Abstract
Endocrine-disrupting chemicals (EDC) are widespread in the built and natural environments. Heightened public awareness of their potential danger has led to concern about whether EDC and their metabolites have significant negative biological effects. Studies have shown that EDC like DDT and other organochlorine pesticides, such as methoxychlor (MXC), have adverse effects on immune cells, but no studies have addressed the impact of HPTE, the primary metabolite of MXC. To elucidate the presence and significance of HPTE adverse effects, this study explored the impact of HPTE on a critical window and component of immune system development, embryonic T-cell development. Lesions at this phase of development can lead to lifelong immune dysfunction and increased incidence of immune disease, such as autoimmunity. Embry-onic thymocytes (GD 16-18) from C57BL/6 mice were subjected to an in vitro differentiation culture that mimicked early steps in thymocyte development in the presence of 0.005, 0.05, 0.5, 5, or 50 μM HPTE, or a model endocrine disruptor, DES. The results indicated that compared to the vehicle control, HPTE- and DES-induced death of thymocytes. Annexin-V staining and Caspase 8, markers of programed cell death, revealed that the loss of cells was due at least in part to induction of apoptosis. Moreover, HPTE-induced cell death not only resulted in selective loss of double positive thymocytes, but also loss of developing CD4 intermediate cells (post-double positive partially differentiated thymocyte population). Phenotypic analysis of thymocyte maturation (T-cell receptor, TCR) and TCR ligation (CD5) surface markers revealed that surviving embryonic thymocytes expressed low levels of both. Taken together these data demonstrate that immature embryonic thymocytes are sensitive to HPTE exposure and that HPTE exposure targets thymocyte populations undergoing critical differentiation steps. These findings suggest HPTE may play a pivotal role in MXC exposure-induced immune dysfunction.
Introduction
Organochlorines are a class of chemicals that persist in the environment, accumulate in humans and other organisms, and have been associated with increased risk of cancer, develop-mental defects, endocrine dysfunction, and alteration of immune competence (Meeker Citation2010; Caserta et al. Citation2011). Globally, organochlorine pesticides represent a significant and ongoing body burden (Schettgen et al. Citation2015) due to their lipid partitioning, bioaccumulation, and resistance to degradation, all of which contribute to their persistence. While the potential hazards of this class of chemicals were first systematically raised by Rachel Carson’s Silent Spring in 1962 (Carson Citation2002), the mechanisms whereby these chemicals mediate their negative effects are still not understood more than 50 years later. Moreover, for nearly two decades, investigators have called attention to the possible relationship between immune dysfunction and environmental toxicants (Ahmed et al. Citation1999; Ahmed Citation2000; Winans et al. Citation2011). However, the impact and mode of action of organochlorines and their metabolites on the immune system have still not been clearly elucidated (Dietert Citation2014; Heindel et al. Citation2016).
Methoxychlor (MXC) is a pesticide and a model compound for more persistent organochlorine pesticides like DDT and its metabolite DDE (p,p′-dichlorodiphenyl-dichloroethylene). While MXC was not re-licensed in 2003, interest in MXC persists due to its continued use through 2007 (as existing supplies were used up), its manufacture beyond 2003, its application on crops imported into the USA, and its potential transgenerational effects (Manikkam et al. Citation2014). During its peak use, the estimated daily intake for females ages 25–30 years was 1.2 ng/kg/d and for two-year-olds was 6.3 ng/kg/d (Gunderson Citation1988). The Canadian total diet study reported detection of MXC in five percent of food samples tested with a mean level of 37.8 ng/g (Rawn et al. Citation2004) and MXC has been detected in blood and adipose tissue (Botella et al. Citation2004), as well as in placenta and breast milk at micromolar concentrations (Damgaard et al. Citation2006; Shen et al. Citation2007). MXC has been associated with developmental, reproductive, neuro-, and immunotoxicity in mammals (CA EPA Citation2010).
Methoxychlor has been shown to be metabolized in a variety of organisms and to enhance its own metabolism (Li et al. Citation1995; Stuchal et al. Citation2006). The primary metabolite of MXC is 1,1,1-trichloro-2,2-bis(4-hydroxyphenyl) ethane (HPTE). While HPTE has been shown to be more estrogenic than MXC (Bulger et al. Citation1978; Ousterhout et al. Citation1981; Shelby et al. Citation1996; Hodges et al. Citation2000), little work has been done to specifically elucidate effects of HPTE on exposed animals or to measure levels of HPTE generated in MXC-treated animals. Without an understanding of conversion efficiency in animals, discrepancies in results are difficult to reconcile, particularly when variations in dose can lead to differences in response. For example, in a uterine cell line, HPTE induced proliferation at nM doses and growth inhibition at μM doses (Hewitt and Korach Citation2011). Lastly, none of the few studies that probed effects of HPTE directly addressed the impact of HPTE on immune system development, even though HPTE is the likely mediator of MXC effects.
A critical component of the adaptive immune response is the T-cell. T-Cell precursors must undergo differentiation in the thymus to become functionally mature. The thymus has been shown to be sensitive to chemical insult by a wide variety of natural (e.g. estrogen) and synthetic chemicals (e.g. diethylstilbestrol [DES], pesticides like MXC, and other so-called endocrine-disrupting chemicals [EDC]). Excess estrogen either due to pregnancy (Rijhsinghani et al. Citation1996; Zoller et al. Citation2007) or added exogenously (Staples et al. Citation1998, Citation1999; Okasha et al. Citation2001; Zoller and Kersh Citation2006) induced thymic atrophy and substantially diminished the presence of double-positive (DP) thymocytes. Studies of estrogen-induced thymic atrophy indicated that cell losses may be due to loss of the earliest thymic progenitors (ETP) or double-negative (DN) thymocytes in the thymus, but not loss of bone marrow progenitors (Zoller et al. Citation2007). Similarly, in DES-exposed animals, the thymus atrophied and the DP population was diminished (Holladay et al. Citation1993; Lai et al. Citation1998; Calemine et al. Citation2002; Fenaux et al. Citation2004; Besteman et al. Citation2005) as well. These data suggested to us that thymocyte populations may be particularly susceptible to elevated estrogen or estrogenic EDC exposure.
Double-positive cells compose the population of thymocytes that is primarily targeted for positive and negative selection, differentiation events that probe thymocyte functionality and the propensity for autoreactivity. By these processes, cells with a functional T-cell receptor (TCR) that is not autoreactive are allowed to survive. Maturation of DP thymocytes results in semi-mature intermediates (CD4 intermediates; Cibotti et al. Citation1997; Kishimoto and Sprent Citation1997) that ultimately give rise to naïve CD4+CD8− or CD4−CD8+ single positive (SP) thymocytes (Singer et al. Citation2008; Hogquist et al. Citation2015; Kurd and Robey Citation2016). Only a single study thus far (Brown et al. Citation2006a) attempted to examine whether in addition to cell loss, differentiation of the DP population might be altered as well in DES-exposed animals. However, interpretation of these data was complicated by the in vivo model used (H–Y TCR transgene). Thus, it remains unclear whether the developmental programing of thymocytes is altered by EDC exposure.
The differentiation process not only involves changes in surface expression of CD4 and CD8 on thymocytes but also in the expression and signaling of TCR on the surface of the cell. When TCR+ thymocytes signal, CD5 expression is induced (Azzam et al. Citation1998). The level of CD5 surface expression depends on the avidity of the TCR (Azzam et al. Citation2001) and serves to dampen the TCR signal intensity during thymocyte development (Tarakhovsky et al. Citation1995). In this manner CD5 serves as both an indicator and regulator of TCR signaling in thymocytes, influencing the normal selection of functional thymocytes.
Aberrations of the normal developmental process by EDC potentially lead to immune dysfunction later in life (Noller et al. Citation1988; Ahmed et al. Citation1999; Heilmann et al. Citation2006). However, none of the studies of other EDC like estrogen and DES, have probed the phenotype (other than basic population determination) or activation status of impacted thymocytes to determine whether cell maturation stage and TCR ligation history might influence EDC susceptibility. Moreover, it is difficult to compare prior studies given that few have used the same route of administration (e.g. intraperitoneal injection, subcutaneous injection, feeding, or gavage), stage of development (e.g. adult, three-week-old, five-week-old, and prenatal, post-natal), duration of treatment (e.g. hours, days, and weeks), or assays to analyze outcomes. Furthermore, in vivo studies have been confounded by endogenous mechanisms for removal of apoptotic cells, making these studies challenging to interpret. Careful and methodical examination of EDC impact is necessary to identify what makes thymocytes susceptible to EDC exposure.
The current study was the first to investigate direct effects of HPTE on thymocytes from one of the most vulnerable populations, i.e. embryos, and to determine whether EDC perturbed normal establishment of the immune system. To achieve this goal the study differed in method and approach from previous research. Specifically, in vitro analyses and use of the metabolite HPTE in culture were employed. These approaches allowed for minimization of confounding variables associated with prior studies, while still examining the dynamic process of embryonic T-cell development. The in vitro differentiation culture mimicked early steps in thymocyte development (Cibotti et al. Citation1997), primarily positive selection, using a combination of surface receptor antibodies that trigger the early steps of maturation and induce partial differentiation of thymocytes from the double positive stage to the CD4 intermediate stage. This study reports on the impact of HPTE exposure on early embryonic thymocyte differentiation, including the induction of apoptosis, the population phenotype and the activation status of embryonic thymocytes most susceptible to HPTE effects.
Materials and methods
Animals
All experiments were approved and conducted under the supervision of the Institution Animal Care and Use Committee, in accordance with federal guidelines for the care and use of live animals in research (Guide, Eighth Edition, NRC Citation2011). C57BL/6 mice were purchased from Simonsen Labs (Gilroy, CA, USA). Timed-matings were performed in house or by the vendor to produce embryos aged 16–18 days post-fertilization (dpf). Mice were maintained in polypropylene cages in a facility maintained at 20–24 °C with a 40–45% relative humidity and a 12-h light cycle. All mice had ad libitum access to standard rodent chow and filtered water.
Chemicals
Endocrine disruptors (i.e., EDC), diethylstilbestrol (DES, purity 100%; Melnick et al. Citation1987; Harris and Waring Citation2012; Reed and Fenton Citation2013) and HPTE (2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (purity 97%; MXC metabolite) were bought from Sigma-Aldrich (St. Louis, MO, USA). MXC is normally metabolized in the liver (Kapoor et al. Citation1970) to form HPTE and 2-(p-hydroxyphenyl)-2-(p-methoxyphenyl)-1,1,1-trichloroethane. As such, here, the metabolite HPTE was used instead of the parent molecule to avoid confounding effects of a lack of metabolism in primary culture.
In vitro differentiation assay
In brief, prior to receiving any cells, 96-well plates were prepared and incubated 24 h at 4 °C with purified antibodies to CD2 (RM2-5, Rat IgG2b) and the T-cell receptor (TCR; H57-597, Armenian Hamster IgG), alone or in combination. Each antibody was purchased from BioLegend (San Diego, CA, USA) and used at 5 and 10 µg/ml, respectively, based on Cibotti et al. (Citation1997) and empirical work. This combination of antibodies has been shown to induce differentiation of DP cells into CD4intermediateCD8low thymocytes (Punt et al. Citation1994; Cibotti et al. Citation1997; Skånland et al. Citation2014). Plates were washed with medium to remove unbound antibody immediately before use with cells.
For the assay, embryonic thymocytes (gestational day [GD] 16–18) were teased into single cell suspensions in complete RPMI 1640 (cRPMI; RPMI 1640 [Caisson Labs, Smithfield, UT, USA] supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 μM β-mercaptoethanol, and 1% penicillin/streptomycin [all Gemini Bioproducts, Sacramento, CA, USA]). Note: Thymocytes were processed and cultured in the absence of phenol red to avoid confounding effects of phenol red estrogenicity (Berthois et al. Citation1986). After counting, the thymocytes were placed in the wells (4 × 105 cells/well) and then incubated for 18–24 h at 37 °C in the antibody-coated plates (signaling culture). At the end of the signal culture period, cells were removed by careful pipetting, washed in cRPMI, and transferred to a fresh, uncoated 96-well plate. Embryonic thymocytes were incubated an additional 18–24 h in the absence of antibodies (recovery culture) to facilitate re-expression of surface markers, such as TCR, and completion of differentiation.
To assess effects of the test agents, cells were exposed to a range of levels (0.005, 0.05, 0.5, 5.0, or 50 μM) of HPTE or DES; these doses were selected based on a survey of published studies and empirical work. HPTE is 20–100 times more estrogenic than the parent molecule and only about 10-fold less potent than estradiol; DES has approximately equivalent estrogenic activity to estradiol (Shelby et al. Citation1996). In general, HPTE or DES were added to in vitro differentiation assays during the signaling culture (signaling alone) then removed when cells were transferred to their recovery cultures. For apoptosis studies, embryonic thymocytes were incubated for 2, 4, 6, 8 or 6, 18, 21, or 24 hr during the signaling culture in the presence of 50 μM HPTE or DES.
Phosphatidylserine staining
During early induction of apoptosis, phosphatidylserine (PS) becomes associated with the outer membrane leaflet. Annexin V-FITC (BioLegend, San Diego, CA, USA) was used to identify cells in early stages of apoptosis as it binds PS. In brief, cells in differentiation cultures were treated with EDC for indicated times, centrifuged to remove the treatment, then stained with Annexin V as per manufacturer protocols. The cells were then washed and stained with propidium iodide (PI, 200 ng/ml final concentration; Sigma, St. Louis, MO, USA), an impermeant dye excluded from membrane-intact cells. The combined stains allowed for identification of live (AnV−PI−), apoptotic (AnV+PI−), late apoptotic or necrotic (AnV+PI+), and necrotic (AnV−PI+) cells. Controls treated with vehicle/diluent only (negative control; , open circles or triangles) or 0.4 μM DEX (positive control; ) were also generated. Water-soluble DEX alone can induce apoptosis and its hallmarks (e.g. PS flipping and caspase 8 activation). The matched negative control for DEX was the “no antibody” condition in medium. In all cases, cells were collected on an Accuri C6 system and analyzed using FlowJo software (both BD Biosciences, San Diego, CA, USA). For each sample, a minimum of 10 000 events was acquired.
Caspase activation
Caspase 8 expression on cells is associated with induction of death via T-cell receptor ligation or Fas/Fas ligand interactions (Pozzesi et al. Citation2014). As caspase 8 has a functional role in DP thymocyte apoptosis (Jiang et al. Citation1999), embryonic thymocytes were probed for any changes in caspase 8 activity using a caspase 8-specific fluorophore-labelled peptide. Cells with activated caspase 8 (presumed apoptotic) were identified by staining with Caspa-Lux®8 L1D2 (Onco-immunin, Gaithersberg, MD, USA) according to manufacturer protocols. In brief, cells in differentia-tion culture were treated with EDC for the indicated times, centrifuged to remove treatment, then resuspended in CaspaLux®8 L1D2 substrate solution and incubated at 37 °C for 1 h in the dark. Fluorescence induced by peptide cleavage (via activated caspase 8) was used to define caspase 8+ cells. Cells were then washed in 1 ml kit-provided dilution buffer and resuspended for flow cytometry collection. Samples were collected on the Accuri C6 system twice, as recommended, in the absence/presence of PI, and analyzed. A minimum of 10 000 events/sample was acquired.
Abs and flow cytometry staining
T-Cell receptor expression levels reflect thymocyte maturational stage. Thymocytes that express no or low levels of TCR are immature; those with high levels are more mature. Following the above treatments, the isolated cells underwent labeling with a cocktail containing the following antibodies (all BioLegend, San Diego, CA, USA) that had been titrated for use to stain freshly isolated and cultured thymocytes. The antibodies used included APC-anti-CD4 (clone RM4-5, rat IgG2a), FITC-anti-CD8 (clone 53-6.7, rat IgG2a), PE-anti-CD5 (clone 53-7.3, rat IgG2a), PE/Cy5-anti-TCR (clone H57–597, Armenian hamster IgG). In brief, the isolated cells were stained with fluorochrome-conjugated antibodies for 30 min in the dark on ice, then washed with phosphate-buffered saline (PBS, pH 7.4) containing 1% FBS. Thereafter, the cells were immediately collected on the Accuri C6 system and analyzed. In each case, a minimum of 10 000 events/sample was acquired, and levels of marker expression (and hence, population dynamics/status) determined for each treatment group.
Statistical analyses
Repeated measures Analysis of Variance (rANOVA) was applied to experiments with a repeated time element (e.g. toxicant was administered to same culture at multiple timepoints). The dependent variable, which represented the experimental outcome, was population mean cell number (, ; DN, DP, CD4, CD8, or total cell population), Annexin V+/PI− mean cell number (, ), mean percent Caspase 8+ cells (, ), or mean percent cells with varying expression levels of CD5 or TCR (, ). One independent variable represented the antibody condition, while the other independent variable is a block designed to control for variation introduced by treating cell populations at different times. The assumptions underlying rANOVA analyses were tested and the dependent variable transformed with the natural log due to non-normality or a violation of sphericity. Tukey–Kramer post hoc tests were also performed when the results from the rANOVA evaluations were significant (p < 0.05).
Table 1. Two-tailed Kolmogorov–Smirnov tests comparing population mean cell numbers of GD16–18 embryonic thymocytes exposed transiently to 50 µM of DES () or HPTE () to controls.
Table 2. Comparison of Annexin V+/PI- mean cell numbers within antibody condition and across hours in treated vs. control embryonic thymocytes.
Table 3. Post hoc analysis of Annexin V+/PI- mean cell numbers in 50 µM DES- or HPTE-treated thymocytes ().
Table 4. Two-tailed Kolmogorov–Smirnov tests comparing Annexin V+/PI− mean cell numbers in treated vs. control embryonic thymocytes.
Table 5. Comparing mean % Caspase 8+ cells between 50 µM DES- or 50 µM HPTE-treated and control cells within antibody condition and across hours ().
Table 6. Post hoc analysis of mean % Caspase 8+ cells in control, 50 μM DES- or HPTE-, and DEX-treated thymocytes ().
Table 7. Post hoc analysis of mean % Caspase 8+ cells in anti-BOTH cultures treated with 50 µM DES or HPTE for durations indicated ().
Table 8. Two-tailed Kolmogorov–Smirnov tests comparing mean percent of embryonic thymocytes expressing CD5 or TCR in untreated and 50 µM DES-treated anti-BOTH differentiation cultures ().
To test if there were differences in the means between doses 0 and 50 μM within each experimental state, non-parametric t-tests, Kolmogorov–Smirnov (KS) tests, were run on each cell type. A Bonferroni correction (c.f., Bland and Altman Citation1995; Altman and Krzywinski Citation2017) was applied to each round of tests to diminish likelihood of Type I errors (finding significance when not present) in analyses testing multiple hypotheses. All rANOVA, post hoc analyses, and KS tests and were conducted in R (R Core Team Citation2013) and graphs were developed in Microsoft Excel. Sphericity tests were completed using SPSS (IBM, Armonk, NY, USA).
Results
In vitro response of primary embryonic thymocytes
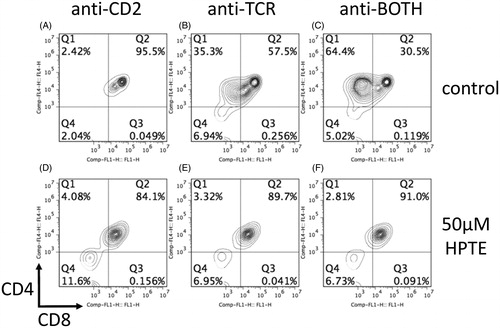
To see if exposure to HPTE abrogated developmental pathways in embryonic thymocytes, primary cells were harvested from normal gestational day (GD) 16–18 C57BL/6 embryos. Embryonic thymocytes were then cultured in vitro in the presence of plate-bound antibodies to CD2, TCR, or both (anti-BOTH) to mimic in vivo signaling events that lead to survival, differentiation, or both, respectively (Cibotti et al. Citation1997). With anti-CD2 alone, thymocytes remained DP in culture (), with anti-TCR alone a small proportion of DP thymocytes differentiated to CD4 intermediate cells (, ∼35%), and with anti-BOTH a large proportion of DP cells differentiated to CD4 intermediate cells (, ∼64%). In cultures where 50 μM HTPE had been added (), CD4 intermediate cells did not develop or died under conditions where they would normally develop (; each ∼3–4% surviving cells).
Figure 1. CD4 intermediate cells did not develop – or died – in differentiation cultures exposed to 50 μM HPTE. Embryonic thymocytes (GD16–18) were differentiated into CD4 intermediate cells in a 2-d differentiation culture. On Day 1, cells were cultured in presence of (A–C) antibodies alone or (D–F) antibodies plus toxicant. On Day 2, cells were washed and transferred to plates without antibodies or toxicant. At the end of Day 2, cells were washed and stained with fluorochrome-conjugated antibodies for CD4 and CD8. Upon stimulation with antibodies against CD2 (A, D), control cells (A) remained DP. Stimulation with antibodies against TCR (B, E) typically resulted in some differentiation into CD4 intermediate cells in controls (B). Cells stimulated with both antibodies (C, F) yielded the most CD4 intermediate cells in controls (C). CD4 intermediate cells were absent in HTPE-treated cells (D–F).
Differences in mean live cell numbers were observed between antibody conditions, primarily for DP and CD4 SP cells (, compared to untreated control in each panel). Anti-TCR and anti-BOTH antibody stimulation increased the number of CD4 intermediate cells that differentiated from the DP pool, with greater numbers of CD4 intermediate cells resulting from anti-BOTH stimulation (, height of bar representing CD4 [crosshatched bar] was greater in subfigures B and F, and greatest in subfigures C, D, G, and H). These differences in population cell numbers between antibody conditions were the expected changes in thymocyte populations that result from antibody stimulation, and verified the effectiveness of the differentiation culture.
Figure 2. Embryonic thymocytes (GD16–18) exposed transiently to DES or HPTE during differentiation culture. Embryonic thymocytes were cultured 18–24 h in presence of antibody listed above each panel and indicated concentrations of (A–D) DES or (E–H) HPTE on Day 1. On Day 2, cells were washed and transferred to plates without the antibody or toxicant. At the end of Day 2, cells were stained with fluorochrome-conjugated antibodies against CD4, CD8, TCR, and CD5. Each bar represents average number of cells in a given population (DN – white, DP – black, CD4 SP – hatched, and CD8 SP – grey) surviving indicated treatment. A blocked two-way ANOVA was used to examine differences across treatments, but within condition and within cell population. *Values significantly different under each condition (antibody type or combination) (see also [DES] and [HPTE]), with post hoc analysis showing the 50 μM dose being significantly different from other doses (0, 0.005, 0.05, 0.5, 5.0 μM DES or HPTE). DES N = 9, HPTE N = 9. Whisker bars are SEM of each population.
![Figure 2. Embryonic thymocytes (GD16–18) exposed transiently to DES or HPTE during differentiation culture. Embryonic thymocytes were cultured 18–24 h in presence of antibody listed above each panel and indicated concentrations of (A–D) DES or (E–H) HPTE on Day 1. On Day 2, cells were washed and transferred to plates without the antibody or toxicant. At the end of Day 2, cells were stained with fluorochrome-conjugated antibodies against CD4, CD8, TCR, and CD5. Each bar represents average number of cells in a given population (DN – white, DP – black, CD4 SP – hatched, and CD8 SP – grey) surviving indicated treatment. A blocked two-way ANOVA was used to examine differences across treatments, but within condition and within cell population. *Values significantly different under each condition (antibody type or combination) (see also Table 1 [DES] and Table 2 [HPTE]), with post hoc analysis showing the 50 μM dose being significantly different from other doses (0, 0.005, 0.05, 0.5, 5.0 μM DES or HPTE). DES N = 9, HPTE N = 9. Whisker bars are SEM of each population.](/cms/asset/8f826a20-095f-4abf-9b57-c76fdd656ea0/iimt_a_1474978_f0002_b.jpg)
A blocked two-way ANOVA revealed no significant difference between mean live cell numbers of cultures treated with 0, 0.005, 0.050, 0.5, or 5.0 μM DES (, first five levels) or HPTE (, first five levels) within each antibody condition (i.e. anti-CD2 [), anti-TCR [], or anti-BOTH [) or control cultures treated with vehicle (anti-BOTH, DMSO []). In contrast, within each antibody condition, effects of the 0 and 50 μM doses were different (, first vs. last level, respectively ( for DES and 2E-2G for HPTE). When analyzed with a Kolmogorov–Smirnov test (), the mean live cell number within each antibody condition was significantly decreased at the 50 μM dose for at least two thymocyte populations, regardless of antibody condition, except anti-BOTH DMSO which received only the vehicle DMSO. Moreover, specific populations of thymocytes, particularly DP and CD4, were consistently affected at 50 μM, with near complete loss of the CD4 intermediate population. DP loss in DES- or HPTE-treated cultures was similarly observed in all antibody conditions. DP loss was statistically significant under all antibody conditions for both toxicants, except in the anti-BOTH condition treated with HPTE where DP loss also occurred but was not statistically significant.
Apoptosis
Annexin V – PS
To determine whether treatment with the EDC-induced apoptosis as measured by Annexin V and PI staining, cultured embryonic thymocytes were harvested 2, 4, 6, and 8 h after the EDC was added to the signaling culture (). Annexin V+/PI− cell counts differed between the controls (open circles) and treatment (closed circles) within anti-CD2 (), anti-TCR (), and anti-BOTH conditions () and positive control DEX (), with EDC- or DEX-treated cells having a larger number of Annexin V+/PI− cells. HPTE treatment appeared to induce PS surface expression earlier (perhaps as early as 4 h, ) and to induce a larger number of cells to express surface PS than DES.
Figure 3. Detection of apoptotic cells in differentiation culture of embryonic thymocytes. Differentiation cultures were treated with (A–C) 50 μM DES, (F–H) 50 μM HPTE, (D, I) 1:2000 dilution of DMSO as negative control, or (E, J) 0.4 μM dexamethasone as positive control. Mean cell number apoptotic (Annexin V+ propidium iodide [PI]−) DP embryonic thymocytes under different antibody conditions is shown. Open symbols, medium only controls; closed symbols, experimental. DES N = 8, HPTE N = 6. Whisker bars are SEM of DP thymocytes.
![Figure 3. Detection of apoptotic cells in differentiation culture of embryonic thymocytes. Differentiation cultures were treated with (A–C) 50 μM DES, (F–H) 50 μM HPTE, (D, I) 1:2000 dilution of DMSO as negative control, or (E, J) 0.4 μM dexamethasone as positive control. Mean cell number apoptotic (Annexin V+ propidium iodide [PI]−) DP embryonic thymocytes under different antibody conditions is shown. Open symbols, medium only controls; closed symbols, experimental. DES N = 8, HPTE N = 6. Whisker bars are SEM of DP thymocytes.](/cms/asset/a126554c-492c-4383-9a74-348808ab112f/iimt_a_1474978_f0003_b.jpg)
Data from DES- or HPTE-treated and control embryonic thymocytes were analyzed by rANOVA (). The results revealed a significant increase in Annexin V+/PI− cells under most conditions (p values within condition) treated with DES or HPTE, except for BOTH–DMSO (the vehicle control), which did not increase.
Comparing all timepoints to each other for the anti-BOTH condition (i.e. differentiated cells), DES-treated cultures did not differ from each other (). However, a significant increase in Annexin V+/PI− cell counts was noted in HPTE-treated cultures at 6- and 8-h compared to at 2 h (); however, outcomes at these two timepoints did not differ from one another () or from those at 4 h (). Two-tailed Kolmogorov–Smirnov tests confirmed significant differences between controls and DES at 6 h in the anti-BOTH condition and DEX (). Whereas, HPTE showed significant increases at 6 hr in all antibody conditions, anti-CD2, anti-TCR, and anti-BOTH, and DEX, compared to controls.
Caspase 8
Caspase 8 appeared to be activated at the earliest analyzed timepoint (i.e. 6 h) in the differentiating (anti-BOTH condition, ) embryonic thymocytes exposed to DES (, closed circles), HPTE (, closed circles), or DEX (, closed triangles). This timing of caspase 8 activation correlated with the rapid appearance of Annexin V+/PI− cells (). Induction of caspase 8 activity peaked at 18 h, remaining high through the 24-h point with DES- or HPTE-treated cultures; in contrast, in DEX-treated cultures, caspase 8 activity declined to control levels by the 24-h point ().
rANOVA analysis of EDC-treated embryonic thymocytes (, p values within condition) revealed statistically significant increases in the percent caspase 8+ cells in DES- or HPTE-treated cells compared to controls under all conditions, except for the BOTH–DMSO (the vehicle control) condition, which did not increase. Significant increases were also observed over time in cultured thymocytes under all antibody conditions (, p values across hours). For DEX, the peak in percent caspase 8+ cells that occurred at 18 h and declined in subsequent timepoints () complicated analysis of significance across hours, yielding contradictory results of no significance (, DES) and significance (, HPTE) for changes across hours. The finding that in the absence of toxicants differentiating thymocytes have a background level of caspase activation over time (, control, anti-BOTH; open circles) was expected. A comparison of controls with and without antibodies showed that antibodies alone produced caspase 8 activation in a small percentage of embryonic thymocytes (, open circles vs. open triangles), as expected, whereas medium alone (, open triangles) or DMSO alone (not shown) did not.
Figure 4. Caspase 8 activation in DP embryonic thymocytes following DES or HPTE exposure. Embryonic thymocytes were incubated in presence of CD2 and TCR antibodies, and medium alone (A, C), 50 μM DES (B and E – closed circles), or 50 μM HPTE (D and F – closed circles) for 6, 18, 21, and 24 h (A–D 18 h only; E and F, all timepoints). Caspase 8 activation measured by mean fluorescence intensity of cleaved target peptide. Cells were counterstained with PI to exclude necrotic cells. Number in lower right quadrant (A–D) indicates % cells with active caspase 8 that have retained membrane integrity (Caspase 8+ PI−). Panels E and F summarize data from five independent experiments (DES or HPTE – closed circles; DEX – closed triangle, antibody alone control – open circles, medium only control – open triangles). Caspase 8 activation detected at 6, 18, 21, and 24 h. DES N = 5, HPTE N = 5. Whisker bars are SEM of DP thymocytes.
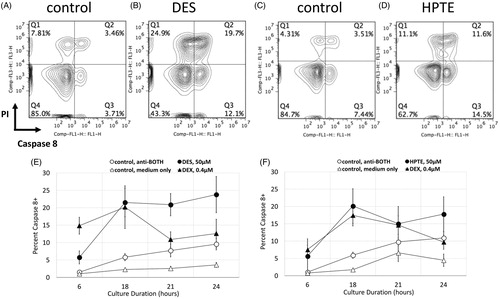
Post hoc analysis comparing EDC-treated embryonic thymocytes to the BOTH–DMSO control (), confirmed that the significant increases by EDC were above and beyond the background. DEX-treated samples were also significantly increased compared to medium control – no antibodies (), but not to EDC (). For the EDC, post hoc tests showed that a 6 hr duration of DES () or HPTE () was significantly different than at any other duration for anti-BOTH conditions.
Phenotypic analysis of markers for maturation and ligation
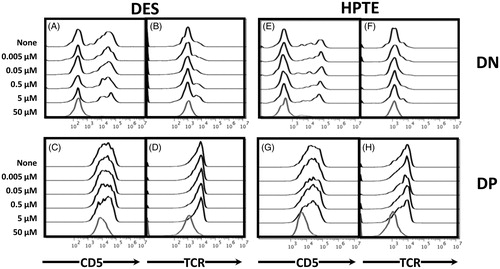
To further understand which stages of development were targeted by EDC, or whether cell death observed was nonspecific, surface TCR and CD5 expression was examined. When the surface phenotype of embryonic thymocytes treated with EDC was probed, DN and DP cells surviving high-dose EDC treatment from differentiation cultures with TCR antibodies (alone or in combination with CD2 antibodies) had lower TCR and CD5 expression levels than untreated or low dose-treated cells (). For DN cells, the CD5high peak was completely lost in cultures treated with DES (, bottom trace) or HPTE (, bottom trace). For DP cells, the mean fluorescence intensity of surviving cells shifted left in cultures treated with 50 µM DES (, bottom trace) or 50 µM HPTE (, bottom trace). TCR expression in surviving cells showed the same leftward shift in DN and DP cells in cultures treated with 50 µM DES (, bottom trace) or 50 µM HPTE (, bottom trace). These changes suggested actively differentiating cells were targeted for death.
Figure 5. Differentiating embryonic thymocytes that survived 50 μM DES or HPTE exposure expressed reduced levels of TCR and CD5 on their surface. Cells were incubated in a 2-d differentiation culture, with exposure to anti-CD2 and anti-TCR antibodies and toxicants on Day 1. Cells were stained at end of Day 2 incubation period with fluorochrome-conjugated antibodies to CD4, CD8, TCR, and CD5. Representative histograms shown (DES N = 9, HPTE N = 10) of CD5 (panels at left) and TCR (panels at right) mean fluorescence intensity from gated DN (top row) or DP (bottom row) embryonic thymocytes treated with indicated concentrations of (A–D) DES or (E–H) HPTE.
For experiments examining DES-treated embryonic thymocytes (), 57% of control DN cells expressed low levels of CD5 (, 0 cluster bar – white portion of bar) compared to 91% of surviving DN cells exposed to 50 μM DES (, 50 μM cluster bar). For DP cells 38% of control cells expressed moderate levels of CD5 (, 0 cluster bar – black portion of bar) compared to 65% of surviving DP cells exposed to 50 μM DES (, 50 μM cluster bar). Analysis of TCR expression () revealed 98% of control DN expressed low levels of TCR (, 0 cluster bar – black portion of bar) compared to 100% of surviving DN cells exposed to 50 μM DES (, 50 μM cluster bar). For DP cells, 87% of DP cells expressed moderate levels of TCR (, 0 cluster bar – black portion of bar) compared to 99% of surviving DP cells exposed to 50 μM DES (, 50 μM cluster bar). The observed significant increases in CD5low and TCRlow expressing cells in the DN and DP populations were balanced by statistically significant decreases in CD5med (in DN) or CD5high (in DP) and TCRhigh expressing cells (, DES). These results indicated a targeted loss of CD5high and TCRhigh cells as a result of DES treatment.
Figure 6. Distribution of embryonic thymocytes expressing different surface levels of CD5 and TCR shifts in cultures that have survived transient concurrent exposure to differentiation signals and toxicants. Cells were incubated in a two-day differentiation culture, with exposure to anti-CD2 and anti-TCR antibodies and toxicants (50 μM) on Day 1, and recovery without antibodies or toxicant on Day 2. Cells were stained at end of Day 2 with fluorochrome-conjugated antibodies to CD4, CD8, TCR, and CD5. Shown are pooled data of the percent distribution of CD5 (low [DN only], medium, high; A, B, E, F) and TCR (low, high; C, D, G, H) from gated DN (A, C, E, G) or DP (B, D, F, H) embryonic thymocytes treated with the indicated concentrations of (A–D) DES or (E-H) HPTE. DES N = 9, HPTE N = 10. Whisker bars are SEM of thymocytes with the indicated level of CD5 or TCR expression.
![Figure 6. Distribution of embryonic thymocytes expressing different surface levels of CD5 and TCR shifts in cultures that have survived transient concurrent exposure to differentiation signals and toxicants. Cells were incubated in a two-day differentiation culture, with exposure to anti-CD2 and anti-TCR antibodies and toxicants (50 μM) on Day 1, and recovery without antibodies or toxicant on Day 2. Cells were stained at end of Day 2 with fluorochrome-conjugated antibodies to CD4, CD8, TCR, and CD5. Shown are pooled data of the percent distribution of CD5 (low [DN only], medium, high; A, B, E, F) and TCR (low, high; C, D, G, H) from gated DN (A, C, E, G) or DP (B, D, F, H) embryonic thymocytes treated with the indicated concentrations of (A–D) DES or (E-H) HPTE. DES N = 9, HPTE N = 10. Whisker bars are SEM of thymocytes with the indicated level of CD5 or TCR expression.](/cms/asset/80a3129d-daac-4c15-8b13-8604271f752e/iimt_a_1474978_f0006_b.jpg)
For experiments examining HPTE-treated embryonic thymocytes (), 57% of control DN cells expressed low levels of CD5 (, 0 cluster bar – white portion of bar) compared to 89% of surviving DN cells exposed to 50 μM DES (, 50 μM cluster bar). For DP cells 27% of control DP cells expressed moderate levels of CD5 (, 0 cluster bar – black portion of bar) compared to 64% of surviving DP cells exposed to 50 μM DES (, 50 μM cluster bar – black). Analysis of TCR expression () revealed 96% of control DN expressed low levels of TCR (, 0 cluster bar – black portion of bar) compared to 100% of surviving DN cells exposed to 50 μM DES (, 50 μM cluster bar). For DP cells, 89% of DP cells expressed moderate levels of TCR (, 0 cluster bar – black) compared to 98% of surviving DP cells exposed to 50 μM DES (, 50 μM cluster bar). The observed statistically significant increases in CD5 low and TCR low expressing cells in the DN and DP populations treated with HTPE, were balanced by statistically significant decreases in CD5 high and TCR high expressing cells (). In the case of HPTE, changes in TCR in the DP population were not significant (, bottom two rows). These results showed a similar targeted loss of CD5high DN and DP cells and TCR high DN cells upon HPTE treatment. Overall, these data suggest that TCR-signaled cells were preferentially targeted by – or were sensitive to – DES or HPTE in the differentiation cultures.
Discussion
Many groups have shown that the adult thymus atrophies and thymocytes die in mice and rats exposed to exogenous estrogen (Luz et al. Citation1969; Luster et al. Citation1984; Silverstone et al. Citation1994; Staples et al. Citation1998; Okasha et al. Citation2001; Zoller and Kersh Citation2006; Zoller et al. Citation2007; Wang et al. Citation2008), DES (Barnes et al. Citation1983; Calemine et al. Citation2002; Brown et al. Citation2006b; Frawley et al. Citation2011), genistein (GEN – Yellayi et al. Citation2002) or MXC (Fukuyama et al. Citation2010, Citation2011). A few studies have examined EDC effects on younger animals treated in vivo during gestation (DES – Luster et al. Citation1979, Holladay et al. Citation1993, Besteman et al. Citation2005, Brown et al. Citation2006b), in vivo during gestation and post natally (DES – Fenaux et al. Citation2004; GEN – Guo et al. Citation2002, Citation2006; MXC – Chapin et al. Citation1997, Takeuchi et al. Citation2002, Citation2004; White et al. Citation2005) or only in vitro (DES – Lai et al. Citation1998, Citation2000). For most of these studies, it has been difficult to identify commonalities in the effects of estrogen or estrogenic EDC exposure on the process of development, as the studies used varied routes of administration, timing of exposure, and outcome measures, predominantly from post-natal or adult cells.
The purpose of this study was to elucidate the presence and significance of any adverse effects of HPTE on embryonic T-cell development, by examining embryonic thymocytes exposed concurrently in vitro to differentiation signals and HPTE. In addition to probing whether HPTE-induced death in embryonic thymocytes, this study evaluated CD4/CD8 surface phenotype and activation status of impacted thymocytes to determine whether maturation stage/TCR ligation history of the cells might influence EDC susceptibility. To accomplish these goals, GD16-18 embryonic thymocytes were harvested and cultured in two-day in vitro differentiation assays that mimicked the early stages of thymocyte development, primarily positive selection. The study here found that a relatively high concentration (≥ 12.5 μM, data not shown and ) of DES or HPTE was required to induce significant cell loss in vitro. In agreement with previous studies of thymocytes from adult mice exposed to MXC (Fukuyama et al. Citation2010, Citation2011), embryonic thymocytes exposed to HPTE or DES in vitro exhibited hallmarks of apoptosis, including rapid up-regulation of caspase 8 activity and PS staining. Prior studies of caspase 8 activation in thymocytes had also showed rapid activation (as early as 3 h post-treatment; Jiang et al. Citation1999). Unlike prior studies of a uterine cell line (Hewitt and Korach Citation2011), nM level doses of HPTE did not induce proliferation in embryonic thymocytes; however, µM doses did induce cell death.
The normal maturation of T-cells requires that thymocytes pass tests of their TCR function and autoreactivity, i.e. positive and negative selection, to yield functionally mature and non-autoreactive cells (Starr et al. Citation2003). The current study found that DP and CD4 intermediate cells were preferentially lost in response to EDC exposure, suggesting targets of positive and negative selection were the most susceptible. The loss of CD4 intermediates may have been due to direct effects on these cells or a blockage of differentiation (i.e. DP cells did not progress to CD4 intermediate stage). Regardless of mechanism, these data were the first to show HPTE could affect differentiation of embryonic DP thymocytes to CD4 intermediate cells, a critical step in thymocyte differentiation. Interestingly, in our hands, Bisphenol A (BPA) or estrogen exposure showed effects similar to HPTE and DES at low and high doses, whereas methylparaben (MPB) had no effect up to a 100 µM dose (data not shown). Thus, many but not all putative estrogenic EDC act to diminish numbers of embryonic thymocytes of the DP and CD4 intermediate stages.
Alterations of CD5 expression occur in concert with thymocyte maturation such that CD5 serves as an indicator and modulator of TCR ligation (Tarakhovsky et al. Citation1995; Azzam et al. Citation1998, Citation2001). Data from phenotypic analysis of HPTE or DES treated thymocytes in this study showed that thymocytes with the highest expression of CD5 and TCR (i.e. thymocytes actively signaling through TCR) were lost. TCR and CD5 phenotypic analysis provided a functional measure of activation status and further supported the hypothesis that HPTE exposure targets thymocyte populations undergoing critical differentiation steps, in agreement with our data on cell maturation stage.
Only one other published study (Brown et al. Citation2006a) has directly investigated whether an EDC might alter thymocyte developmental programing. Those authors treated six-week-old H–Y-specific TCR transgenic mice with DES twice by subcutaneous injection. A presence of the H–Y TCR transgene allowed a large proportion of thymocytes to be signaled for differentiation simultaneously and ultimately give rise to CD8 T-cells. In female mice (where H–Y antigen is absent) expression of the transgene results in a preponderance of CD8 SP cells, whereas in males (H–Y antigen present) the CD8 SP are mostly absent. In DES-treated H–Y TCR transgenic mice, the ratio of CD8 SP to other cells changed, but the absolute numbers of cells remained the same (calculated from reported data) in females (control ∼28 × 106 vs. DES ∼21 × 106) and in males (control ∼4.3 × 106 vs. DES ∼4.0 × 106). These data suggested that in H–Y TCR transgenic mice, preexisting CD8 SP were resistant to treatment with - or that differentiation of DP thymocytes into CD8 SP was unaffected by – DES treatment. However, DP thymocyte cell numbers were reduced in females (control ∼200 × 106 vs. DES ∼97 × 106) as well as in males (control ∼15 × 106 vs. DES ∼0.1 × 106). Reductions in DN (by 16% of control cell numbers) and CD4 SP (by 92% of control) were only seen in DES-treated males. From the data, one can conclude thymocytes strongly stimulated through the TCR (H–Y males, antigen present) experienced broader losses of populations than thymocytes (H–Y females, no antigen) that were not. If strongly stimulated thymocytes were depleted by DES, one might expect a reduction in the proportion of TCRhigh cells three days after injection. Their results show, however, enrichment for TCRhigh cells instead of any reduction. These data contrast with the present findings of a preferential loss of TCRhigh thymocytes.
Reconciling these data is complicated. Brown et al. (Citation2006a) used injections into young adult mice. In that model, precursor immigration from bone marrow to thymus continued during the course of the experiment, shifting the ratio of treated:untreated cells in the thymus over time. Other work from that group (Brown et al. Citation2006b) suggested embryonic thymocytes could recover from DES injection after 2 d. The H–Y TCR thymocytes in Brown et al. (Citation2006a) were examined 3 d after injection, potentially allowing time for recovery. Further, H–Y TCR transgenic mouse thymocytes prematurely express fully functional TCR. While it is unclear if early presence of a fully functional H–Y TCR during development alters the dynamics of responses to DES, it is clear that appropriate timing of TCR expression (Shortman et al. Citation1991) correlates with normal differentiation of T-cells in the thymus. When expression is altered by a presence of transgenes (Baldwin et al. Citation2005; Serwold et al. Citation2007), aberrant development occurs. In the case of the H–Y TCR transgene system, transgene expression is four to five times greater than in wild-type mice and expression begins in the DN2, rather than DN3, stage seen with wild-type TCR (Baldwin et al. Citation2005). These alterations lead to earlier than normal signaling (of greater intensity) through the TCR; this may explain the effects on DN thymocytes in Brown’s H–Y TCR transgenic males and the reduced effects on embryonic DN thymocytes seen here. For these reasons, data from an H–Y TCR transgene system may not best reflect the impact of EDC, like DES, on non-transgenic thymocyte differentiation in vivo or in vitro. However, taken together, the current data and that of Brown et al. suggest thymocytes undergoing strong signaling may be more susceptible to EDC-induced death. Conversely, one would predict already-signaled thymocytes might be resistant.
An alternative hypothesis to TCR signal crosstalk as a mechanism of enhancing EDC-induced death is that EDC induce up-regulation of Fas/Fas L, and thus death. Fas/Fas ligand has been shown to play a pivotal role in gestational T-cell development as well. In mice, Fleck et al. (Citation1998) identified a window of sensitivity to Fas from gestational day (GD) 15–17. In their study, the highest levels of Fas ligand were detected at Day 15, the same day apoptosis was first detected (in the medulla), whereas Fas (receptor) increased between Day 15 and 17. More mature DP and SP thymocytes became resistant to Fas-mediated apoptosis after GD17, but low avidity TCR interactions induced resistance to Fas-mediated death before GD17. Mixed cultures of thymocytes containing immature DN, DP, and mature SP cells support the idea that estrogen-induced thymic atrophy is also dependent upon Fas/Fas L interactions (Do et al. Citation2002). However, when cortical DP thymocytes were separated from more mature medullary CD4+CD8- thymocytes, death of the DP thymocytes was not Fas/FasL dependent (Kishimoto and Sprent Citation2001). Reconciliation of these data is likely due to the fact that more mature CD4+CD8− thymocytes appear in measurable numbers later in gestation (beginning between GD16 and GD18), rather than changes in sensitivity of DP thymocytes to Fas-mediated death.
Both estrogen (Mor et al. Citation2001) and DES (Singh et al. Citation2012) are known to induce Fas and Fas L expression in the thymus. The Fas-mediated death pathway (of immature CD4+CD8− thymocytes) is represented in a small cohort of cells undergoing death; the DP thymocyte population undergoes death by a Fas-independent mechanism. Indeed, it has been shown that caspase 8, activated in DP thymocytes (Jiang et al. Citation1999) in a Fas-independent manner (Pozzesi et al. Citation2014), is not an indicator of Fas-mediated death. Any use of mixed cultures of thymocytes would make discerning the role of Fas more difficult due to the differential susceptibility of the thymocyte populations. In the current study, these confounding variables were avoided by harvesting and culturing embryonic thymocytes at Days 16–18 gestation. At these timepoints, the majority of cells in the thymus are DP (70–90%) and the remaining cells are DN. Virtually none of the cells have matured beyond the DP stage. The natural enrichment of the embryonic thymocytes allowed for probing of the DP population, the primary target for selection events.
It is not clear from the literature what role thymocytes play (as opposed to thymic stromal cells [TSC]: Brown Citation2005; Melichar et al. Citation2015) and which population(s) participate in responses to EDC. Data from chimeras (Staples et al. Citation1999) suggest that ERα on stromal cells may be responsible for the decrease in thymic cellularity rather than ERα on thymocytes themselves. If this is so, it would suggest stromal cells were inducing an extrinsic cell death pathway via FasL. The present study showed that DES and HPTE have direct effects on embryonic thymocytes. It is important to note this study separated thymocytes from TSC. The results thus cannot exclude a likelihood that TSC have a role in EDC-mediated toxicities in vivo (Kaplan et al. Citation2015). Rather, it shows that in addition to potential TSC effects, EDC (like DES and HPTE) have direct effects on embryonic thymocytes similar to sustained thymocyte–thymocyte interactions that support negative selection (Melichar et al. Citation2015).
Examining embryonic thymocytes in an in vitro differentiation culture (in the absence of stromal cells) made it possible to probe the role of thymocytes in EDC-derived effects, to determine whether differentiation processes have an impact on embryonic thymocyte sensitivity to DES or HPTE, and to identify specific populations of thymocytes that are more susceptible to EDC. Based on results here, DP and CD4 intermediate (semi-mature) thymocytes with high level expression of CD5 and TCR were most susceptible to death induced by DES or HPTE.
Conclusions
Because of the dearth of studies on the susceptibility of thymocyte developmental programing to EDC-induced perturbation and the near ubiquity of exposure to EDC, the impact of HPTE and other EDC on human health and the environment is likely underestimated. Moreover, the potential for EDC to create a dysfunctional immune system (Winans et al. Citation2011; Dietert Citation2014) highlights how critical it is to understand mechanisms of action of this class of molecules. What is learned from pesticides of concern like MXC/HPTE can be used to determine what endogenous mechanisms (e.g. maturation and activation) might influence EDC susceptibility among embryonic thymocytes during development. Findings here, in turn, could enhance the ability to both understand effects of MXC exposure and potentially serve as a generalizable model for EDC action on the development of the immune system.
While others have shown that thymocytes die by apoptosis in response to exogenous estrogen, DES, or MXC, this is the first study to have examined the impact of HPTE (primary metabolite and effective agent of MXC) on embryonic thymocyte development. This study showed HPTE-induced death in embryonic thymocytes; significant Annexin V staining occurred faster (4 h) with HPTE than with DES (6 h), and HPTE-exposed cultures had larger numbers of surviving cells, suggesting differences in ability to induce cell death.
This was also the first EDC study to focus on understanding intrinsic qualities that make differentiating embryonic thymocytes susceptible to these agents. The plate-based in vitro differentiation assay mimicked in vivo signaling events that lead to survival and differentiation of thymocytes, while avoiding confounding variables such as stromal cell/thymocyte interactions and bone marrow precursor immigration. Thus, this study was able to examine contributions of embryonic thymocyte interactions on HPTE and DES toxicity. The findings suggested to us that actively differentiating embryonic thymocytes (high TCR and CD5 expression levels) were most susceptible to HPTE. An interesting corollary to these findings is whether completion of differentiation provides any protection from exposure.
A key question that remains is the mechanism of action of EDC, like HPTE, in this context. Chemicals such as DES and HPTE have been lumped together based on their known estrogenic properties (Folmar et al. Citation2002), with the underlying assumption that the mode of action of these chemicals would be mediated by genomic estrogenic activity through classical estrogen receptors (ER). Indeed HPTE has been shown to act as an ERα agonist and an ERβ antagonist (Gaido et al. Citation1999, Citation2000). However, with regard to thymocyte development, estrogenic compounds have proven difficult to link solely to classical estrogenic activity. For example, ERα knockout mice still undergo atrophy when treated with estrogen (Staples et al. Citation1999; Erlandsson et al. Citation2001). Additional studies suggest that different receptors, such as GPER/GPR 30, may play a role in death of thymocytes at different stages (Wang et al. Citation2008). Moreover, xenoestrogens have long been known to trigger nongenomic estrogenic responses (Watson et al. Citation2007; reviewed in Levin and Hammes Citation2016 and Xu et al. Citation2017) and to trigger cellular toxicity at high doses.
However, the nature of estrogen and xenoestrogen activity is complex (reviewed in Xu et al. Citation2017). Estrogenic signaling leads to genomic and nongenomic responses, including regulation of miRNA (reviewed in Klinge Citation2015). For non-genomic effects, extranuclear receptors like plasma-membrane associated ER, ER splice variants (ERa 36 or 46), or GPER/GPR30 are implicated as potentially responsible for rapid intracellular signaling leading to enzymatic pathway induction (including Fas/Fas L signaling) or interactions with transcriptional co-regulators. In addition, EDC have been shown to have other toxic effects, potentially unrelated to their estrogenic activity. Gu et al. (Citation2002) identified four mechanisms of toxicity, including damage to DNA, protein, membranes, and oxidative status.
Given the rapid response observed in this study (4 h for apoptosis with HPTE), it seems unlikely that HPTE (or DES) is acting through classical genomic ER pathways. The preferential loss of embryonic thymocytes with high TCR and CD5 expression (indicating active differentiation) suggests a non-genomic signaling-mediated mechanism, perhaps influenced by TCR signaling crosstalk. However, the findings thus far do not rule out the possibility of a partially or strictly toxicity-mediated mechanism induced by damage to cellular components or alteration of the oxidative status of embryonic thymocytes. Further studies are underway to address these potential mechanisms.
Acknowledgements
The authors thank Jerome Garcia and Jeniffer Hernandez for reading and commenting on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed S. 2000. The immune system as a potential target for environmental estrogens (endocrine disrupters): A new emerging field. Toxicology. 150:191–206.
- Ahmed S, Hissong B, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. 1999. Gender and risk of autoimmune diseases: Possible role of estrogenic compounds. Environ. Health Perspect. 107(S5):681–686.
- Altman N, Krzywinski M. 2017. Points of significance: p-Values and the search for significance. Nat Methods. 14:3–4.
- Azzam H, DeJarnette J, Huang K, Emmons R, Park C, Sommers C, Love P. 2001. Fine tuning of TCR signaling by CD5. J Immunol. 166:5464–5472.
- Azzam H, Grinberg A, Lui K, Shen H, Shores E, Love P. 1998. CD5 expression is developmentally regulated by T-cell receptor (TCR) signals and TCR avidity. J Exp Med. 188:2301–2311.
- Baldwin T, Sandau M, Jameson S, Hogquist K. 2005. The timing of TCR alpha expression critically influences T-cell development and selection. J Exp Med. 202:111–121.
- Barnes D, Page D, Duke S, White K. 1983. Subchronic toxicology of diethystilbestrol in the mouse. Drug Chem Toxicol. 6:455–485.
- Berthois Y, Katzenellenbogen J, Katzenellenbogen B. 1986. Phenol red in tissue culture media is a weak estrogen: Implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83:2496–2500.
- Besteman E, Zimmerman K, Holladay S. 2005. Diethylstilbestrol (DES)-induced fetal thymic atrophy in C57BL/6 mice: Inhibited thymocyte differentiation and increased apoptotic cell death. Int J Toxicol. 24:231–239.
- Bland J, Altman D. 1995. Multiple significance tests: The Bonferroni method. BMJ 310:170.
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano M, Olea N. 2004. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 96:34–40.
- Brown N, Nagarkatti M, Nagarkatti P. 2006a. Diethylstilbestrol alters positive and negative selection of T-cells in the thymus and modulates T-cell repertoire in periphery. Toxicol Appl Pharmacol. 212:119–126.
- Brown N, Nagarkatti M, Nagarkatti P. 2006b. Induction of apoptosis in murine fetal thymocytes following perinatal exposure to diethylstilbestrol. Int J Toxicol. 25:9–15.
- Brown N. 2005. Chapter V. Diethylstilbestrol-induced apoptosis requires T-cell-stromal cells interactions. In: The mechanism of T-cell Dysfunction Induced by Diethylstilbestrol [Doctoral dissertation]. Virginia Commonwealth University.
- Bulger W, Muccitelli R, Kupfer D. 1978. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 27:2417–2423.
- CA EPA (California Environmental Protection Agency). 2010. Public Health Goal for Methoxychlor in Drinking Water. Pesticide and Environmental Toxicology Branch Office of Environmental Health Hazard Assessment.
- Calemine J, Gogal R, Lengi A, Sponenberg P, Ahmed S. 2002. Immunomodulation by diethyl-stilbestrol is dose and gender related: Effects on thymocyte apoptosis and mitogen-induced proliferation. Toxicology. 178:101–118.
- Carson R. 2002. Silent Spring. 40th anniversary edition. New York: Mariner Books.
- Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C, Moscarini M. 2011. Environment and women's reproductive health. Human Reprod Update. 17:418–433.
- Chapin R, Harris M, Davis B, Ward S, Wilson R, Mauney M, Lockhart A, Smialowicz R, Moser V, Burka L, et al. 1997. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 40:138–157.
- Cibotti R, Punt J, Dash K, Sharrow S, Singer A. 1997. Surface molecules that drive T-cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity 6:245–255.
- Damgaard I, Skakkebaek N, Toppari J, Virtanen H, Shen H, Schramm K. and the Nordic Cryptorchidism Study Group. 2006. Persistent pesticides in human breastmilk and crypt-orchidism. Environ Health Perspect. 114:1133.
- Dietert R. 2014. Developmental immunotoxicity, perinatal programming, and non-communicable diseases: Focus on human studies. Adv Med. 2014:18.
- Do Y, Ryu S, Nagarkatti M, Nagarkatti P. 2002. Role of death receptor pathway in estradiol-induced T-cell apoptosis in vivo. Toxicol Sci. 70:63–72.
- Erlandsson M, Ohlsson C, Gustafsson J, Carlsten H. 2001. Role of oestrogen receptors α and β in immune organ development and in estrogen-mediated effects on thymus. Immunology. 103:17–25.
- Fenaux J, Gogal R, Ahmed S. 2004. Diethylstilbestrol exposure during fetal development affects thymus: Studies in fourteen-month-old mice. J Reprod Immunol. 64:75–90.
- Fleck M, Zhou T, Tatsuta T, Yang P, Wang Z, Mountz J. 1998. Fas/Fas ligand signaling during gestational T-cell development. J Immunol. 160:3766–3775.
- Folmar L, Hemmer M, Denslow N, Kroll K, Chen J, Cheek A, Richman H, Meredith H, Grau E. 2002. A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquat Toxicol. 60:101–110.
- Frawley R, White K, Brown R, Musgrove D, Walker N, Germolec D. 2011. Gene expression alterations in immune system pathways in the thymus after exposure to immunosuppressive chemicals. Environm Health Perspect. 119:371.
- Fukuyama T, Kosaka T, Tajima Y, Hayashi K, Shutoh Y, Harada T. 2011. Detection of thymocytes apoptosis in mice induced by organochlorine pesticides methoxychlor. Immunopharmacol Immunotoxicol. 33:193–200.
- Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T, Kosaka T. 2010. Apoptosis in immunocytes induced by several types of pesticides. J Immunotoxicol. 7:39–56.
- Gaido K, Leonard L, Maness S, Hall J, McDonnell D, Saville B, Safe S. 1999. Differential inter-action of methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors α and β. Endocrinology. 140:5746–5753.
- Gaido K, Maness S, McDonnell D, Dehal S, Kupfer D, Safe S. 2000. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: Structure-activity studies. Mol Pharmacol. 58:852–858.
- Gu M, Min J, Kim E. 2002. Toxicity monitoring and classification of endocrine-disrupting chemicals (EDC) using recombinant bioluminescent bacteria. Chemosphere. 46:289–294.
- Gunderson E. 1988. FDA Total Diet Study, April 1982-April 1984, dietary intakes of pesticides, selected elements, and other chemicals. J Assoc. 71:1200–1209.
- Guo T, Chi R, Zhang X, Musgrove D, Weis C, Germolec D, White K. 2006. Modulation of immune response following dietary genistein exposure in F0 and F1 generations of C57BL/6 mice: Evidence of thymic regulation. Food Chem Toxicol. 44:316–325.
- Guo T, White K, Brown R, Delclos K, Newbold R, Weis C, Germolec D, McCay J. 2002. Genistein modulates splenic natural killer cell activity, antibody-forming cell response, and phenotypic marker expression in F0 and F1 generations of Sprague–Dawley rats. Toxicol Appl Pharmacol. 181:219–227.
- Harris R, Waring R. 2012. Diethylstilboestrol-a long-term legacy. Maturitas. 72:108–112.
- Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. 2006. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 3:e311.
- Heindel J, Balbus J, Birnbaum L, Brune-Drisse M, Grandjean P, Gray K, Landrigan P, Sly P, Suk W, Slechta D, et al. 2016. Developmental origins of health and disease: Integrating environmental influences. Endocrinology. 156:3416–3421.
- Hewitt S, Korach K. 2011. Estrogenic activity of bisphenol A and 2,2-bis-(p-hydroxy-phenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect. 119:63.
- Hodges L, Bergerson J, Hunter D, Walker C. 2000. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 54:355–364.
- Hogquist K, Xing Y, Hsu F, Shapiro V. 2015. T-Cell adolescence: Maturation events beyond positive selection. J Immunol. 195:1351–1357.
- Holladay S, Blaylock B, Comment C, Heindel J, Fox W, Korach K, Luster M. 1993. Selective prothymocyte targeting by prenatal diethylstilbesterol exposure. Cell Immunol. 152:131–142.
- Jiang D, Zheng L, Lenardo M. 1999. Caspases in T-cell receptor-induced thymocyte apoptosis. Cell Death Diff. 6:402–411.
- Kaplan B, Li J, LaPres J, Pruett S, Karmaus P. 2015. Contributions of non-hematopoietic cells and mediators to immune responses: Implications for immunotoxicology. Toxicol Sci. 145:214–232.
- Kapoor I, Metcalf R, Nystrom R, Sangha G. 1970. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 18:1145–1152.
- Kishimoto H, Sprent J. 1997. Negative selection in the thymus includes semimature T cells. J Exp Med. 185:263–272.
- Kishimoto H, Sprent J. 2001. A defect in central tolerance in NOD mice. Nat Immunol. 2:1025.
- Klinge C. 2015. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol Cell Endocrinol. 418:273–297.
- Kurd N, Robey E. 2016. T-Cell selection in the thymus: A spatial and temporal perspective. Immunol Rev. 271:114–126.
- Lai Z, Fiore N, Gasiewicz T, Silverstone A. 1998. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and diethylstilbestrol affect thymocytes at different stages of development in fetal thymus organ culture. Toxicol Appl Pharmacol. 149:167–177.
- Lai Z, Fiore N, Hahn P, Gasiewicz T, Silverstone A. 2000. Differential effects of diethylstilbestrol and 2,3,7,8-tetrachlorodibenzo-p-dioxin on thymocyte differentiation, proliferation, and apoptosis in bcl-2 transgenic mouse fetal thymus organ culture. Toxicol Appl Pharmacol. 168:15–24.
- Levin E, Hammes S. 2016. Nuclear receptors outside the nucleus: Extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 17:783–797.
- Li H, Dehal S, Kupfer D. 1995. Induction of the hepatic CYP2B and CYP3A enzymes by the proestrogenic pesticide methoxychlor and by DDT in the rat. Effects on methoxychlor metabolism. J Biochem Toxicol. 10:51–61.
- Luster M, Faith R, McLachlan J, Clark G. 1979. Effect of in utero exposure to diethylstilbestrol on the immune response in mice. Toxicol Appl Pharmacol. 47:279–285.
- Luster M, Hayes H, Korach K, Tucker A, Dean J, Greenlee W, Boorman G. 1984. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol. 133:110–116.
- Luz N, Marques M, Ayub A, Correa P. 1969. Effects of estradiol upon the thymus and lymphoid organs of immature female rats. Am J Obstet Gynecol. 105:525–528.
- Manikkam M, Haque M, Guerrero-Bosagna C, Nilsson E, Skinner M. 2014. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PloS One 9:e102091.
- Meeker J. 2010. Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas. 66:236–241.
- Melichar H, Ross J, Taylor K, Robey E. 2015. Stable interactions and sustained TCR signaling characterize thymocyte–thymocyte interactions that support negative selection. J Immunol. 194:1057–1061.
- Melnick S, Cole P, Anderson D, Herbst A. 1987. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. New Engl J Med. 316:514–516.
- Mor G, Muñoz A, Redlinger R, Silva I, Song J, Lim C, Kohen F. 2001. The role of the Fas/Fas ligand system in estrogen-induced thymic alteration. Am J Reprod Immunol. 46:298–307.
- National Research Council. 2011. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press.
- Noller K, Blair P, O’Brien P, Melton L, Offord J, Kaufman R, Colton T. 1988. Increased occurrence of autoimmune disease among women exposed in utero to diethylstilbestrol. Fertil Steril. 49:1080–1082.
- Okasha S, Ryu S, Do Y, McKallip R, Nagarkatti M, Nagarkatti P. 2001. Evidence for estradiol-induced apoptosis and dysregulated T-cell maturation in the thymus. Toxicology. 163:49–62.
- Ousterhout J, Struck R, Nelson J. 1981. Estrogenic activities of methoxychlor metabolites. Biochem Pharmacol. 30:2869–2871.
- Pozzesi N, Fierabracci A, Liberati A, Martelli M, Ayroldi E, Riccardi C, Delfino D. 2014. Role of caspase-8 in thymus function. Cell Death Diff. 21:226–233.
- Punt J, Osborne B, Takahama Y, Sharrow S, Singer A. 1994. Negative selection of CD4+CD8+ thymocytes by T-cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 179:709–713.
- R Core Team. 2013. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Rawn D, Cao X, Doucet J, Davies D, Sun W, Dabeka R, Newsome W. 2004. Canadian Total Diet Study in 1998: Pesticide levels in foods from Whitehorse, Yukon, Canada, and corresponding dietary intake estimates. Food Additives Contam. 21:232–250.
- Reed C, Fenton S. 2013. Exposure to diethylstilbestrol during sensitive life stages: A legacy of heritable health effects. Birth Defects Res C Embryo Today. 99:134–146.
- Rijhsinghani A, Bhatia S, Tygrett L, Waldschmidt T. 1996. Effect of pregnancy on thymic T-cell development. Am J Reprod Immunol. 35:523–528.
- Schettgen T, Alt A, Esser A, Kraus T. 2015. Current data on background burden to the persistent organochlorine pollutants HCB, p,p′-DDE as well as PCB 138, PCB 153 and PCB 180 in plasma of the general population in Germany. Int J Hyg Environ Health. 218:380–385.
- Serwold T, Hochedlinger K, Inlay M, Jaenisch R, Weissman I. 2007. Early TCR expression and aberrant T-cell development in mice with endogenous prerearranged T-cell receptor genes. J Immunol. 179:928–938.
- Shelby M, Newbold R, Tully D, Chae K, Davis V. 1996. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environ Health Perspect. 104:1296–1300.
- Shen H, Main K, Virtanen H, Damggard I, Haavisto A, Kaleva M, Toppari J. 2007. From mother to child: Investigation of prenatal and postnatal exposure to persistent bioaccumulating toxicants using breastmilk and placenta biomonitoring. Chemosphere. 67:S256–S262.
- Shortman K, Vremec D, Egerton M. 1991. The kinetics of T-cell antigen receptor expression by subgroups of CD4 + CD8+ thymocytes: Delineation of CD4 + 8+3(2+) thymocytes as post-selection intermediates leading to mature T-cells. J Exp Med. 173:323–332.
- Silverstone A, Frazier D, Fiore N, Soults J, Gasiewicz T. 1994. Dexamethasone, β-estradiol, and 2,3,7,8-tetrachlorodibenzo-p-dioxin elicit thymic atrophy through different cellular targets. Toxicol Appl Pharmacol. 126:248–259.
- Singer A, Adoro S, Park J. 2008. Lineage fate and intense debate: Myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 8:788–801.
- Singh N, Singh U, Nagarkatti P, Nagarkatti M. 2012. Prenatal exposure of mice to diethylstilbestrol disrupts T-cell differentiation by regulating Fas/Fas ligand expression through estrogen receptor element and NF-κB motifs. J Pharmacol Exp Ther. 343:351–361.
- Skånland S, Moltu K, Berge T, Aandahl E, Taskén K. 2014. T-Cell co-stimulation through the CD2 and CD28 co-receptors induces distinct signalling responses. Biochem J. 460:399–410.
- Staples J, Fiore N, Frazier D, Gasiewicz T, Silverstone A. 1998. Overexpression of the anti-apoptotic oncogene, bcl-2, in the thymus does not prevent thymic atrophy induced by estradiol or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 151:200–210.
- Staples J, Gasiewicz T, Fiore N, Lubahn D, Korach K, Silverstone A. 1999. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 163:4168–4174.
- Starr T, Jameson S, Hogquist K. 2003. Positive and negative selection of T cells. Annu Rev Immunol. 21:139–176.
- Stuchal L, Kleinow K, Stegeman J, James M. 2006. Demethylation of the pesticide methoxy-chlor in liver and intestine from untreated, methoxychlor-treated, and 3-methylcholanthrene-treated channel catfish (Ictalurus punctatus): Evidence for roles of CYP1 and CYP3A family isozymes. Drug Metab Disposit. 34:932–938.
- Takeuchi Y, Kosaka T, Hayashi K, Ishimine S, Ohtsuka R, Kuwahara M, Yoshida T, Takahashi N, Chiba Y, Takeda M, et al. 2004. Alterations in the developing immune system of the rat after perinatal exposure to methoxychlor. J Toxicol Pathol. 17:165–170.
- Takeuchi Y, Kosaka T, Hayashi K, Takeda M, Yoshida T, Fujisawa H, Teramoto S, Maita K, Harada T. 2002. Thymic atrophy induced by methoxychlor in rat pups. Toxicol Lett. 135:199–207.
- Tarakhovsky A, Kanner S, Hombach J, Ledbetter J, Muller W, Killeen N, Rajewsky K. 1995. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 269:535.
- Wang C, Dehghani B, Magrisso I, Rick E, Bonhomme E, Cody D, Elenich L, Subramanian S, Murphy S, Kelly M, et al. 2008. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 22:636–648.
- Watson C, Bulayeva N, Wozniak A, Alyea R. 2007. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 72:124–134.
- White K, Germolec D, Booker C, Hernendez D, McCay J, Delclos K, Newbold R, Weis C, Guo T. 2005. Dietary methoxychlor exposure modulates splenic natural killer cell activity, antibody-forming cell response and phenotypic marker expression in F0 and F1 generations of Sprague Dawley rats. Toxicology. 207:271–281.
- Winans B, Humble M, Lawrence P. 2011. Environmental toxicants and the developing immune system: A missing link in the global battle against infectious disease? Reprod Toxicol. 31:327–336.
- Xu Z, Liu J, Gu L, Huang B, Pan X. 2017. Biological effects of xenoestrogens and the functional mechanisms via genomic and non-genomic pathways. Environ Rev. 25:306–322.
- Yellayi S, Naaz A, Szewczykowski M, Sato T, Woods J, Chang J, Segre M, Allred C, Helferich W, Cooke P. 2002. The phytoestrogen genistein induces thymic and immune changes: A human health concern? Proc Natl Acad Sci USA 99:7616–7621.
- Zoller A, Kersh G. 2006. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol. 176:7371–7378.
- Zoller A, Schnell F, Kersh G. 2007. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology. 121:207–215.
