Abstract
Benzo[a]pyrene (BaP) can induce developmental and reproductive toxicity; however, the full scope of its immunotoxic effects remains unknown. This study aimed to assess effects of lactational exposure to low-dose BaP (comparable to human exposure) on potential allergic\non-allergic immune responses in murine offspring. Lactating C3H/HeJ dams were orally dosed with BaP at 0, 0.25, 5.0, or 100 pmol/animal/week) at post-natal days [PND] 1, 8, and 15. Five-weeks-old pups then received intratracheally ovalbumin (OVA) every 2 weeks for 6 weeks. Following the final exposure, mice were processed to permit analyses of bronchoalveolar lavage (BAL) fluid cell profiles as well as levels of lung inflammatory cytokines and chemokines, serum OVA-specific immunoglobulin, and mediastinal lymph node (MLN) cell activation/proliferation. In OVA-sensitized male offspring, lactational low-dose BaP exposure led to enhanced (albeit not significantly) macrophage, neutrophil, and eosinophil infiltration to, and increased T-helper (TH)-2 cytokine production in, the lungs. In females, BaP exposure, regardless of dose, led to slightly enhanced lung levels of macrophages and eosinophils, and of inflammatory molecules. Protein levels of interleukin (IL)-33 in the OVA + BaP (middle dose) group, and interferon (IFN)-γ in the OVA + BaP (low dose) group, were higher than that of the OVA (no BaP) group. Ex vivo studies showed lactational exposure to BaP partially induced activation of T-cells and antigen-presenting cells (APCs) in the MLN cells of both male and female offspring, with or without OVA sensitization. Further, IL-4 and IFNγ levels in MLN culture supernatants were elevated even without OVA-re-stimulation in OVA + BaP groups. In conclusion, lactational exposure to low-dose BaP appeared to exert slight effects on later allergic and non-allergic immune responses in offspring by facilitating development of modest TH2 responses and activating MLN cells. In addition, lactational exposures to BaP might give rise to gender differences in allergic/non-allergic immune responses of offspring.
Introduction
Benzo[a]pyrene (BaP) is a well-known polycyclic aromatic hydrocarbon (PAH) and a ubiquitous environmental pollutant that is primarily derived from cigarette smoke, diesel exhaust particles (DEP), industrial wastes, and food products (Birnbaum Citation1994; Rubin Citation2001). Although BaP is recognized as an environmental mutagenic and carcinogenic PAH, several reports have demonstrated that BaP also is an adjuvant immunoglobulin E (IgE) producer in allergen-immunized mice. Mizutani et al. (Citation2007) suggested that BaP exposure aggravates allergic rhinitis in a guinea pig model. Intranasal (Kadkhoda et al. Citation2005) and oral (Kadkhoda et al. Citation2004) administration of BaP enhances allergen-specific IgE production and systemic T-helper (TH)-1/TH2 responses. Recently, our research group reported that intratracheal exposure to low-dose BaP can partly contribute to exacerbation of allergic airway inflammation through lung TH2 cytokine and chemokine expression and activation/proliferation of mediastinal lymph node (MLN) cells (Yanagisawa et al. Citation2016).
Importantly, fetuses and infants are more vulnerable than adults to the adverse effects of environmental pollutants because they are at the most sensitive stages of human development (WHO Citation2010). Compared to adults, infants have greater exposure to BaP from multiple sources, such as breast milk, food, soil, and ambient air. Although breastfeeding is the best source of nutrition for newborn infants, and affords a number of benefits for growth, immunity, and development (Gordon Citation1997; Anderson et al. Citation1999; Oddy Citation2001; WHO Citation2010), breast milk and infant formulas often contain numerous environmental toxicants, including PAHs. Santonicola et al. (Citation2017) reported that the average concentration of total PAHs was 114.93 μg/kg in breast milk and 53.68 μg/kg in infant formula. The average BaP content was 0.81 μg/kg in breast milk and 2.77 μg/kg in infant formula samples.
Several animal studies have shown that lactational exposure to “high dose” (2–25 mg/kg) BaP disrupts the reproductive (Liang et al. Citation2012) and central nervous systems (Bouayed et al. Citation2009). However, there have been few studies on the effects of BaP on offspring immune responses. Similarly, there have been very few studies on the immunomodulatory effects of low-dose BaP exposure in humans. An epidemiological report showed that the concentration of 1-hydroxypyrene (a PAH) in urine at 2-years-of-age may be associated with food allergy in children (Jerzynska et al. Citation2017). Santos et al. (Citation2014) reported that early postnatal exposure to 1,2-NQ, a quinone compound in DEP, aggravates allergen-induced pulmonary inflammation in male mice offspring. However, it remains unclear whether lactational exposure to BaP is associated with aggravation of allergic diseases, such as allergic asthma.
Accordingly, this study aimed to determine the effects of lactational exposure to low-dose BaP on potential allergic\non-allergic immune responses (in mouse allergic airway inflammation model) in offspring. In addition, this study investigated whether there were gender-specific differences in immune responses following lactational exposure to BaP.
Materials and methods
Animals
C3H/HeJSlc pregnant mice (gestation day 15) were purchased from Japan SLC Inc. (Shizuoka, Japan) for use in the experiments. Mice were provided commercial diet (CE-2; Japan CLEA Co., Tokyo, Japan) and water ad libitum while housed in a facility maintained at 22–26 °C and 40–69% relative humidity under a 12 h light/dark cycle.
BaP (: B1760, Sigma, St. Louis, MO) was dissolved in olive oil (Nacalai Tesque Inc., Kyoto, Japan). The predicted average oral exposure in humans has been estimated at 0.00044 μg/kg/d (Ministry of Environment, Government of Japan Website), equivalent to a dose of 0.3 pmol/25 g BW/week. Therefore, this study used three doses of BaP, i.e. 0.25 (low; BaP-L), 5 (medium; BaP-M), and 100 (high; BaP-H) pmol/mouse/week to treat the lactating mother mice by oral gavage on PND 1, 8, and 15. Mothers in the vehicle group received only olive oil. A previous study showed that BaP is transferred to offspring from lactating mice at a parental dose of 0.7% after 6 h of nursing, and that this toxic compound is absorbed and distributed throughout the body (Ho and Blume Citation1976).
Delivery day was designated postnatal day (PND) 0. After PND 1, litters were culled to four or five pups and kept with their mothers until weaning at 3-weeks-old. At 5-weeks-old, pups were randomly allocated into eight groups, four treated with OVA (10 μg/ml; Sigma) and the other four with phosphate-buffered saline (PBS, pH 7.4; carrier for OVA). For exposures, mice were anesthetized with 4% halothane (Takeda Chemical Industries Ltd., Osaka, Japan), and intratracheally administered a 100 μl aliquot of the suspension every 2 weeks from 6-weeks-old.
All procedures were approved by the Animal Care and Use Committee of the National Institute for Environmental Studies, and this study was conducted in accordance with the guide-lines for the Care and Use of Laboratory Animals of the National Institute for Environmental Studies. All animals were treated humanely, and care was taken to alleviate suffering.
Blood retrieval and bronchoalveolar lavage (BAL)
All mice were euthanized under anesthesia 24 h after their final intratracheal instillation (4–8 mice/group). Blood was retrieved by cardiac puncture and serum prepared/harvested using standard protocols. Thereafter, the lungs were lavaged three times with 1 ml sterile saline at 37 °C by syringe and the lavage fluid harvested by gentle aspiration (average volume was 90% of the amount instilled). The lavage fluid was centrifuged at 300 × g for 10 min at 4 °C to recover all cells and the pellet was re-suspended in 1 ml saline. Total cell counts were then determined using a hemocytometer. Differential cell counts were prepared using Autosmear (Sakura Seiki, Tokyo, Japan), the slides were stained with Diff-Quik (International Reagents Co., Kobe, Japan), and a total of 500 cells/slide/mouse were then counted using a light microscope (AX80; Olympus, Tokyo). The lavaged lungs were then retrieved and similar to the isolated serum, were stored at −80 °C until further analysis.
Histopathological examination
The lungs for the four groups that received OVA were fixed in 10% phosphate-buffered formalin (pH 7.4) 24 h after the final intratracheal instillation. Tissue sections from five animals per group were embedded in paraffin and cut into 3 μm thick slices. The histological specimens were stained with hematoxylin and eosin (H&E) to evaluate eosinophil and lymphocyte accumulation in the peribronchial and perivascular regions. The sections were also subjected to periodic acid–Schiff (PAS) staining to evaluate goblet cell proliferation in bronchial epithelium. Histological changes were assessed using an Olympus AX80 microscope. The degree of eosinophil and lymphocyte infiltration or goblet cell proliferation was graded in a blinded fashion as follows: 0 = not present, 0.5 = slight, 1 = mild, 1.5 = mild to moderate, 2 = moderate, 2.5 = moderate to marked, and 3 = marked. Although 0.5 was defined as an inflammatory reaction affecting <10% of the airways or goblet cells stained with PAS 1 = 10–20%, 1.5 = 20–30%, 2 = 30–40%, 2.5 = 40–50%, and 3= >50% of the airways or goblet cells affected.
Enzyme-linked immunosorbent assay (ELISA)
Lavaged lungs (4–8 mice/group) were each homogenized in 3 ml of 10 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA; Sigma), 0.1 mM phenylmethanesulfonyl fluoride (PMSF; Nacalai Tesque), 1 μM pepstatin, and 2 μM leupeptin (Peptide Institute, Osaka, Japan). The homogenates were subsequently centrifuged at 105 000 × g for 1 h at 4 °C. Resulting supernatants were isolated and stored at −80 °C. Enzyme-linked immunosorbent assay (ELISA) kits were used to quantify levels of interleukin (IL)-4, and -5 (Thermo Fisher Scientific, Chicago, IL), IL-13 and -33 (R&D Systems, Minneapolis, MN), interferon (IFN) γ (e-Bioscience, San Diego, CA) in each supernatant. The total protein level of each cytokine in the lungs was calculated by multiplying the corresponding measured value by the total supernatant volume, as calculated in previous studies (Takano et al. Citation1997; Yanagisawa et al. Citation2006). Kits were also used to assess IL-4, IL-5, IFNγ (all e-Bioscience) levels in MLN cell culture supernatants. OVA-specific IgE was measured using a mouse anti-OVA IgE ELISA Kit (Shibayagi Co., Gunma, Japan). Serum OVA-specific IgG1 was measured as described in Yanagisawa et al. (Citation2006).
Preparation of mouse MLN cells
MLN cells (4–8 mice/group) of each individual mouse were isolated from their MLNs that were passed through sterile stainless wire mesh. Released cells were centrifuged (400 × g, 5 min, 20 °C), washed with PBS, and re-suspended in R10 medium, i.e. RPMI 1640 (Invitrogen, Grand Island, NY) plus 10% heat-inactivated fetal bovine serum (MP Biomedical, Eschwege, Germany), 100 U penicillin/ml, 100 µg streptomycin/ml (Sigma), and 50 µM 2-mercaptoethanol (Invitrogen). Cell number and viability were determined by trypan blue (Invitrogen) exclusion.
Fluorescence-activated cell sorting (FACS) analysis
The following monoclonal antibodies were used for fluorescence-activated cell sorting (FACS) analysis. Hamster anti-mouse: CD11c (HL3, IgG1 λ2, PE-conjugated), rat anti-mouse-: MHC Class II, I-A/I-E (2G9, IgG2a, κ, FITC-conjugated), CD86 (GL1, IgG2a, κ, PE-conjugated), TCR β-chain (H57–597, FITC-conjugated), CD4 (RM4–5, PE-conjugated) (all BD Biosciences, San Diego, CA), or PDCA-1 (JF05–1C2.4.1, IgG2b, FITC-conjugated), CD8 (53–6.7, PE-conjugated, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Aliquots of the isolated lymph node cells (2–5 × 105) were pelleted and then re-suspended in 100 µl FACS buffer (PBS containing 0.3% bovine serum albumin and 0.05% sodium azide (Wako Pure Chemicals, Osaka, Japan)), before receiving 0.15–1 µg of individual antibody (company-recommended amount). All samples were then incubated for 30 min on ice in the dark. After the incubation, the cells were washed with FACS buffer and fluorescence signal was immediately measured with a BD FACSCalibur using BD CellQuest Pro software (Becton, Dickinson and Co., Parsippany, NJ). Fluorescence data from 10 000 cells in each sample was acquired and signal-positive cells were counted and expressed as cell numbers. All data were analyzed using FlowJo software (TOMY Digital Biology, Tokyo, Japan).
MLN proliferation assay
MLN cells (2 × 105) were placed into wells of 96-well plates and then treated with 200 µl R10 medium containing 0 or 20 µg OVA. All cultures were performed in triplicate. After 25 h of incubation at 37 °C, cell proliferation was examined by adding 20 µl of a 100 µM solution of 5-bromo-2′-deoxyuridine (BrdU; Roche Molecular Biochemicals, Mannheim, Germany) to each well and then allowing the cells to incubate a further 20 h before evaluation. Specifically, proliferation was measured using a Cell-Proliferation-ELISA Kit (Roche), according to manufacturer instructions.
Statistical analysis
Data are expressed as means ± SE. Differences among the groups were determined by two-way analysis of variance (ANOVA), followed by a Scheffe or Kruskal–Wallis test, followed by a Steel multiple comparison test using Ekuseru-Toukei 2010 statistical software (Social Survey Research Information Co. Ltd., Tokyo, Japan). For experiments limited to OVA administration, differences between the OVA + BaP groups and the OVA group were evaluated using one-way ANOVA, followed by a Dunnett’s or a Steel test, followed by a Kruskal–Wallis test. A p value < 0.05 was considered statistically significant.
Results
Effects of lactational exposure to BaP on cellular profiles in BAL fluid and pulmonary histopathological findings
Examination of cell infiltration into BAL fluid 24 h after the final intratracheal instillation showed that BaP exposure during the lactation period had no significant effect on pulmonary inflammation in male and female offspring. In the four groups that received OVA, the administration resulted in increased infiltration of eosinophils, neutrophils, and lymphocytes into the lungs compared with vehicle (PBS) administration. In male offspring, while not statistically significant, accumulation of inflammatory cells was greater in the OVA + BaP-L group than in the OVA group (heretofore, this term refers to the offspring who were breastfed from olive oil-treated mothers and who, in turn, underwent OVA instillation) (). Macrophage accumulation was greater in the OVA + BaP-L group than in the vehicle group (heretofore, this term refers to offspring who were breastfed from olive oil-treated mothers and who, in turn, underwent PBS instillation) (p < 0.01). In female offspring, lactational exposure to BaP slightly enhanced the filtration of eosinophils in the OVA-sensitized groups, regardless of dose, although this was not statistically significant (). Next, histopathological analysis in the lung was performed on OVA-sensitized offspring. Maternal exposure to BaP slightly enhanced eosinophil and lymphocyte accumulation in the peribronchial and perivascular regions compared with vehicle exposure in male offspring. Goblet cell hyperplasia and mucus hypersecretion in bronchial epithelium were greater in the OVA + BaP-M group than in the OVA group (p < 0.05, ). In female offspring, OVA + BaP-L caused slight accumulation of inflammatory cells and significant proliferation of goblet cells compared with OVA alone (p < 0.05, ).
Table 1. Number of inflammatory cells in bronchoalveolar lavage (BAL) fluid and pulmonary histopathological findings in male offspring.
Table 2. Number of inflammatory cells in the bronchoalveolar lavage (BAL) fluid and pulmonary histopathological findings in female offspring.
Effects of lactational exposure to BaP on lung cytokine expression
Effects of lactational exposure to BaP on lung protein levels of pro-inflammatory molecules 24 h after the final intratracheal instillation were also evaluated. BaP exposure during the lactation period had no significant effect on cyto/chemokine expression in both male and female offspring without OVA sensitization ( and , Supplemental Material Tables 1 and 2). OVA administration caused a significant increase in protein levels of IL-5, IL-13, IL-33, eotaxin, MCP-1, and RANTES in the lungs compared with vehicle administration in male and female offspring (p < 0.01). In male offspring, IL-5 levels tended to be higher in the OVA + BaP-L group than in the OVA group (p = 0.17). In female offspring, IFNγ levels were also greater in the OVA-treated groups than in the vehicle-treated group (). Furthermore, IL-33 levels in the OVA + BaP-M group and IFNγ levels in the OVA + BaP-L group were significantly higher than in the OVA group (, p < 0.05; IL-33 and IFNγ). IL-4 levels did not undergo any significant changes in either group.
Table 3. Protein levels of Th1/Th2 cytokines in the lungs following exposure to BaP on allergic airway inflammation in male offspring.
Table 4. Protein levels of Th1/Th2 cytokines in the lungs following exposure to BaP on allergic airway inflammation in female offspring.
Effects of lactational exposure to BaP on serum OVA-specific immunoglobulin
To evaluate the effects of lactational exposure to BaP on OVA-induced Ig production, OVA-specific IgE and IgG1 in serum were measured 24 h after the final instillation. Compared with vehicle, OVA significantly increased serum OVA-specific IgE and IgG1 in male and female (; p < 0.01). No significant changes were observed in the OVA-IgE and IgG1 levels in male offspring, whereas in female offspring, OVA-IgG1 production tended to be higher in the OVA + BaP-L group than in the OVA group (p = 0.07).
Figure 2. Ovalbumin (OVA)-specific Ig antibody levels in serum following lactational expo-sure to benzo[a]pyrene in allergic airway inflammation in offspring. Serum OVA-specific IgE and IgG1 levels were assessed 24 h after the respective final OVA instillation. Data shown are means ± SE for 4–8 animals/group. **p < 0.01 vs. vehicle group.
![Figure 2. Ovalbumin (OVA)-specific Ig antibody levels in serum following lactational expo-sure to benzo[a]pyrene in allergic airway inflammation in offspring. Serum OVA-specific IgE and IgG1 levels were assessed 24 h after the respective final OVA instillation. Data shown are means ± SE for 4–8 animals/group. **p < 0.01 vs. vehicle group.](/cms/asset/600120ae-134a-4086-b553-c6f4bfb6ccd0/iimt_a_1442379_f0002_b.jpg)
Effects of lactational exposure to BaP on MLN cell activation/proliferation
Examination of total MLN cell numbers 24 h after the final instillation indicated that, compared with PBS administration, OVA administration resulted in increased MLN cells in male and female offspring (). The total MLN cells in males were significantly increased in the OVA + BaP-L group compared with the OVA group (p < 0.05).
Figure 3. Total cell number and cell surface molecule expression in mediastinal lymph node cells: male offspring. Data is from FACS sorting of cells harvested 24 h after final intratracheal instillation. (A) Total cell number. (B–D) ovalbumin (OVA)− groups. (E–G) OVA+ groups. (B,E) CD11c+PDCA-1+ and CD11c+PDCA-1− cells. (C,F) CD28+, TCRβ+, and CD28+TCRβ+ cells. (D,G) CD86+, MHC Class II+, and MHC Class II+CD86+ cells. Data shown are means ± SEM of 4–8 animals for total cell number or 4 or 5 animals for FACS analysis, respectively. **p < 0.01 vs. vehicle group, #p < 0.05 vs. OVA group.
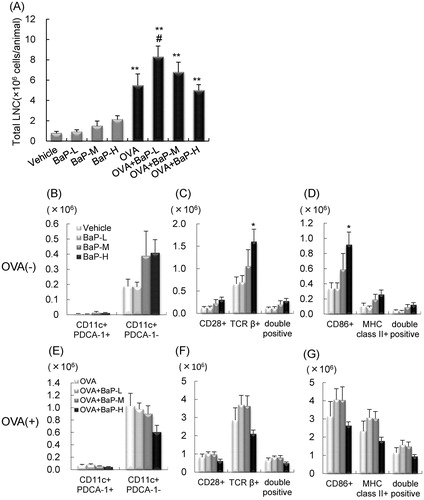
Figure 4. Total cell number and cell surface molecule expression in mediastinal lymph node cells: female offspring. Data is from FACS sorting of cells harvested 24 h after final intratracheal instillation. (A) Total cell number. (B–D) ovalbumin (OVA)(−) groups. (E–G) OVA(+) groups. (B,E) CD11c+PDCA-1+ and CD11c+PDCA-1− cells. (C,F) CD28+, TCRβ+, and CD28+TCRβ+ cells. (D,G) CD86+, MHC Class II+, and MHC Class II+CD86+ cells. Data shown are means ± SEM of 4–8 animals for total cell number or 4 animals for FACS analysis, respectively. *p < 0.05, **p < 0.01 vs. vehicle group, #p < 0.05 vs. OVA group.
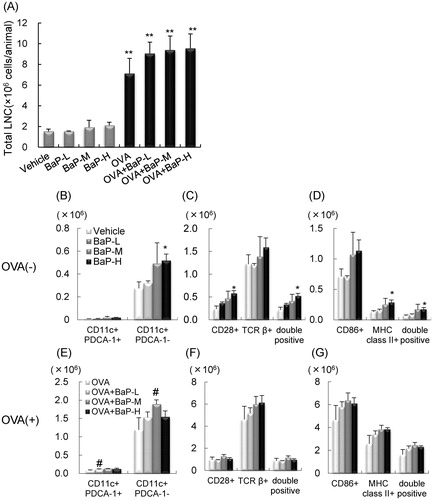
This study next evaluated the effects of lactational exposure to BaP on MLN cell activation/proliferation. In male offspring, BaP-H exposure significantly increased numbers of TCRβ+ and CD86+ cells compared with vehicle (olive oil) exposure (; p < 0.05). Increases in CD11c+PDCA-1− [conventional dendritic cell (cDC)], CD11c+PDCA-1+ [plasmacytoid dendritic cell (pDC)], MHC Class II+CD86+ (activated APC), and TCR β+CD28+ (activated T-cell) levels were also seen in the MLN from BaP-H groups compared to the vehicle group (), although these changes were not significant. In OVA-sensitized mice, lactational exposure to BaP exposure had no significant changes (). OVA administration led to enhanced MLN cell proliferation during OVA-re-stimulation for 45 h, and there was no effect of BaP exposure during the lactation period (). IL-4 and IFNγ levels were higher in the OVA + BaP-L and OVA + BaP-M groups than in the OVA (dam saline) without OVA-re-stimulation (). In contrast, no significant differences were found in IL-4, IL-5, and IFNγ levels in culture supernatants of the OVA-restimulated MLN cells.
Table 5. Proliferation/Activation of mediastinal lymph node cells in male offspring.
In female offspring, lactational exposure to BaP-H increased numbers of CD11c+PDCA-1−, TCRβ+CD28+, and MHC Class II+CD86+ cells compared with vehicle exposure () (p < 0.05). In OVA-sensitized mice, a significant increase in CD11c+PDCA-1− cells in the OVA + BaP-M group and CD11c+PDCA-1+ cells in the OVA + BaP-L group compared with the OVA group () was observed. There were no significant changes in the number of TCRβ+CD28+ and MHC Class II+CD86+ cells (). The OVA + BaP-M group enhanced MLN cell proliferation with OVA re-stimulation compared to the OVA group (, p < 0.05). There was no change in MLN cell proliferation without OVA re-stimulation in either group. IL-4, IL-5, and IFNγ levels in culture supernatants of the OVA-re-stimulated MLN cells showed no significant differences between the OVA + BaP groups and OVA group (). Without OVA re-stimulation, levels of IL-4 and IFNγ, but not IL-5, were higher in MLN cell cultures of the OVA + BaP groups than in those from the OVA group. Additionally, the percentage of positive cells were similar (e.g. CD86+ cells in the OVA + BaP-H group compared with that in the OVA group in males and CD11c+PDCA− cells in the OVA + BaP-M group compared with that in the OVA group in females; data not shown).
Table 6. Proliferation/Activation of mediastinal lymph node cells in female offspring.
Discussion
These results are the first to indicate that low doses of BaP, comparable to human exposure levels during the lactation period, can partially enhance allergen-induced pulmonary inflammation and lung TH2 cytokine expressions in both male and female offspring. In ex vivo studies, lactational exposure to BaP with/without OVA-sensitization partially enhanced activation of T-cell and activated antigen-presenting cell (APC) in MLN cells. Remarkably, the enhancement of allergic responses was greater with a lower dose of BaP (comparable to human exposure) in offspring of both genders, although differences in the dose response of BaP were observed.
BaP is a PAH that induces immune responses including adjuvant effects. Previous studies reported that intranasal or oral administration of BaP enhanced allergen-specific IgE production in mite allergen-immunized mice (Kadkhoda et al. Citation2004, Citation2005). Intranasal BaP exposure also aggravated Japanese cedar pollen-induced allergic rhinitis in guinea pigs (Mizutani et al. Citation2007). It was recently shown that intratracheal exposure to low-dose BaP during the juvenile period of development exacerbated allergic airway inflammation via lung TH2 cytokine/chemokine expression and proliferation/activation of MLN cells (Yanagisawa et al. Citation2016).
The main route for BaP exposure in humans is oral. According to the Total Human Environmental Exposure Study, the range and magnitude of dietary exposures (2–500 ng/d) were much greater than those resulting from inhalation (10–50 ng/d) (Waldman et al. Citation1991). Hattemer-Frey and Travis (Citation1991) estimated that the long-term average daily intake of BaP by the general population of the United States was ≈2.2 μg/d. In Japan, the predicted average oral exposure to BaP is ≈0.00044 μg/kg/d (equivalent to 0.3 pmol/25 g BW/week). Therefore, this study evaluated effects of exposure levels corresponding to human exposure of 0.25, 5, and 100 pmol BaP/mouse/week by oral gavage. It is important to note that infants are exposed to environmental chemicals via breast milk, food, soil, and ambient air. Due to their body sizes, the total dosages of such agents (at equal amounts) are in turn relatively amplified relative to calculated levels in adults. Thus, it is important to consider the increased potential vulnerability of neonates with regard to risk assessment of many PAHs, such as BaP.
This study showed that OVA + BaP-L enhanced macrophage infiltration compared with vehicle in male offspring. In female offspring, OVA-induced macrophage accumulation was greater than that of male offspring, although lactational exposure to BaP had no effects. Alveolar macrophages are major cellular mediators of allergic lung inflammation in animal models and humans. In a murine model of allergic lung inflammation, female mice showed aggravated eosinophilic inflammation, increased lung TH2 cytokines and serum IgE compared with male mice following allergen challenge (Melgert et al. Citation2005; Blacquiere et al. Citation2010). The gender differences in macrophage accumulation may be due to gender differences in OVA responses.
Lung protein levels of select TH1/TH2 pro-inflammatory molecules were then examined. Lactational exposure to low-dose BaP tended to increase lung TH2 cytokines levels in OVA-administered male offspring. In female offspring, IL-33 levels in the OVA + BaP-M group was significantly higher than in the OVA group. TH2 cytokines such as IL-5, IL-13, and IL-33, play important roles in the pathogenesis of allergic asthma (Galli et al. Citation2008). IL-5 is essential for eosinophil migration (Faccioli et al. Citation1996) and specifically supports eosinophil differentiation, maturation, and activation (Clutterbuck and Sanderson Citation1988). IL-13 is released from TH2 lymphocytes and promotes mucous secretion and goblet cell hyperplasia in the bronchial epithelium (Tesfaigzi Citation2008). IL-33, an IL-1 family cytokine, contributes to development of type 2 immune responses by inducing IL-5, IL-13, and IgE production (Liew et al. Citation2010; Oboki et al. Citation2010). IL-33 is constitutively and abundantly expressed in human and mouse barrier tissues, including lung epithelial cells. Furthermore, in lungs from female offspring, IFNγ levels were higher in the OVA + BaP-L group than in the vehicle group. IFNγ, associated with TH1 responses, is considered a prime target for modulating autoimmunity. Moore et al. (Citation2014) reported that levels of non-TH2 factors such as IFNγ often correlate with severe asthma phenotypes and resistance to corticosteroid treatment. Intranasal exposure to BaP with mite allergen induces TH1 (IL-12p70 and IFNγ) as well as TH2 (IL-4 and IL-10) cytokine production in splenocyte culture supernatants (Kadkhoda et al. Citation2005). Our recent study reported that intratracheal BaP administration activated TH1 and TH2 responses in OVA-sensitized mice (Yanagisawa et al. Citation2016). These results suggested to us that exposure to BaP during the lactation period may, in part, enhance TH2-polarization – resulting in aggravation of allergic pulmonary inflammation in offspring. Furthermore, low doses of BaP, comparable to that of human exposures, appear to be able to activate both TH1 and TH2 responses in female offspring, in contrast to in male offspring.
This study next examined MLN cell phenotypes and functions. BaP exposure during lactation period activated T-cells and APC (including cDC subsets) in offspring, in the presence and absence of allergen. DCs are major lung APC that contribute to asthma development. Two major subsets, cDC (CD11c+PDCA-1−) and pDC (CD11c+PDCA-1+), are known; cDC are implicated in pro-allergic responses. OVA-exposed bone marrow-derived cDC can induce allergic airway inflammation and hyper-responsiveness (Lombardi et al. Citation2012). On the other hand, pDC can suppress allergen-derived TH2 responses (Kool et al. Citation2009). Kadkhoda et al. (Citation2005) reported that bone marrow-derived DC from naive male mice was involved in secretion of TH1/TH2 cytokines following exposure to OVA + BaP compared to OVA alone.
MHC Class II (Niederhuber and Shreffler Citation1977) and co-stimulatory CD86 (Freeman et al. Citation1993) are essential for antigen presentation. A previous study from our group showed that intratracheal BaP exposure increased expression of both on MLN cells during OVA-induced airway allergic inflammation in male mice (Yanagisawa et al. Citation2016). In addition, IL-4 and IFNγ levels in MLN cell culture supernatants were significantly higher in cells from OVA + BaP groups than those from OVA-only groups – even without OVA re-stimulation, particularly in female offspring in this study. OVA administration (without maternal BaP exposure) markedly induced cytokine expression after OVA re-stimulation; in comparison, remarkable differences between the OVA + BaP and OVA-only groups in this study were not detected. However, the OVA + BaP groups tended to show an increase in total MLN cell number relative to that in OVA group. Thus, total amounts of cytokines may yet be greater in the OVA + BaP mice.
In this study, lactational exposure to BaP without OVA sensitization also induced activation of T-cells and APC in the MLN cells. Though any persistence of effects due to lactational BaP exposure in mice offspring after they grow up remains unknown, one report demonstrated that immunological parameters were still strongly modulated for up to 18 months after maternal exposure to BaP in in utero-exposed offspring (IPCS Citation1998). Prenatal exposure to ambient PAH in mice alters cortical DNA methylation in adult mice (Miller et al. Citation2016). Data from other studies, together with the current study data, suggest that lactational exposure to BaP may contribute to disrupted allergic/non-allergic immune responses, in part, through the activation of immune cells and DNA methylation.
In this study, gender differences in allergic responses following lactational BaP exposure were observed. Gender-specific differences in TH2 cytokine production have been reported in both asthmatic patients and mice. Levels of TH2 cytokines (e.g. IL-4, IL-5, and IL-13) in lung lavage fluid from female mice were significantly higher than those from male mice in a murine model of allergic asthma (Okuyama et al. Citation2008). Female-dominant TH2 cytokine production was observed in lung and bronchial lymph node cells (Okuyama et al. Citation2008; Wada et al. Citation2010). It is not yet fully understood how gender differences in TH2 cytokine production are generated in the development of allergic responses and diseases like asthma. A previous study reported that maternal exposure to low-dose di-(2-ethylhexyl) phthalate (DEHP), widely used in polyvinyl chloride products, during lactation but not fetal periods, enhanced atopic dermatitis-like skin lesions resulting from mite allergen (Yanagisawa et al. Citation2008), and the severity of these outcomes were also gender-dependent.
Our group has previously reported how poly-brominated diphenyl ethers, a common additive in flame retardants, facilitated inflammatory responses by activating nuclear receptors such as the aryl hydrocarbon receptor (AhR) and thyroid hormone receptor in bronchial epithelial cells (Koike et al. Citation2014). AhR is a regulator that senses and responds to environmental chemicals and plays a role in immune system regulation (Quintana and Sherr Citation2013), including allergic asthma (Zhu et al. Citation2011; Zhou et al. Citation2014; Xia et al. Citation2015). The observed gender difference may be partly explained by the involvement of hormone receptor-/nuclear receptor-mediated processes in environmental chemical-induced early postnatal effects on allergic responses and diseases. Furthermore, such gender differences in immune responses might also be attributed to lung CYP1a1 expression. CYP1a1 is a member of the CYP family inducible by PAHs, including those found in DEP and TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), and mediated by AhR. Among lung cancer patients, female smokers have been found to have higher levels of PAH-related DNA adduct levels and CYP1a1 gene expression in their normal lung tissue, compared to male smokers (Ryberg et al. Citation1994; Mollerup et al. Citation1999, Citation2006). Gender differences in the current study may reflect differences in activation/activation status of AhR and/or CYP1a1 between female and male offspring following lactational exposure to BaP. Future studies should investigate changes in environmental chemical-induced nuclear receptor signaling in the respiratory and immune systems.
In conclusion, this is the first study to demonstrate that lactational exposure to BaP, even at human-relevant exposure levels, may exert slight effects on allergic and non-allergic immune responses in these exposed offspring through changes in lung TH2 cytokine expression and MLN proliferation/activation. Different susceptibility and hormonal factors may play a role in the gender-specific effects of BaP.
Rie_Yanagisawa_et_al_supplemental_content.zip
Download Zip (30.4 KB)Acknowledgments
The authors wish to thank S. Abe for technical assistance. The authors also thank Enago (http://www.enago.jp) and Genius Plus (https://genius.jp.net/) for the review of the English in this manuscript.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Anderson J, Johnstone B, Remley D. 1999. Breast-feeding and cognitive development: A meta-analysis. Am J Clin Nutr. 70:525–535.
- Birnbaum L. 1994. The mechanism of dioxin toxicity: Relationship to risk assessment. Environ Health Perspect. 102:157–167.
- Blacquiere M, Hylkema M, Postma D, Geerlings M, Timens W, Melgert B. 2010. Airway inflammation and remodeling in two mouse models of asthma: Comparison of males and females. Int Arch Allergy Immunol. 153:173–181.
- Bouayed J, Desor F, Rammal H, Kiemer A, Tybl E, Schroeder H, Rychen G, Soulimani R. 2009. Effects of lactational exposure to benzo(α)pyrene (B[a]P) on postnatal neurodevelopment, neuronal receptor gene expression, and behavior in mice. Toxicology. 259:97–106.
- Clutterbuck E, Sanderson C. 1988. Human eosinophil hematopoiesis studied in vitro by means of murine eosinophil differentiation factor (IL5): Production of functionally active eosinophils from normal human bone marrow. Blood. 71:646–651.
- Faccioli L, Mokwa V, Silva C, Rocha G, Araujo J, Nahori M, Vargaftig B. 1996. IL-5 drives eosinophils from bone marrow to blood and tissues in a guinea pig model of visceral larva migrans syndrome. Med Inflamm. 5:24–31.
- Freeman G, Borriello F, Hodes R, Reiser H, Gribben J, Ng J, Kim J, Goldberg J, Hathcock K, Laszlo G, et al. 1993. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T-cell proliferation and interleukin 2 production. J Exp Med. 178:2185–2192.
- Galli S, Tsai M, Piliponsky A. 2008. The development of allergic inflammation. Nature. 454:445–454.
- Gordon N. 1997. Nutrition and cognitive function. Brain Dev. 19:165–170.
- Hattemer-Frey H, Travis C. 1991. Benzo(α)pyrene: Environmental partitioning and human exposure. Toxicol Ind Health. 7:141–157.
- Ho W, Blume M. 1976. Transmission of 14C-benzo(α)pyrene from lactating mice to their offspring. Proc West Pharmacol Soc. 19:28–31.
- International Program on Chemical Safety (IPCS). 1998. Selected non-heterocyclic polycyclic aromatic hydrocarbons. [accessed 2018 Feb 2]. www.inchem.org/documents/ehc/ehc/ehc202.htm
- Jerzynska J, Podlecka D, Polanska K, Hanke W, Stelmach I, Stelmach W. 2017. Prenatal and postnatal exposure to polycyclic aromatic hydrocarbons and allergy symptoms in city children. Allergol Immunopathol. 45:18–24.
- Kadkhoda K, Pourfathollah A, Pourpak Z, Kazemnejad A. 2005. The cumulative activity of benzo(a)pyrene on systemic immune responses with mite allergen extract after intranasal instillation and ex vivo response to ovalbumin in mice. Toxicol Lett. 157:31–39.
- Kadkhoda K, Pourpak Z, Akbar Pourfathallah A, Kazemnejad A. 2004. The ex vivo study of synergistic effects of polycyclic aromatic hydrocarbon, benzo(α)pyrene with ovalbumin on systemic immune responses by oral route. Toxicology. 199:261–265.
- Koike E, Yanagisawa R, Takigami H, Takano H. 2014. Penta- and octa-bromodiphenyl ethers promote pro-inflammatory protein expression in human bronchial epithelial cells in vitro. Toxicol In Vitro. 28:327–333.
- Kool M, van Nimwegen M, Willart M, Muskens F, Boon L, Smit J, Coyle A, Clausen B, Hoogsteden H, Lambrecht B, et al. 2009. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 183:1074–1082.
- Liang J, Zhu H, Li C, Ding Y, Zhou Z, Wu Q. 2012. Neonatal exposure to benzo[a]pyrene decreases the levels of serum testosterone and histone H3K14 acetylation of the StAR promoter in the testes of SD rats. Toxicology. 302:285–291.
- Liew F, Pitman NI, McInnes I. 2010. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol. 10:103–110.
- Lombardi V, Speak A, Kerzerho J, Szely N, Akbari O. 2012. CD8α+β− and CD8α+β+ plasma-cytoid dendritic cells induce Foxp3+ regulatory T-cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 5:432–443.
- Melgert B, Postma D, Kuipers I, Geerlings M, Luinge M, van der Strate B, Kerstjens H, Timens W, Hylkema M. 2005. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 35:1496–1503.
- Miller R, Yan Z, Maher C, Zhang H, Gudsnuk K, McDonald J, Champagne F. 2016. Impact of prenatal polycyclic aromatic hydrocarbon exposure on behavior, cortical gene expression and DNA methylation of theBdnfgene. Neuroepigenetics. 5:11–18.
- Mizutani N, Nabe T, Ohtani Y, Han H, Fujii M, Yoshino S, Hirayama T, Kohno S. 2007. Polycyclic aromatic hydrocarbons aggravate antigen-induced nasal blockage in experimental allergic rhinitis. J Pharmacol Sci. 105:291–297.
- Mollerup S, Berge G, Baera R, Skaug V, Hewer A, Phillips D, Stangeland L, Haugen A. 2006. Sex differences in risk of lung cancer: Expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Intl J Cancer. 11:741–744.
- Mollerup S, Ryberg D, Hewer A, Phillips D, Haugen A. 1999. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 59:3317–3320.
- Moore W, Hastie A, Li X, Li H, Busse W, Jarjour N, Wenzel S, Peters S, Meyers D, Bleecker E, et al. 2014. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 133:1557–1563.
- Niederhuber J, Shreffler D. 1977. Anti-Ia serum blocking of macrophage function in the in vitro humoral response. Transplant Proc. 9:875–879.
- Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. 2010. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 59:143–160.
- Oddy W. 2001. Breastfeeding protects against illness and infection in infants and children: A review of the evidence. Breastfeed Rev. 9:11–18.
- Okuyama K, Wada K, Chihara J, Takayanagi M, Ohno I. 2008. Sex-related splenocyte function in a murine model of allergic asthma. Clin Exp Allergy. 38:1212–1219.
- Quintana F, Sherr D. 2013. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev 65:1148–1161.
- Rubin H. 2001. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: A bio-historical perspective with updates. Carcinogenesis. 22:1903–1930.
- Ryberg D, Hewer A, Phillips D, Haugen A. 1994. Different susceptibility to smoking-induced DNA damage among male and female lung cancer patients. Cancer Res. 54:5801–5803.
- Santonicola S, de Felice A, Cobellis L, Passariello N, Peluso A, Murru N, Ferrante M, Mercogliano R. 2017. Comparative study on occurrence of polycyclic aromatic hydrocarbons in breastmilk and infant formula and risk assessment. Chemosphere. 175:383–390.
- Santos K, Florenzano J, Rodrigues L, Favaro R, Ventura F, Ribeiro M, Teixeira S, Ferreira H, Brain S, Damazo A, et al. 2014. Early postnatal, but not late, exposure to chemical ambient pollutant 1,2-naphthoquinone increases susceptibility to pulmonary allergic inflammation at adulthood. Arch Toxicol. 88:1589–1605.
- Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, Sagai M. 1997. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med. 156:36–42.
- Tesfaigzi Y. 2008. Regulation of mucous cell metaplasia in bronchial asthma. Curr Mol Med. 8:408–415.
- Wada K, Okuyama K, Ohkawara Y, Takayanagi M, Ohno I. 2010. Gender differences in transcriptional regulation of IL-5 expression by bronchial lymph node cells in a mouse model of asthma. Respirology. 15:629–635.
- Waldman J, Lioy P, Greenberg A, Butler J. 1991. Analysis of human exposure to benzo(a)-pyrene via inhalation and food ingestion in the Total Human Environmental Exposure Study (THEES). J Expo Anal Environ Epidemiol. 1:193–225.
- World Health Organization (WHO). 2010. Persistent organic pollutants: Impact on child health. Geneva: World Health Organization.
- Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee S, Chatila Z, Daher N, Sioutas C, Chatila T. 2015. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 136:441–453.
- Yanagisawa R, Koike E, Win-Shwe T, Ichinose T, Takano H. 2016. Low-dose benzo(α)pyrene aggravates allergic airway inflammation in mice. J Appl Toxicol. 36:1496–1504.
- Yanagisawa R, Takano H, Inoue K, Koike E, Sadakane K, Ichinose T. 2008. Effects of maternal exposure to di-(2-ethylhexyl) phthalate during fetal/neonatal periods on atopic dermatitis in male offspring. Environ Health Perspect. 116:1136–1141.
- Yanagisawa R, Takano H, Inoue K, Ichinose T, Sadakane K, Yoshino S, Yamaki K, Yoshikawa T, Hayakawa K. 2006. Components of diesel exhaust particles differentially affect TH1/TH2 response in a murine model of allergic airway inflammation. Clin Exp Allergy. 36:386–395.
- Zhou Y, Mirza S, Xu T, Tripathi P, Plunkett B, Myers A, Gao P. 2014. Aryl hydrocarbon receptor (AhR) modulates cockroach allergen-induced immune responses through active TGFβ1 release. Med Inflamm. 2014:591479.
- Zhu J, Cao Y, Li K, Wang Z, Zuo P, Xiong W, Xu Y, Xiong S. 2011. Increased expression of aryl hydrocarbon receptor and IL-22 in patients with allergic asthma. Asian Pacific J Allergy Immunol. 29:266–272.