in the last 6 months and those with infections or neoplastic diseases were not included. Those who met the above criteria and saw a doctor between August and October 2015 were included. There was a total of 32 patients (five male, 27 female) including 16 (2 M, 14 F) who had been treated with Enbrel® and 16 (3 M, 13 F) with Etanar®. After having received informed consent of each individual, inflamed painful joints were examined. Clinical and laboratory data were registered and blood taken for ADAb analysis (see below).
Blood analyses
For serum analyses, a 10-ml blood sample from basilica vein was taken from each patient at time of enrollment. The samples were centrifuged immediately and the serum obtained transported within 12 h [at 2–8 °C] before being aliquoted (100 μl) and then frozen at –20 °C in a Thermo Revco UXF freezer (Waltham, MA) until needed. For actual analyses, thawed samples were analyzed with a commercial Promonitor anti-ETN ELISA kit (Progenika Biopharma, Barcelona, Spain) and following manufacturer protocols. Each kit contained ETN as antigen [used as active pharmaceutical ingredient in Enbrel®]. All sera were analyzed at dilutions of 1:2; final measures were taken at 450 nm in an iMark® microplate reader (Bio-Rad, Hercules, CA).
Given the research conditions and the goal to analyze reactogenicity in Colombian patients vis-à-vis the ETN molecule, it was important to establish a cutoff point other than the one recommended in the kit. Said point was obtained using the mean of absorbance values +3 SD from the negative controls (sera from two healthy volunteers not receiving treatment with ETN nor any other anti-TNF molecule) from two experiments done at different times. In addition, the ELISA kit provided two different negative controls (i.e. sera from healthy volunteers without any kind of treatment and a mix of reagents without sera).
Statistical analysis
The continuous variables were described with the usual minimum statistics, first quartile, mean, average, third quartile, maximum and standard deviation. The calculation for each variable was done on the basis of the amount of effective data and the amount of missing data. A normality test was done on each continuous variable. The categorical variables are reported at absolute and relative frequencies for each one of the categories. The total was calculated on the effective size of the variable, i.e. on the total number of available data. To evaluate the statistical associations between the ELISA variable and the rest of the variables, the Kruskal–Wallis and Chi-square tests were used. The first was used to evaluate differences between the distributions of each continuous variable given within each one of the variable ELISA categories. A Chi-square test was used to evaluate independence of the ELISA variable from another categorical variable. A logistic regression analysis was done to build a model to explain the variation seen in the seropositivity of the ELISA test with respect to other relevant variables from both the clinical and statistical points of view. A more parsimonious model than the above – which was obtained through a stepwise algorithm based on the Akaike criterion (Venables & Ripley Citation2002) only preserved the following variables: disease activity score by C-reactive protein (DAS28-CRP), brand of drug, and time of treatment with drug. All statistical analyses were done using R v.3.3.1 software (R Foundation, Vienna, Austria).
Results
There were 32 individuals with a diagnosis of RA in the study. Of these, 84.4% were women. The average and mean ages were 58.9 and 58.0 years, respectively, with an interquartile range of 14.8. The average duration of the disease was 13.2 years while the average duration of the treatment was 34.8 months. Of the patients, 59.3% were under treatment with corticosteroids, 81.2% with conventional disease-modifying anti-rheumatic drugs (DMARDc), i.e. methotrexate, and 25.0% of them had received treatment with biological drugs before study entry.
The most frequently associated chronic diseases were cardiovascular disease (CVD), which was present in 31% of the individuals included and hypothyroidism in 22% of them. No thrombotic events, neoplasms nor depression were observed in the subjects evaluated. With respect to the characteristics of RA, the majority of the patients presented positive for rheumatoid factor (RF) or for anti-cyclic-citrullinated peptide antibodies (anti-CCP). Only one patient was negative for both autoantibodies, but bone erosion that is characteristic of RA was seen in X-rays of the hands. Erosive disease was presented by 68% of the patients; rheumatoid nodules as an extra-articular manifestation were identified in only two patients.
General characteristics of the population and drug treatments are provided in . ELISA absorbance and DAS-28 score for all patients organized by time of treatment with each drug are provided in . Of the 32 patients, six (18.8%) were positive in the ELISA. There were no significant differences between patients being treated with Enbrel® and with Etanar® with respect to age or clinical manifestations such extra-articular compromise, erosiveness or CVD. There were also no significant differences in differences in RF, anti-CCP, ANA titers, erythrocyte sedimentation rate or C-reactive protein values.
Table 1. Clinical, laboratory and treatment characteristics.
Patients under treatment with Enbrel® had a longer disease duration as well as a longer treatment time in comparison to the patients treated with Etanar®. The disease activity score by erytrocyte sedimentation rate (DAS28-ESR) and DAS28-CRP scores were higher in the Etanar® group although without significant differences between the two groups (). The DAS28-ESR and DAS28-CRP scores were higher in the group with positive ELISA test although without significant differences between the two groups (). In the Enbrel® group, 5/16 (31.2%) ADAb-positive sera were found and 1/16 (6.2%) in the Etanar® group, an outcome that was significantly different (). The proportion of patients with disease activity defined as a DAS28-CRP score greater than or equal to 2.3 was higher in patients with positive sera on the ELISA test (4/6 = 0.67) than in those patients with negative sera (9/26 = 0.35). shows the distribution of DAS28-CRP values based on ELISA reactogenicity in a box-and-whisker plot. A logistic regression model of the association between ELISA [given in average absorbance values], and DAS28-CRP scores is shown in .
Figure 1. Reactogenicity in ELISA (i.e. ADAb-positive outcomes) among samples from patients in the Enbrel® (N = 16) and in the Etanar® (N = 16) groups.
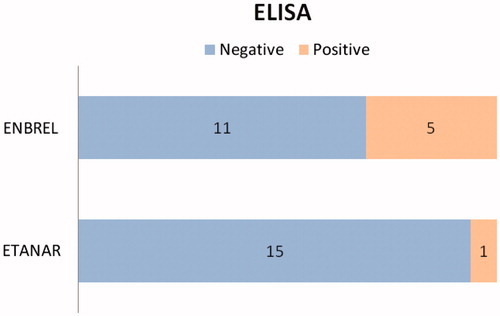
Figure 2. Distribution of DAS28-PCR (disease activity score by C-reactive protein) values based on positive or negative reactogenicity in ELISA. Dark line shows mean for each group of values and box shows values between first and third quartiles. Upper and lower value limits for each group are likewise shown. pos: positive. neg: negative.
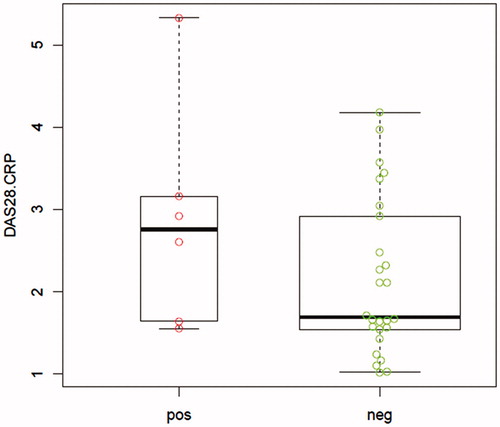
Figure 3. Effect plot of DAS28-CRP on ELISA seropositivity. Gray area correspond to 95% prediction interval. DAS28-CRP was a variable preserved from the logistic regression analysis build to explain the variation in seropositivity of the ELISA test with respect to other variables, after applying the stepwise algorithm based on the Akaike information criterion (Venables & Ripley Citation2002).
Table 2. ELISA absorbance and DAS-28 scores for all patients organized by time of treatment with Enbrel® or Etanar®.
Table 3. Average of DAS28 scores for each sub-group of patients according to drug.
Table 4. Average of DAS28 scores for patients negative or positive in the ELISA test.
Discussion
This study was the first to evaluate a presence of anti-ETN ADAb in a Colombian population and also to include patients under treatment with Etanar® in the search. The clinical characteristics of RA and accompanying medical status of the patients included in this study were the ones expected for this type of population based on gender and age. The analyses here found there were no significant differences between the patients under treatment with Enbrel® or Etanar® with respect to age (normal distribution), gender (female:male) and clinical or laboratory manifestations, i.e. there were strong similarities between the two groups.
However, it was noted that the patients under treatment with Enbrel® had a longer disease duration as well as a longer treatment time in comparison to the patients treated with Etanar®. The above could be explained by the fact that Etanar® entered the market in this country at a later date than the original drug; as a result, its use is not as extensive as Enbrel®. The DAS28-ESR and DAS28-CRP scores were higher in Etanar® subjects, though these differences were not significant.
Six of the 32 patients were seen to test positive on the ELISA assay which is equivalent to 18.75%. For the Enbrel® group, five of the 16 in this group were positive which corresponds to 31.2%. These numbers are higher than the values in other studies that report low ETN immunogenicity (Anderson Citation2005; Aarden et al. Citation2008; Emi Aikawa et al. Citation2010; Atzeni et al. Citation2013; Vincent et al. Citation2013). Low immunogenicity has also been reported in RA patients with ETN treatment (Moreland et al. Citation1999; Weinblatt et al. Citation1999; Bathon et al. Citation2000; Keystone et al. Citation2004; Dore et al. Citation2007; Klareskog et al. Citation2011; Hoshino et al. Citation2012; Jamnitski et al. Citation2012). As this was a study with a limited number of patients, these percentages should be confirmed in future studies that include a larger number of individuals.
As a cutoff point for the ELISA test was defined that was different from the one recommended by the producers of the commercial kit used in this study, new assays should be done with different ELISA test characteristics. These results in the seropositivity of the test for ADAb, although limited by the number, are relevant for the clinical implications that would be derived if confirmed by future studies. What turns out to be equally important is the discovery of the relationship between this ELISA positivity, which would identify the presence of ADAb against ETN, and the greater disease activity which is defined by the DAS28-CRP score in this study. This finding is also in contradiction with what has been reported in other studies in which the presence of between 0.01% and 5.6% non-neutralizing antibodies has been described without these being related to modifications in the clinical outcomes evaluated including disease activity (Weinblatt et al. Citation1999; Bathon et al. Citation2000; Keystone et al. Citation2004; Klareskog et al. Citation2004; Dore et al. Citation2007).
Immunogenicity in patients treated with anti-TNF agents has been examined for infliximab, adalimumab and ETN. Recently, a meta-analysis revealed a presence of ADAb in the cases of infliximab and adalimumab that was related to a lower rate of response to the drug. For ETN, no ADAb were detected in the same meta-analysis (Garces et al. Citation2013). In the current study, 5/16 (31.2%) ADAb-positive sera were identified in the Enbrel® group and 1/16 (6.2%) in the Etanar® group. A difference was also seen between absorbance values for these two groups; this difference was the only significantly different one. The lower absorbance values seen in sera from patients treated with Etanar® could be explained by a low reactogenicity of the sera to a standardized ELISA with an Enbrel® ETN molecule. Though this hypothesis is considered probable, there are other explanations equally possible, such as lower immunogenicity of Etanar® in comparison to Enbrel® – whether this would be due to characteristics of the molecule itself or a shorter exposure time to Etanar® in this group of patients. Given the sample size as well as the absence of an ELISA test specifically standardized for Etanar®, it is difficult to ratify or rule out this hypothesis.
The small number of patients as a sample size is one of the limitations of this study even though they were representative of the Colombian patients with RA under treatment with ETN. Another point to note is the test used to measure the ADAb. ELISA assays are the most common laboratory test among the majority of tests that evaluate immunogenicity. However, as is well-known, it is associated with a higher rate of false positives, which could explain the higher frequency of ADAb in this study. Another limitation on this study is the cross-sectional evaluation of ADAb in the patients. A longitudinal evaluation that includes ETN sera levels would provide very valuable additional information.
The search for immunogenicity in biopharmaceutical products should be a constant and active task after they are put on the market. This should be done the same way for both original drugs and biosimilars since it is not possible to foresee with any significant degree of certainty the immunogenicity of these proteins from the tests done during the development stages prior to being placed on the market. Extrapolating from the pre- and post-market immunogenicity results with the original is not recommended either since small variations in both the molecule and its auxiliary agents as well as in manufacturing, transportation, storage and preservation processes could bring about different immunological responses.
We believe that the absence of demonstrable clinical consequences or the neutralizing nature of the ADAb are insufficient standards by which to deny the existence of immunogenicity or immunotoxicity phenomena. Therefore, doing laboratory tests and other in vitro tests in search of these types of antibodies as well as careful interpretation of them should be considered one of the many tasks for active pharmaco-vigilance of these products.
Conclusions/recommendations
The results of the ELISA assay for this group of patients showed evidence of a greater frequency of ADAb in patients treated with ETN than what has been reported to date. Greater disease activity was observed in those patients in whose serum ADAb was detected. Significant differences were found between the sera from the patients treated with Enbrel® and that from the ones treated with Etanar® in the ELISA reactogenicity. Some of the factors that could explain this difference are the length of the treatment time with the drug, the commercial ELISA kit used to detect ADAb, or the immunogenicity of each product itself. Given this was a cross-sectional evaluation, it is necessary to monitor the patients included in the current study. Results obtained from different analyses of immunogenicity would make it possible to gain knowledge and collect evidence in order to interchange protein molecules.
Acknowledgements
The authors express their sincere gratitude to the Immunotoxicology Research Group, with special thanks to Y. Dominguez for her technical support with the ELISA. The authors wish to extend sincere thanks to Dr. Juan Manuel Anaya and the team (Carolina Ramírez, Yenny Acosta, Diana Monsalve, Nicolás Molano) at the Center for Autoimmune Diseases Research (CREA) in the School of Medicine and Health Sciences at Universidad del Rosario for assistance in conducting the experimental phase of this study. Sincerest thanks to Dr. Rubén Mantilla and Juan C. Sarmiento in FUNINDERMA medical center for their clinical continuous support.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Aarden L, Ruuls S, Wolbink G. 2008. Immunogenicity of anti-tumor necrosis factor antibodies: Toward improved methods of anti-antibody measurement. Curr Opin Immunol. 20:431–435.
- Anderson P. 2005. Tumor necrosis factor inhibitors: Clinical implications of their different immunogenicity profiles. Sem Arthritis Rheum. 34:19–22.
- Arnett F, Edworthy S, Bloch D, McShane D, Fries J, Cooper N, Healey L, Kaplan S, Liang M, Luthra H, et al. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumat. 31:315–324.
- Atzeni F, Talotta R, Salaffi F, Cassinotti A, Varisco V, Battellino M, Ardizzone S, Pace F, Sarzi-Puttini P. 2013. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev. 12:703–708.
- Bathon J, Martin R, Fleischmann R, Tesser J, Schiff M, Keystone E, Genovese M, Wasko M, Moreland L, Weaver A, et al. 2000. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 343:1586–1593.
- Descotes J. 2009. Immunotoxicity of monoclonal antibodies. MAbs. 1:104–111.
- Dore R, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A, Zhou L, Peloso P. 2007. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin Exp Rheumatol. 25:40–46.
- Emi Aikawa N, de Carvalho J, Artur Almeida Silva C, Bonfá E. 2010. Immunogenicity of anti-TNF-α agents in autoimmune diseases. Clin Rev Allergy Immunol. 38:82–89.
- Garces S, Demengeot J, Benito-Garcia E. 2013. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: A systematic review of the literature with a meta-analysis. Ann Rheum Dis. 72:1947–1955.
- Geiler J, Buch M, McDermott M. 2011. Anti-TNF treatment in rheumatoid arthritis. Curr Pharm Des. 17:3141–3154.
- Hoshino M, Yoshio T, Onishi S, Minota S. 2012. Influence of antibodies against infliximab and etanercept on the treatment effectiveness of these agents in Japanese patients with rheumatoid arthritis. Modern Rheumatol. 22:532–540.
- Jamnitski A, Krieckaert C, Nurmohamed M, Hart M, Dijkmans B, Aarden L, Voskuyl A, Wolbink G. 2012. Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann Rheum Dis. 71:88–91.
- Kelley B. 2009. Industrialization of mAb production technology: The bioprocessing industry at a crossroads. MAbs. 1:443–452.
- Keystone E, Schiff M, Kremer J, Kafka S, Lovy M, DeVries T, Burge D. 2004. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: Results of a multi-center, randomized, double-blind, placebo-controlled trial. Arthritis Rheumat. 50:353–363.
- Klareskog L, Gaubitz M, Rodríguez-Valverde V, Malaise M, Dougados M, Wajdula J. 2011. Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol. 29:238–247.
- Klareskog L, van der Heijde D, de Jager J, Gough A, Kalden J, Mola E, Martín Mola E, Pavelka K, Sany J, Settas L, et al. 2004. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: Double-blind randomised controlled trial. Lancet. 363:675–681.
- Moreland L, Schiff M, Baumgartner S, Tindall E, Fleischmann R, Bulpitt K, Weaver A, Keystone E, Furst D, Mease P, et al. 1999. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 130:478–486.
- Roger S. 2010. Biosimilars: Current status and future directions. Expert Opin Biol Ther. 10:1011–1018.
- Rondon F. 2010. Etanar therapy in real-life patients with rheumatoid arthritis [abstract]. Arthritis Rheumat. 62(Suppl 10):1811.
- Santos-Moreno P. 2015. Clinical outcomes in a cohort of Colombian patients with rheumatoid arthritis treated with Etanar, a new biologic type rhTNFR:Fc. Clin Exp Rheumatol. 33:858–862.
- Santos-Moreno P. 2016. Direct comparative effectiveness among 3 anti-tumor necrosis factor biologics in a real-life cohort of patients With rheumatoid arthritis. J Clin Rheumatol. 22:57–62.
- Shukla A, Thömmes J. 2010. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 28:253–261.
- Singh J, Christensen R, Wells G, Suarez-Almazor M, Buchbinder R, Lopez-Olivo M, Ghogomu E, Tugwell P. 2009. Biologics for rheumatoid arthritis: An overview of Cochrane reviews. Cochrane Database Syst Rev. 4:CD007848.
- van der Laan J, van Loveren H. 2005. Current status and burning issues in immunotoxicity testing of drugs. Toxicol Appl Pharmacol. 207:435–440.
- Venables W, Ripley B, editors. 2002. Modern applied statistics with issues of accuracy and scale. New York: Springer.
- Vincent F, Morand E, Murphy K, Mackay F, Mariette X, Marcelli C. 2013. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: A real issue, a clinical perspective. Ann Rheum Dis. 72:165–178.
- Weinblatt M, Kremer J, Bankhurst A, Bulpitt K, Fleischmann R, Fox R, Jackson C, Lange M, Burge D. 1999. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 340:253–259.
