Abstract
Fluoro-edenite (FE) is an asbestiform mineral fiber spotted in the lava rocks excavated from a stone quarry in Biancavilla (Italy). The derived material had been employed locally for building purposes. Previous studies found evidence that exposure to asbestos may induce autoimmunity, with frequency of anti-nuclear autoantibodies (ANA). The aim of this study was to explore the relationship between FE exposure and autoimmune responses in an exposed population. For the study, 60 subjects living in the area of Biancavilla and 60 subjects as control group were randomly invited to participate. A free medical check, including spirometry and a high-resolution computer tomography chest scan, was given to all participants. ANA were determined by indirect immunofluorescence. On medical check, no subject showed any sign and/or symptoms of illness. Prevalence for samples positive to ANA were 70% (n = 42) and 25% (n = 15), respectively, for exposed and non-exposed subjects (p < 0.05). The presence of pleural plaques (PP) was found in 21 (30%) of the exposed subjects and in 2 (3%) of the non-exposed participants. PP subjects were always ANAs positive. In conclusion, as already it was observed with exposure to asbestos fibers, levels of ANA seemed to significantly increase in subjects who had been exposed to FE. Furthermore, all subjects showing PP were also ANA-positive. This first finding in subjects exposed to FE should encourage researchers to further investigate associations between autoimmune unbalance and environmental exposure to asbestiform fibers.
Introduction
Natural asbestiform fibers are called “naturally occurring asbestos” (NOA) as natural components of soils or rocks (Lee et al. Citation2008; Wei et al. Citation2012; Abakay et al. Citation2016). The release of NOA fibers into the air by human work activities or natural weathering processes is a potential risk for the general population. These fibers have been detected in various parts worldwide (Sullivan Citation2007; Bayram et al. Citation2013; Abakay et al. Citation2016; Ledda, Loreto, Bracci, et al. Citation2016) such as Greece, Turkey, Cyprus, Corsica, New Caledonia, Afghanistan, Russia, Montana (USA) and Italy (Paoletti et al. Citation2000; Comba et al. Citation2003; Sullivan Citation2007; Bayram et al. Citation2013; Miozzi et al. Citation2016). In particular in Italy in the 1990s, a significantly increased mortality rate from malignant mesothelioma (MM) was reported in Biancavilla, a town on the southwest slope of Mt. Etna in Italy, Sicily (Paoletti et al. Citation2000).
Environmental investigations have shown the presence of an asbestiform mineral fiber, called fluoro-edenite (FE), in the lava rocks excavated from a local stone quarry. The stone material from the quarry had been employed locally for about 50 years for houses and road construction (Paoletti et al. Citation2000; Comba et al. Citation2003; Ledda, Loreto, Bracci, et al. Citation2016). The quarry was shut down in 1998 (Miozzi et al. Citation2016). FE (NaCa2Mg5[Si7Al]O22F2) is a new mineral species recognized by the Commission on New Minerals and Mineral Names (CNMMN; IMA: code 2000–049) in 2001” (Gianfagna and Oberti Citation2001). FE fibers share size and morphology similarities with certain amphibolic asbestos fibers (including antophyllite, actinolite, and tremolite) (DeNardo et al. Citation2004; Ballan et al. Citation2014). Based upon in vitro, in vivo, and epidemiological studies (Soffritti et al. Citation2004; Martinez et al. Citation2006; Loreto et al. Citation2008; Bernstein et al. Citation2013 Grosse et al. Citation2014). FE has been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC; Lyon, France) (WHO Citation2015).
The relationship between exposure to asbestos and adverse health effects has been extensively studied (Ledda, Loreto, Matera, et al. Citation2016; Ledda, Loreto, Pomara, et al. Citation2016; Ledda, Pomara, Bracci, et al. Citation2016). However, little has been established with regard to FE-related diseases (Biggeri et al. Citation2004; Loreto et al. Citation2009; Musumeci et al. Citation2010; Fazzo et al. Citation2012; Rapisarda, Ledda, Migliore, et al. Citation2015; Rapisarda, Ledda, Ricceri et al. Citation2015; Rapisarda et al. Citation2017; Ledda and Rapisarda Citation2016; Ledda, Cocuzza, Salerno, et al. Citation2017; Ledda, Costa, Matera, et al. Citation2017) apart from epidemiological studies that have shown that these fibers may cause MM (Comba et al. Citation2003; Fazzo et al. Citation2012), pleural plaques (PP), and chronic obstructive lung disease (Biggeri et al. Citation2004; Ledda, Loreto, Matera, et al. Citation2016; Ledda, Loreto, Pomara, et al. Citation2016; Ledda and Rapisarda Citation2016; Rapisarda, Ledda, Ricceri et al. Citation2015). It has been demonstrated that exposure to asbestos determines unbalanced inflammatory processes as an early response to inhaled fibers (Ledda, Loreto, Pomara, et al. Citation2016, Ledda, Costa, Matera, et al. Citation2017; Sayan and Mossman Citation2016). Historically, immune system cells have been regarded as the main players in initiating acute or chronic inflammation (Brown et al. Citation2005; Cooper et al. Citation2008; Pfau et al. Citation2014). However, recent studies have shown that epithelial cells of the respiratory tract and mesothelial cells lining the body cavities are capable of initiating inflammatory events after exposure to pathogenic fibers in the absence of immune system cells (Pfau et al. Citation2014).
Autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus seem to be the product of a complex, poorly understood interaction between environmental exposures and genetic predisposition (Galffy et al. Citation1999; Brown et al. Citation2005; Adachi et al. Citation2006; Gwinn Citation2011). Autoantibodies may be markers of subclinical diseases (Del Papa et al. Citation1992; Chizzolini et al. Citation2002). Epidemiological studies have tried to detect autoantibodies in populations exposed to environmental agents to spot a possible interaction between genes and environment (Brown et al. Citation2005; Gwinn Citation2011). Previous studies found evidence that exposure to asbestos may induce autoimmunity, with high frequency and titers of anti-nuclear autoantibodies (ANA), compared to a reference population (Pfau et al. Citation2005). The objective of this study was to explore the relationship between FE exposure and autoimmune responses in an exposed population.
Materials and methods
Ethical statement
The research protocol received the approval of Catania University Hospital Ethics Committee (Catania, Italy). Informed consent of all subjects was acquired before recruiting them in the study.
Study population
A total of 60 subjects living in the area of Biancavilla (Sicily, Italy) were randomly invited to participate in this study. In the same period, 60 subjects living and working at least 40 km away from the area of Biancavilla, were recruited as control group. All subjects were chosen within the framework of periodic occupational surveillance and invited to take part in this study. Inclusion criteria for exposed population were born during 1950–1980 and lived/still living in the Biancavilla municipality. Exclusion criteria were a history of bronchopulmonary disease (i.e. asthma, bronchopneumonia, or tuberculosis), systemic diseases, previous asbestos exposure, and or not having been born and/or lived/living in the municipality. A questionnaire was used to obtain information about medical and occupational history, use of medications, smoking and drinking habits, hobbies, etc. Questions on tobacco consumption enabled participants to be classified as current smokers, ex-smokers (those who had not smoked for >1 year) and nonsmokers (those who had never smoked). A free medical check, including spirometry and a high-resolution computer tomography (HRCT) chest scan, was given to all participants.
Pleural plaque assessment and spirometry
Subjects underwent HRCT total chest scanning using an Optima CT 580 W (GE Healthcare, Little Chalfont, CT) without contrast medium. Radiologists also received specific training in the interpretation of HRCT scans by experienced chest radiologists and occupational physicians. Double reading (and triple reading in case of disagreement) focused on benign asbestos-related abnormalities. PP were defined as circumscribed quadrangular elevations with sharp borders and density comparable to tissue, with/without signs of calcification. Respiratory function tests were conducted using a bell spirometer (Biomedin, Padova, Italy). Equipment, calibration and maneuvers met American Thoracic Society guidelines (ATS 2005). Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), peak expiratory flow (PEF), maximal expiratory flow rate at 25–75% of the vital capacity (MEF25–75%), and total lung capacity (TLC) were measured and expressed as a proportion of European Coal and Steel Community reference values adjusted for individual characteristics (age, weight, and height) recorded at the time of testing (Miller et al. Citation2005).
Laboratory tests
Venous blood was collected in the morning (following overnight fasting) to determine red blood cell (RBC) counts, hematocrit, and hemoglobin levels, white blood cell (WBC) counts, erythrocyte sedimentation rate, C-reactive protein levels, and liver enzyme (aspartate and alanine aminotransferases) levels. Serum was isolated from each sample and then the presence/levels of any ANA were determined by indirect immunofluorescence (400× magnification on a Nikon fluorescence microscope (city, NY), HEp2 cells (BioRad, Hercules, CA), and serum samples diluted 1:80. Nuclear staining patterns were evaluated by two independent expert readers (three readings in case of disagreement). Staining was identified by possible patterns: (1) speckled, (2) homogeneous, (3) centromere, and (4) nucleolar. Positive and negative quality controls provided by the manufacturer were included on each slide. Results were also classified as positive or negative for statistical analysis. The prevalence of ANA was determined by dividing the number of positive samples by the total number of subjects tested for exposed and non-exposed subjects.
Statistical analysis
Data were summarized as mean ± SD for continuous variables and frequencies for categorical variables. Normality was checked using a Kolmogrov–Smirnov test and homogeneity of variance by a Levene’s test. A t-test was used for analyzing continuous variance, and a Fischer test for categorical variables. The ANA and PP results were arranged in contingency tables to determine odds ratio (OR). Binary logistic regression was used to compare the odds of PP and ANA. Statistical significance was taken at p < 0.05. All analyzes were performed using Prism software v.6.0 (GraphPad, San Diego, CA).
Results
A total of 120 subjects were enrolled in this study, of which 60 were resident in Biancavilla (exposed) and 60 living and working at least 40 km away from Biancavilla (non-exposed). None of the subjects met the exclusion criteria. The two groups under examination, i.e. exposed and non-exposed, were homogeneous as far as age, smoking habits, number of cigarettes and BMI were concerned. Both groups had similar socioeconomic status and milieu. reports the main features of both groups. On medical check, no subject showed any sign and/or symptoms of illness.
Table 1. Main characteristics of study populations.
The mean values of spirometry values (e.g. FVC, FEV1, PEF, MEF25–75%, TLC) in both the exposed and control (non-exposed) subjects were within normal ranges. Pulmonary function test percentage values were also normal in all the study subjects (data not shown). Laboratory tests values fell within normal ranges for each parameter evaluated and there were significant differences observed between the groups for any endpoint ().
Table 2. Spirometry and laboratory tests.
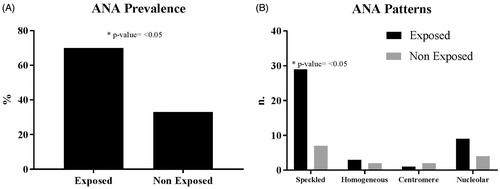
With regard to the presence of any ANA in the subjects, the incidence of serum samples that were ANA-positive was 70% (42/60) for the exposed and 25% (15/60) for the non-exposed subjects (p < 0.05). For this endpoint, the OR was calculated to be 7.00 (95% CI: 3.13–15.64) (). The predominant ANA staining pattern observed during analyzes was speckled in the exposed population (29/60 cases) ().
Figure 1. (A) Prevalence of ANA positive tests. (B) ANA pattern profile distribution. Values shown are absolute values for each population (from n = 60 in each). Types of patterns observed are indicated across X-axis in (B).
The HRCT scans revealed the presence of PP in 21 (30%) of the 60 exposed subjects but just in 2 (3%) of the 60 non-exposed counterparts; the OR here was calculated to be 15.62 (CI 95%: 3.46–70.41). Binary logistic regression highlights that PP frequency and ANA-positive status were significantly associated (p < 0.05).
Discussion
Several studies have revealed a link between exposure to silicas and development of autoimmune disease (Hess Citation2002; Brown et al. Citation2005; Gwinn Citation2011). Though less extensively studied, there is also evidence to suggest that exposure to asbestos is associated with development of autoimmune responses, and these are often manifest in terms of increases in levels of serum immunoglobulins, positive ANA tests and immune complex deposition (Turner-Warwick and Parks Citation1970; Lange et al. Citation1974; Nigam et al. Citation1993; Pfau et al. Citation2005; Bunderson-Schelvan et al. Citation2011). In the present study, a significantly greater number of the Biancavilla general population exposed to FE were found to be ANA-positive (ANA+; 70%) compared to control group (25%).
Similar results were observed by Marchand et al. (Citation2012) and Pfau et al. (Citation2005) who reported a high frequency of ANA in a small set of samples from the Libby population by demonstrating that over 61% of samples tested were ANA+. Normally, these autoantibodies are present in < 30% of the population (Tan et al. Citation1997). In general, ANA are associated with systemic autoimmune diseases, although their contribution to the actual disease is not clear (Marchand et al. Citation2012). In particular, a speckled ANA pattern seems to be associated with inflammatory processes affecting connective tissues. If the presence of these autoantibodies precedes systemic autoimmune diseases, as suggested by the Libby studies, the Biancavilla population may be more prone to develop these diseases owing to their high levels of ANA.
As in Libby study (Marchand et al. Citation2012), ANA test positivity appears to bind with pleural abnormalities (positive association with PP frequencies), raising the possibility that an autoimmune process is contributing to disease (Pfau et al. Citation2005). It is hypothesized that autoantibodies may be targeting pulmonary tissues, leading to specific tissue cytotoxicity (Marchand et al. Citation2012). Pfau et al. (Citation2005) showed that the Libby samples gave rise to a significantly higher frequency of positive ANA and ENA tests, increased mean fluorescence intensity and titers of the ANAs, and higher serum IgA, compared with control samples. Our results and Libby observations may lead us to hypothesize that pleural mesothelial cells may be potential targets for autoantibodies that might play a role in pleural lesions (Peipins et al. Citation2003; Whitehouse Citation2004).
Exposure to asbestos has been associated with the production of ANA (Lange et al. Citation1974), possibly through a mechanism similar to that hypothesized for silica, which includes release of antigens from dying cells (Gwinn Citation2011). Amphibole fibers are bio-persistent and cytotoxic, possibly resulting in repeated exposure of the immune system to cellular debris. Fibers reaching the pleural cavity may damage mesothelial cells lining it: over time, mesothelial debris could lead not only to production of ANAs, but also to autoantibodies towards mesothelial surface proteins as observed by Brown et al. (Citation2005). Previous studies demonstrated that exposure to Libby fiber increased ANA expression in two Lewis rat models (Salazar et al. Citation2012, Citation2013). As in the Salazar et al. (Citation2013) observations, the predominant ANA staining pattern was speckled (29/42 were positive).
This study established that significant differences existed in the frequency of ANA-positive titers in an FE exposed cohort, an outcome that is in line with that previously described for humans who had been exposed to asbestos. These data support a hypothesis that exposure to asbestiform fibers like FE are likely to be associated with autoimmune responses. It is well-known that exposure to asbestos and all asbestiform fibers may generate inflammatory processes (Sayan and Mossman Citation2016; Ledda, Costa, Matera, et al. Citation2017). Nevertheless, as few studies have explored the interaction between exposure to asbestos and autoimmune-like disorders, this does not allow us to compare our results in depth with other studies. These difficulties have previously been addressed by Pfau et al. (Citation2005); apart from these limitations, those Authors also identified the problem of comparing the results and the gender difference. In fact, most studies have a predominantly male, professionally-exposed population, while in Pfau et al. (Citation2005) and in the current study, women were widely present.
Another dilemma with the nonprofessional environmental exposure is represented by the impossibility to determine how much and how long the general population has been exposed to FE fibers (Ledda, Loreto, Bracci, et al. Citation2016). In the latter case, conventionally, subjects exposed are defined as population residing in a contaminated area (Rapisarda, Ledda, Ricceri et al. Citation2015; Ledda, Loreto, Matera, et al. Citation2016). It seems that a continuous stimulus produced by repeatedly inhaling the fibers and their persistence in the body can act as trigger both in prompting inflammatory processes and the immune system (Gangemi et al. Citation2005; Ledda, Loreto, Pomara, et al. Citation2016, Ledda, Costa, Matera, et al. Citation2017). Probably, the low level of exposure (referred to as low concentration of fibers and/or discontinuous exposure) can generate responses only for immunological induction and not for systemic autoimmune disease (Brown et al. Citation2005).
Conclusions
The significance of elevated ANA levels in subjects exposed to fibers is unknown. However, additional studies can provide opportunities to establish a correlation between autoimmunity and environmental exposure to fibers. To our knowledge, the work reported here is the first study that highlights increased prevalence of ANA in humans that had been exposed to FE. Further studies need to be done with a greater number of subjects exposed to FE in order to be able to detect specific autoantibodies (i.e. anti-SS-A/Ro, anti-SS-B/La, anti-Sm, anti-RNP, etc.) and if there are also mesothelial cell autoantibodies.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Abakay A, Tanrikulu A, Ayhan M, Imamoglu M, Taylan M, Kaplan M, Abakay O. 2016. High-risk mesothelioma relation to meteorological and geological condition and distance from naturally occurring asbestos. Environ Health Prev Med. 21:82–90.
- Adachi Y, Aoki C, Yoshio-Hoshino N, Takayama K, Curiel D, Nishimoto N. 2006. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int J Cancer. 119:1303–1311.
- American Thoracic Society (ATS). 1995. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 152:S77–S121.
- Ballan G, Del Brocco A, Loizzo S, Fabbri A, Maroccia Z, Fiorentini C, Travaglione S. 2014. Mode of action of fibrous amphiboles: The case of Biancavilla (Sicily, Italy). Ann Ist Super Sanita. 50:133–138.
- Bayram M, Dongel I, Bakan N, Yalçin H, Cevit R, Dumortier P, Nemery B. 2013. High risk of malignant mesothelioma and pleural plaques in subjects born close to ophiolites. Chest. 143:164–171.
- Bernstein D, Dunnigan J, Hesterberg T, Brown R, Velasco J, Barrera R, Hoskins J, Gibbs A. 2013. Health risk of chrysotile revisited. Crit Rev Toxicol. 43:154–183.
- Biggeri A, Pasetto R, Belli S, Bruno C, Di Maria G, Mastrantonio M, Trinca S, Uccelli R, Comba P. 2004. Mortality from chronic obstructive pulmonary disease and pleural mesothelioma in an area contaminated by natural fiber (fluoro-edenite). Scand J Work Environ Health. 30:249–252.
- Brown J, Pfau J, Pershouse M, Holian A. 2005. Silica, apoptosis, and autoimmunity. J Immunotoxicol. 1:177–187.
- Bunderson-Schelvan M, Pfau J, Crouch R, Holian A. 2011. Nonpulmonary outcomes of asbestos exposure. J Toxicol Environ Health B Crit Rev. 14:122–152.
- Chizzolini C, Raschi E, Rezzonico R, Testoni C, Mallone R, Gabrielli A, Facchini A, Del Papa N, Borghi M, Dayer J, et al. 2002. Autoantibodies to fibroblasts induce a pro-adhesive and pro-inflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum. 46:1602–1613.
- Comba P, Gianfagna A, Paoletti L. 2003. Pleural mesothelioma cases in biancavilla are related to a new fluoro-edenite fibrous amphibole. Arch Environ Health. 58:229–232.
- Cooper G, Gilbert K, Greidinger E, James J, Pfau J, Reinlib L, Richardson B, Rose N. 2008. Recent advances and opportunities in research on lupus: Environmental influences and mechanisms of disease. Environ Health Perspect. 116:695–702.
- Del Papa N, Meroni P, Barcellini W, Sinico A, Radice A, Tincani T, D'Cruz D, Nicoletti F, Borghi M, Khamashita M, et al. 1992. Antibodies to endothelial cells in primary vasculitides mediate in vitro endothelial cytotoxicity in the presence of normal peripheral blood mononuclear cells. Clin Immunol Immunopathol. 63:267–274.
- DeNardo P, Bruni B, Paoletti L, Sirianni B. 2004. Pulmonary fiber burden in sheep living in the Biancavilla area (Sicily): Preliminary results. Sci Total Environ. 325:51–58.
- Fazzo L, Minelli G, Santis M, Bruno C, Zona A, Marinaccio A, Conti S, Pirastu R, Comba P. 2012. Mesothelioma mortality surveillance and asbestos exposure tracking in Italy. Ann Inst Super Sanita. 48:300–310.
- Galffy G, Mohammed K, Nasreen N, Ward M, Antony V. 1999. Inhibition of interleukin-8 reduces human malignant pleural mesothelioma propagation in nude mouse model. Oncol Res. 11:187–194.
- Gangemi S, Rapisarda V, Minciullo P, Di Pasquale G, Lombardo G, Valentino M, Fenga C. 2005. Circulating levels of IL-18 in asbestos-exposed workers. Toxicol Ind Health. 21:125–129.
- Gianfagna A, Oberti R. 2001. Fluoro-edenite from Biancavilla (Catania, Sicily, Italy): Crystal chemistry of a new amphibole end-member. Am Mineral. 86:1489–1493.
- Grosse Y, Loomis D, Guyton K, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, et al. 2014. Carcinogenicity of fluoro-edenite, silicon carbide fibers and whiskers, and carbon nanotubes. Lancet Oncol. 15:1427–1428.
- Gwinn M. 2011. Multiple modes of action of asbestos and related mineral fibers. J Toxicol Environ Health B Crit Rev. 14:1–2.
- Hess E. 2002. Environmental chemicals and autoimmune disease: Cause and effect. Toxicology. 181:65–70.
- Lange A, Smolik R, Zatoński W, Szymańska J. 1974. Autoantibodies and serum immunoglobulin levels in asbestos workers. Intl Arch Arbeitsmed. 32:313–325.
- Ledda C, Cocuzza S, Salerno M, Senia P, Matera S, Rapisarda V, Loreto C. 2017. Occupational exposure to Mount Etna's basaltic dust: Assessment of mutagenic and cytotoxic effects. Mol Med Rep. 15:3350–3354.
- Ledda C, Costa C, Matera S, Puglisi B, Costanzo V, Bracci M, Fenga C, Rapisarda V, Loreto C. 2017. Immunomodulatory effects in workers exposed to naturally-occurring asbestos fibers. Mol Med Rep. 15:3372–3378.
- Ledda C, Loreto C, Bracci M, Mangano D, Migliore M, Ricceri V, Musumeci A, Costa C, Pomara C, Rapisarda V. 2016. High risk of pleural plaques and parenchymal abnormalities in women living in Biancavilla (Italy). Future Oncol. 12:63–65.
- Ledda C, Loreto C, Matera S, Massimino N, Cannizzaro E, Musumeci A, Migliore M, Fenga C, Pomara C, Rapisarda V. 2016. Early effects of fluoro-edenite: Correlation between IL-18 serum levels and pleural and parenchymal abnormalities. Future Oncol. 12:59–62.
- Ledda C, Loreto C, Pomara C, Rapisarda G, Fiore M, Ferrante M, Bracci M, Santarelli L, Fenga C, Rapisarda V. 2016. Sheep lymph nodes as a biological indicator of environmental exposure to fluoro-edenite. Environ Res. 147:97–101.
- Ledda C, Pomara C, Bracci M, Mangano D, Ricceri V, Musumeci A, Ferrante M, Musumeci G, Loreto C, Fenga C, et al. 2016. Natural carcinogenic fiber and pleural plaques assessment in a general population: A cross-sectional study. Environ Res. 150:23–29.
- Ledda C, Rapisarda V. 2016. Malignant pleural mesothelioma: The need to move from research to clinical practice. Arch Med Res. 47:407.
- Lee R, Strohmeier B, Bunker K, van Orden D. 2008. Naturally occurring asbestos: A recurring public policy challenge. J Hazard Mater. 153:1–21.
- Loreto C, Carnazza M, Cardile V, Libra M, Lombardo L, Malaponte G, Martinez G, Musumeci G, Papa V, Cocco L. 2009. Mineral fiber-mediated activation of phosphoinositide-specific phospholipase C in human bronchoalveolar carcinoma-derived alveolar epithelial A549 cells. Intl J Oncol. 34:371–376.
- Loreto C, Rapisarda V, Carnazza M, Musumeci G, Valentino M, Fenga C, Martinez G. 2008. Fluoro-edenite fibres induce lung cell apoptosis: An in vivo study. Histol. Histopathol. 23:319–326.
- Marchand L, St-Hilaire S, Putnam E, Serve K, Pfau J. 2012. Mesothelial cell and anti-nuclear autoantibodies associated with pleural abnormalities in an asbestos exposed population of Libby, MT. Toxicol Lett. 208:168–173.
- Martinez G, Loreto C, Rapisarda V, Masumeci G, Valentino M, Carnazza M. 2006. Effects of exposure to fluoro-edenite fiber pollution on the respiratory system: An in vivo model. Histol Histopathol. 21:595–601.
- Miller M, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten C, Gustafsson P, et al. 2005. Standardization of spirometry. Eur Respir J. 26:319–338.
- Miozzi E, Rapisarda V, Marconi A, Costa C, Polito I, Spandidos D, Libra M, Fenga C. 2016. Fluoro-edenite and carbon nanotubes: The health impact of 'asbestos-like' fibres ). Exp Ther Med. 11:21–27.
- Musumeci G, Loreto C, Cardile V, Carnazza M, Martinez G. 2010. Immunohistochemical expression of retinoblastoma and phospho-retinoblastoma protein in lung sheep exposed to fluoro-edenite fibers. Anat Sci Intl. 85:74–78.
- Nigam S, Suthar A, Patel M, Karnik A, Dave S, Kashyap S, Venkaiah K. 1993. Humoral immunological profile of workers exposed to asbestos in asbestos mines. Indian J Med Res. 98:274–277.
- Paoletti L, Batisti D, Bruno C, Di Paola M, Gianfagna A, Mastrantonio M, Nesti M, Comba P. 2000. Unusually high incidence of malignant pleural mesothelioma in a town of eastern Sicily: An epidemiological and environmental study. Arch Environ Health. 55:392–398.
- Peipins L, Lewin M, Campolucci S, Lybarger J, Miller A, Middleton D, Weis C, Spence M, Black B, Kapil V. 2003. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, MT, USA. Environ Health Perspect. 111:1753–1759.
- Pfau J, Sentissi J, Weller G, Putnam E. 2005. Assessment of autoimmune responses associated with asbestos exposure in Libby, MT, USA. Environ Health Perspect. 113:25–30.
- Pfau J, Serve K, Noonan C. 2014. Autoimmunity and asbestos exposure. Autoimmune Dis. 2014:782045
- Rapisarda V, Caltabiano R, Musumeci G, Castrogiovanni P, Ferrante M, Ledda C, Lombardo C, Graziano A, Cardile V, Loreto C. 2017. Analysis of fibulin-3 after exposure to asbestos-like fibers. Environ Res. 156:381–387.
- Rapisarda V, Ledda C, Migliore M, Salemi R, Musumeci A, Bracci M, Marconi A, Loreto C, Libra M. 2015. FBLN-3 as a biomarker of pleural plaques in workers occupationally exposed to carcinogenic fibers: A pilot study. Future Oncol. 11:35–37.
- Rapisarda V, Ledda C, Ricceri V, Arena F, Musumeci A, Marconi A, Fago L, Bracci M, Santarelli L, Ferrante M. 2015. Detection of pleural plaques in workers exposed to inhalation of natural fluoro-edenite fibers. Oncol Lett. 9:2046–2052.
- Salazar K, Copeland C, Luebke R. 2012. Effects of Libby amphibole asbestos exposure on two models of arthritis in the Lewis rat. J Toxicol Environ Health. 75:351–365.
- Salazar K, Copeland C, Wood C, Schmid JE, Luebke R. 2013. Evaluation of anti-nuclear antibodies and kidney pathology in Lewis rats following exposure to Libby amphibole asbestos. J Immunotoxicol. 10:329–333.
- Sayan M, Mossman B. 2016. The NLRP3 inflammasome in pathogenic particle and fiber-associated lung inflammation and diseases. Part Fiber Toxicol. 2016:13.
- Soffritti M, Minardi F, Bua L, Degli Esposti D, Belpoggi F. 2004. First experimental evidence of peritoneal and pleural mesotheliomas induced by fluoro-edenite fibers present in Etnean volcanic material from Biancavilla (Sicily, Italy). Eur J Oncol. 9:169–175.
- Sullivan P. 2007. Vermiculite, respiratory disease, and asbestos exposure in Libby, Montana: Update of a cohort mortality study. Environ Health Perspect. 115:579–585.
- Tan E, Feltkamp T, Butcher B, Dawkins R, Fritzler M, Gordon T, Hardin J, Kalden J, Lahita R, et al. 1997. Range of anti-nuclear antibodies in 'healthy' individuals. Arthritis Rheum. 40:1601–1611.
- Turner-Warwick M, Parkes W. 1970. Circulating rheumatoid and antinuclear factors in asbestos workers. Br Med J. 3:492–495.
- Wei B, Jia X, Ye B, Yu J, Zhang B, Zhang X, Lu R, Dong T, Yang L. 2012. Impacts of land use on spatial distribution of mortality rates of cancers caused by naturally-occurring asbestos. J Exposure Sci Environ Epidemiol. 22:516–521.
- Whitehouse A. 2004. Asbestos-related pleural disease due to tremolite associated with progressive loss of lung function: Serial observations in 123 miners, family members, and residents of Libby, Montana. AmJInd Med. 46:219–225.
- World Health Organization (WHO). 2015. Asbestos: Elimination of asbestos-related diseases. Geneva (Switzerland): WHO.
