Abstract
The immunotoxic potential of drug candidates is assessed through the examination of results from a variety of studies and endpoints. While the functional assessment of CD8+ cytotoxic T-lymphocytes (CTL) is well-characterized in the clinic, the lack of a robust macaque CTL functional assay has been an important hurdle in evaluating and accurately quantifying cell-mediated CD8+ T-cell effector responses in the nonclinical setting. This paper describes the development of an assay to measure CTL activity in peripheral blood mononuclear cells (PBMC) isolated from Cynomolgus macaques. A human EGFR/CD3 Bispecific T-cell Engager (BiTE®) was used to mount a robust CD8+ T-cell response in the presence of target-expressing cells. Upon target engagement, degranulation of CD107a and production of interferon (IFN)-γ both reliably indicated a robust functional response in CD8+ T-cells. The BiTE®-mediated stimulation method proved to be favorable when compared to other methods of stimulation in the absence of target cells. These studies demonstrated acceptable longitudinal variability of the functional assay and sensitivity to dexamethasone-mediated immunosuppression. Taken together, the results indicated an assay leveraging CD3-bispecific antibodies and target-expressing cells can provide a robust approach to the in vitro or ex vivo assessment of CTL function in Cynomolgus macaques. Because the impairment of CTL activity by immunomodulators is recognized to be an important contributor to decreased antiviral defense and increased carcinogenicity risk, we believe that this novel assay to be a valuable addition to the immunotoxicology assessment of therapeutic drug candidates.
Introduction
The evaluation of potential adverse effects on the immune system is an important component of drug development. Immunotoxicity encompasses multiple potential adverse effects including impairments of humoral or cellular immune function (i.e. immunosuppression) resulting in decreased host defense against infection and/or increased cancer risk (Brennan et al. Citation2010). As stated in the ICH S8 Guideline on “Immunotoxicity Studies for Human Pharmaceu-ticals” (ICH Citation2005), standard toxicity studies contribute to the initial assessment of the immunotoxic potential of drug candidates. ICH S8 also indicates that if there are causes for concern, additional studies including functional tests may be warranted. It is fairly common, when such causes for concern have been identified, to complement standard toxicity studies with the evaluation of peripheral blood immunophenotyping (B-lymphocytes, T-helper [TH] lympho-cytes, cytotoxic T-lymphocytes [CTL], and natural killer [NK] cells, and sometimes subsets of these populations) and functional assays such as the T-cell-dependent antibody response (TDAR) and NK cell cytotoxicity assays (ICH Citation2005; http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S8/Step4/S8_Guideline.pdf). A thorough immunotoxicology safety assessment evaluates all three arms of the immune response: innate, cell-mediated, and humoral immunity. Assays currently exist for evaluating innate and humoral-mediated immunity in the Cynomolgus macaque. The lack of a robust macaque CTL functional assay has been an important barrier in evaluating and accurately quantifying cell-mediated CD8+ T-cell effector responses in the non-clinical setting. For a subset of immunomodulatory drugs, understanding their impact on CTL function could contribute to their risk assessment, including carcinogenicity risk assessment (Lebrec et al. Citation2016). This study presents a method for evaluation of CD8+ T-cell-mediated immunity.
A variety of CTL functional assays utilizing multiple approaches, including antigen-specific responses, and T-cell receptor (TCR)-mediated non-antigen specific activation methods (Kay Citation1991; Lamers et al. Citation1992; Walsh et al. Citation1992; Levine et al. Citation1996; Frauwirth and Thompson Citation2002; Trickett and Kwan Citation2003; Shi et al. Citation2013) are used in the clinical setting. For antigen-specific CTL activation, cytomegalovirus (CMV) and influenza recall responses can be measured if the donors have been previously exposed to the viruses (Turner et al. Citation2001; Klenerman and Oxenius Citation2016). However, these assays require patient-specific human leukocyte antigen (HLA) typing and viral epitope sequences so a viral antigen-specific recall response can be stimulated. Aside from these antigen-specific assays, a variety of methods based on non-antigen specific stimulation of CTL activity exist, including stimulation by anti-CD3/CD28 antibody-coated beads or plates, phytohemagglutinin (PHA), superantigen Staphylococcus enterotoxin B (SEB), phorbol myristate acetate (PMA), and ionomycin (ION) (Ceuppens et al. Citation1988; Walsh et al. Citation1992; McLeod et al. Citation1998; Trickett and Kwan Citation2003; Shi et al. Citation2013).
Measuring viral antigen-specific T-cell response has been previously suggested for non-clinical assessment in Cynomolgus macaques (Kamperschroer et al. Citation2014). However, due to lack of pre-dose knowledge of non-human primate (NHP) HLA haplotype and viral infection history, it is not practical to conduct antigen-specific CTL assays in GLP studies (O’Connor et al. Citation2007). For anti-CD3/CD28 and various other agents of mitogenic stimulation, the T-cells are activated with limited trigger of co-stimulatory molecules due to the absence of specific target cell engagement (Teschner et al. Citation2011).
The overall objective of these studies was to develop a robust, reproducible, and sensitive CD8+ T-cell functional assay suitable for in vitro or ex vivo application that could inform on the immunotoxic potential of therapeutics in non-clinical toxicology studies. This paper reports results of a study from our laboratories in which a BiTE® was utilized to mount a marked CD8+ T-cell response in Cynomolgus macaque PBMC. The anti-CD3 arm of the BiTE® cross-reacts with Cynomolgus macaque CD3+ T-cells, and the other arm of the BiTE® specifically binds to human epidermal growth factor receptor (EGFR) (Smith et al. Citation2016; Ross et al. Citation2017). Target cell cytotoxicity ensues when a specific cytolytic effector response is redirected against human colon tumor (HCT)-116 cells co-cultured with Cynomolgus macaque PBMC in the presence of BiTE®. In the presence of target cells, BiTE®-induced T-cell activation mimics a physiological antigen-specific T-cell activation. The magnitude of CTL response was compared between BiTE®-mediated activation versus other common stimuli. The degranulation marker CD107a (lysosomal-associated membrane protein, LAMP-1) was chosen as the primary parameter because of the direct correlation with cytolytic activity in human (Betts et al. Citation2003; Rubio et al. Citation2003; Betts and Koup Citation2004; Burkett et al. Citation2005; Aktas et al. Citation2009) and the robustness of the response induced by BiTE® and target cell engagement.
Materials and methods
Antibodies and additional reagents used for immunophenotpying
BD Biosciences (San Diego, CA) was the source of the monoclonal antibodies unless otherwise stated. The following anti-human mouse monoclonal antibodies (with indicated conjugate) were used: anti-CD3 (clone SP34-2, BUV395), anti-CD4 (clone L200, PerCP-Cy5.5), anti-CD8 (clone RPA-T8, AF700), anti-CD25 (clone M-A251, BV421), anti-CD127 (clone HIL7RM21, FITC), anti-CD45RA (clone 5H9, APC-H7), anti-CCR7 (clone G043H7, BV650), anti-CD107a (clone H4A3, APC), anti-IFNγ (B27, FITC), and anti-TNFα (clone MAb 11, PerCP-Cy5.5, eBioscience, ThermoFisher Scientific, Waltham, MA). Live/Dead Fixable NIR (ThermoFisher Scientific) was also purchased for use. Lineage markers (conjugated with PE-Cy7) against CD11c (clone 3.9), CD123 (clone 7G3), CD159a (clone Z199), CD16 (3G8), CD14 (M5E2), and CD20 (2H7), as well as Live/Dead Fixable Blue (Thermo Fisher Scientific) were also bought. PharmLyse (BD Biosciences) was employed to lyse red blood cells. Cytofix/Cytoperm (BD Biosciences) served as a fixative and permeabilization reagent for intracellular cytokine staining. Staining buffer was purchased (BD Biosciences) as a solution of 2% bovine serum albumin and 0.1% sodium azide in phosphate buffered saline (PBS).
Isolation of PBMC
Peripheral blood was drawn directly into sodium heparin-coated Vacutainer tubes (BD Biosciences). All blood was obtained from adult (4–11 years-old, male and female) Cynomolgus macaques (Macaca fascicularis) of Mauritius origin housed at AlphaGenesis (Yemassee, SC) and delivered overnight at ambient temperature to Amgen facilities located in South San Francisco, CA. All in vivo work/blood sampling was conducted under an IACUC approved protocol in an AAALAC-accredited facility.
PBMC were subsequently isolated using Ficoll (GE Healthcare, Chicago, IL) density gradient sedimentation. In brief, blood samples were diluted with an equal volume of RPMI 1640 media (Gibco, Grand Island, NY), layered onto either 90% Ficoll (male donors) or 95% Ficoll (female donors), and then centrifuged at 1830g for 30 min at room temperature. These differences in the need for Ficoll strengths were based upon empirical testing. The resulting mononuclear cell fraction in each case was washed with media, and then any remaining red blood cells in the pellets were lysed using PharmLyse for 10 min at room temperature. Resulting PBMC were either used immediately or cryopreserved in Recovery™ Cell Culture Freezing Medium (Invitrogen, Waltham, MA), placed in a Biocision Freezing Container at −80 °C for >24 h prior to transfer to a −150 °C freezer for long-term storage.
Longitudinal variability of Cynomolgus macaque CTL responses
Three male and three female untreated macaques were bled every 2 weeks, for a period spanning 6 weeks. All blood samples collected at the same timepoints were analyzed the day after collection for T-cell immunophenotyping before PBMC were isolated and subjected to further in vitro cytotoxicity assays. All blood sampling was within typical blood volume sampling guidelines.
In vitro BiTE®-mediated CTL assay
Human colon cancer cells (HCT-116) sourced from ATCC (Manassas, VA) were transduced with NucLight-mKate2 (Essen Bioscience, Ann Arbor, MI) with an estimated multiplicity of infection (MOI) of one, according to manufacturer recommendations. The labeled adherent cells were then lifted from the culture vessel with Cell Dissociation Buffer (Gibco) before washing with PBS, counting (and checked for viability), and subsequently being added to co-cultures. In brief, 105 viable PBMC were co-cultured with HCT-116 cells at an effector to target ratio (E:T) equal to 5:1 in the presence or absence of 200 pM(EC90) recombinant anti-EGFR × anti-CD3 bispecific T-cell engager (BiTE®, Amgen) that cross-binds Cynomolgus CD3 antigen on T-cells in 96-well flat-bottom tissue culture plates. The EC90 value was pre-determined in a cytotoxicity assay (Lutterbuese et al. Citation2010). Cells were then incubated at 37 °C for 24–72 h. At 5 h prior to harvest, APC-conjugated anti-human CD107a (H4A3), GolgiStop, and GolgiPlug (BD Biosciences) were added to the cells. Cells were then subjected to Live/Dead Fixable NIR staining for 30 min at 4 °C. After washing, the cells were fixed and permeabilized with CytofIx/Cytoperm according to manufacturer instructions. After permeabilization, cells were stained with BUV395-anti-CD3 (SP34-2), PE-anti-CD4 (L200), BV421-anti-CD8 (RP8-T8), FITC-anti-IFNγ (B27), and PerCP-Cy5.5-anti-TNFα (Mab 11) for 30 min on ice protected from light. After a thorough washing with Stain Buffer, the cells were re-suspended in Stain Buffer and promptly analyzed in an LSR II flow cytometer (BD Biosciences). Data were initially acquired using DiVa acquisition software (BD Biosciences) before final analysis using FlowJo v10.4. A minimum of 10 000 events was acquired for each sample.
T-cell stimulation by other methods
PBMC were treated with both immobilized anti-human CD3 (SP34, 1 µg/ml) and soluble anti-human CD28 (CD28.2, 1 µg/ml) or soluble PHA (1 µg/ml, Sigma, St. Louis, MO) for 48 h, or with SEB (1 µg/ml, Toxin Technology, Sarasota, FL) for 6 hr. Cells were then harvested and processed for surface and intracellular staining as above.
Isolation of regulatory T-cells and CTL
For the sorting of regulatory T (Treg) cells, CD4 T-lymphocytes from the macaque donors were further enriched from PBMC using negative immunomagnetic bead selection (EasySep, Stem Cell Technology, Vancouver, Canada) using biotinylated anti-human: CD8 (RPA-T8), CD11b (ICRF44), CD11c (3.9), CD14 (M5E2), CD16 (3G8), CD20 (2H7), CD33 (AC104.3E3), and CD123 (7G3), along with streptavidin-microbeads. For cytotoxic T-lymphocyte enrichment, cells were stained with the same cocktail except for the omission of biotinylated anti-human CD4 (L200) followed by biotinylated anti-human CD8 (RPA-T8) as described above. After CD4 enrichment, the cells were stained with PerCP-Cy5.5-anti-CD4 (L200), BV421-anti-CD8 (RPA-T8), PE-anti-CD25 (M-A251), and FITC-anti-CD127 (HIL7RM21). TO-PRO-3 (Thermo Fisher Scientific) was used to discriminate dead cells. After a final washing with Dulbecco’s PBS (DPBS, Gibco), the cells were re-suspended in DPBS supplemented with 2% Fetal Bovine Serum (GE Healthcare) and 25 mM HEPES (Sigma), and sorted on a FACS Aria System (BD Biosciences) using FACSDiva software (BD Biosciences). Final isolates of Treg cells (CD4+CD25+CD127lo) were promptly used for co-culture experiments.
Statistical analyses
Statistical analyses were performed using a multiple t-test. A p values ≤0.05 was considered as statistically significant.
Results
Human EGFR BiTE® stimulated a robust CTL response measured by membrane CD107a and intracellular IFNγ expression in Cynomolgus macaque PBMC
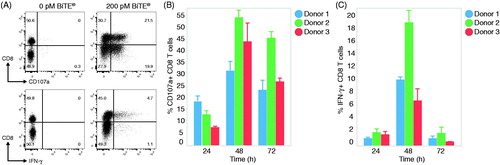
To stimulate a CD8+ cytotoxic T-lymphocyte response, Cynomolgus macaque PBMC were cultured with HCT-116 and human EGFR BiTE® for 48 h. Without the addition of BiTE®, there was no CD107a degranulation and IFNγ production detected within CD8+ T-cells (). Upon stimulation with a predetermined optimal concentration of BiTE®, there were 41% CD8+ T-cells degranulating CD107a and 10% CD8+ T-cells producing IFNγ (). Because BiTE® can stimulate all CD3+ T-cells, it was noted that CD4+ T-cells also degranulated CD107a and produced IFNγ after co-culture, albeit to a lesser extent compared to CD8+ cells (Supplementary Figure 1). Further, target cell cytotoxicity was consistent with T-cell activation (Supplementary Figure 2). Because killing of target cells is an aggregate effect of stimulating both CD8+ and CD4+ T-cells and not a CD8+ CTL-specific endpoint, CD107a degranulation was selected as the preferred endpoint in subsequent studies. The kinetics of T-cell degranulation, cytokine production, and target cell killing were then examined at 24, 48, and 72 h after co-culture. CD107a and IFNγ production was detected as early as 24 h and showed a peak response at 48 h (). A total of 30–55% of CD8+ T-cells became CD107a+ and 6–20% of CD8+ T-cells produced IFNγ at 48 h. Despite expected donor-to-donor variability in the magnitude of CD8+ T-cell responses, the kinetics from all three animals was consistent over the stimulation cultures.
Figure 1. BiTE® induces robust CTL response in Cynomolgus macaque PBMC. PBMC and HCT-116 target cells were co-cultured with 200 pM EGFRxCD3 BiTE® antibody or media alone at an effector to target (E:T) ratio of 5:1. After 24, 48, or 72 h, cells were harvested and stained for CD8, CD107a, and IFNγ. Values shown represent percentage CD8+ T-cells. (A) Flow cytometry dot-plots of CD3+ gated T-cell responses of representative donor cells after 48 h in the presence or absence of BiTE®. (B) Graphs of CD107a and IFNγ expression on CD8+ T-cells from three animals across the kinetic timepoints. Data shown are representative of multiple experiments (n = 28) across multiple donors (n = 85). Data shown are means ± SD.
BiTE®-mediated CTL activation is more robust in comparison with traditional methods of T-cell stimulation
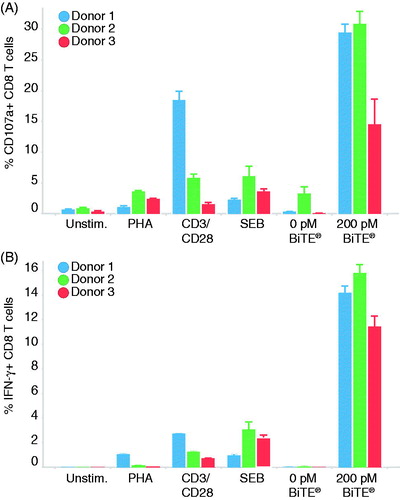
Human CTL responses under in vitro experimental conditions are commonly induced by the cross-linking of surface CD3 with CD28 or by using various mitogens or superantigens. To compare the magnitude of the BiTE®-mediated CTL response with other methods of activation, Cynomolgus macaque PBMC were stimulated with PHA, CD3/CD28, and SEB (). The staining on unstimulated PBMC provided the level of background T-cell activation. Due to T-cell activation induced cell death, PBMC were only allowed to incubate with SEB for 6 h. The BiTE®-mediated CD8+ T-cell degranulation response ranged from 12% to 30% within three animals, whereas T-cell degranulation resulting from other stimuli was below 10% (with exception of Donor 1 under CD3/CD28 co-stimulation) (). Similarly, all donors’ cells presented substantially higher IFNγ production upon BiTE®-mediated activation (11–15%) than with other methods of stimulation (0.1–3%) (). Given the low magnitude of CTL response from the traditional methods of stimulation, the assessment of the impact of a drug on the CD107a and IFNγ endpoints could be unreliable.
Figure 2. BiTE®-mediated CTL response in Cynomolgus macaque PBMC is greater compared to other methods of stimulation. HCT-116 cells were co-cultured with PBMC with an E:T ratio of 5:1 in the presence of 200 pM EGFRxCD3 BiTE® or media alone for 48 h. Alternatively, PBMC were stimulated with 1 µg/ml each of co-stimulatory antibodies against CD3/CD28, soluble mitogen PHA, or SEB. Values shown represent percentage CD8+ T-cells expressing (A) CD107a or (B) IFNγ. Data shown are means ± SD.
BiTE®-mediated CTL activation is minimally impacted by changes in CD8+ T-cell frequency
The purpose of developing a Cynomolgus macaque CTL assay was to monitor alterations of CD8+ T-cell function during or after administration of test articles in the context of toxicology studies. In this assay, a BiTE® molecule was used to reveal the maximal potential of CD8+ T-cell effector function. In this context, the level of CTL activation was dependent on the number of target cells engaged. PBMC were used without adjustment of the number of CD8+ T-effector cells. Some therapeutics may cause an expansion or reduction of CD8+ T-cell numbers in peripheral blood that could lead to subsequent alterations of the CTL to target cell ratio. To assess if the BiTE®-mediated CTL assay was affected by increased or decreased CD8+ T-cell frequencies in PBMC, various amounts of magnetically-purified CD8+ T-cells were titrated against a fixed amount of HCT-116 target cells simulating E:T ratio ranges from 2.5 to 40:1. The results indicated that when the E:T ratio was <20:1, variation in the E:T ratio was not associated with discernible changes in % CTL degranulation (Supplementary Figure 3, ). Only when the E:T ratio ranged >20:1, was a trend of increasing CTL activation observed. The enhancement of CTL activity at this very high E:T ratio was likely due to the high density of cells in each culture well.
Table 1. Parameters evaluated to assess impact on assay performance.
Another technical aspect that was taken into consideration was whether the functional readouts for this assay obtained from freshly isolated PBMC were comparable to the results generated from previously frozen PBMC. It is common practice during Cynomolgus macaque in vivo studies that PBMC purified from fresh blood are preserved frozen until the assay dates. It was anticipated that the freeze/thaw process may impact the viability and functionality of immune cells based on examples in the public domain (Mazur Citation1984; Bull et al. Citation2007; Axelsson et al. Citation2008; Jeurink et al. Citation2008; Mallone et al. Citation2011). To determine the impact of processing upon CTL performance, freshly-purified PBMC and previously-frozen PBMC isolated from the same donor were compared. Comparable levels of CD107a degranulation and IFNγ production were observed across the fresh and cryopreserved samples (Supplementary Figure 4, ). The amount of IFNγ production was less robust than CD107a expression observed in this donor.
Immunomodulatory drugs may also induce changes in the frequency of regulatory immune cells that can indirectly affect CD8+ T-cell function. To evaluate if an increased presence of FoxP3+CD4+ Treg cells could suppress CTL function in vitro, CD8+ T-cells and Treg cells were enriched and co-cultured at different ratios with HCT-116 cells and BiTE® for 48 h. Impairment of CTL function was not observed upon increasing Treg cell presence to a CTL:Treg ratio of 5:1 from a range of 20:1–70:1 seen in normal healthy Cynomolgus macaque donors (Supplementary Figure 5, ). Based on these findings, it is possible to suggest that the BiTE®-mediated CTL functional assay primarily measures the intrinsic CTL function that can be affected by drug treatment and is not affected by changes in frequencies of immune subsets.
CTL longitudinal variability assessment as measured by the BiTE®-mediated assay
Longitudinal variability of Cynomolgus macaque CTL functional response as measured using the BiTE®-mediated CTL assay was determined on PBMC from an untreated cohort isolated every two weeks for a total of four time points. Comparing each individual animal CTL response through the course of the study revealed a level of longitudinal variability (). CD107a degranulation responses ranged from 25% to 35%, 29% to 54%, and 40% to 48% for the three male donors and 26% to 45%, 22% to 40%, and 10% to 21% for the three female donors. Longitudinal IFNγ production throughout the study ranged from 5% to 36%, 7% to 2%3, and 11% to 46% for the male donors and 12% to 23%, 3% to 7%, and 3% to 13% for the females. Overall, during this timeframe of assessment, it was found that CTL degranulation (endpoint) was less variable than IFNγ production. BiTE®-mediated CD107a and IFNγ responses from individual animals are summarized in .
Figure 3. BiTE®-mediated CTL response is longitudinally stable. Three male and three female untreated macaques were sampled every two weeks for a total of four collections. PBMC from each collection were then co-cultured with HCT-116 target cells with an E:T ratio of 5:1 in the presence of 200 pM EGFRxCD3 BiTE® or media alone for 48 h. Values shown represents percentage CD8+ T-cells expressing CD107a or IFNγ. Each line represents an individual animal.
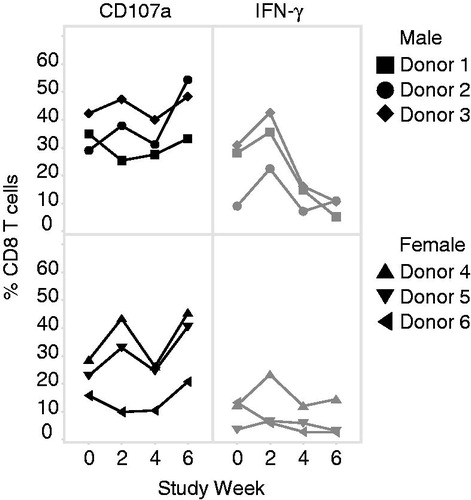
Table 2. Longitudinal CTL responses from cells isolated from individual animals.
Dexamethasone suppressed the BiTE®-mediated CTL activation
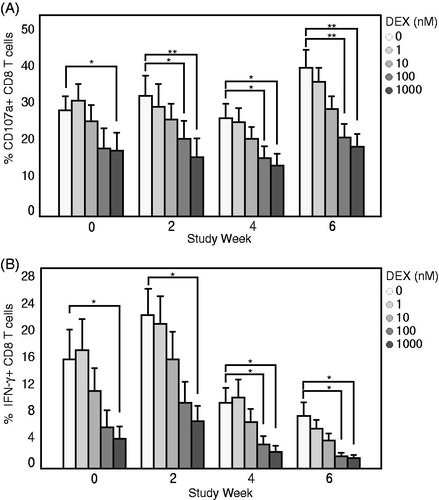
To determine if the BiTE®-mediated CTL functional assay was able to detect induced immunosuppression of T-cell responses, dexamethasone – a known glucocorticoid immunosuppressant, was concomitantly added to the cell culture of Cynomolgus macaque PBMC containing HCT-116 and BiTE®. At 48 h, CTL degranulation and production of IFNγ was dose-dependently reduced by dexamethasone (). Dexamethasone at 10 nM was able to cause measurable suppression; at a concentration of 100 nM, maximum reduction, that is, to a level comparable to 1000 nM, was observed in CTL CD107a degranulation and IFNγ production. These results demonstrated the BiTE®-mediated CTL method was sensitive for detecting immunosuppression of T-cell responses in vitro.
Figure 4. In vitro dexamethasone induced immunosuppression of Cynomolgus macaque CTL function is detected with BiTE® activation. PBMC isolated from donors in longitudinal study were co-cultured with HCT-116 target cells in the presence of 200 pM BiTE® and subjected to concomitant dose-titration of dexamethasone ranging from 0 to 1000 nM for 48 h. Values represent average percentage CD8+ T-cells expressing (A) CD107a or (B) IFNγ at each timepoint. Data shown are means ± SD. *p < 0.05, **p < 0.05 (multiple t-test).
Discussion
Assessment of T-cell functions and associated immunosuppression in pre-clinical NHP studies is essential to evaluate cell-mediated responses and is acknowledged as an informative endpoint for conducting cancer risk assessments of immunomodulators (Lebrec et al. Citation2016). In this study, a Cynomolgus CTL functional assay was developed to predict immunotoxicity, with an emphasis on immunosuppression. Here, adult Cynomolgus macaque PBMC were co-cultured with HCT-116 target cells and huEGFR BiTE®. The engagement of target cells and BiTE® strongly activated CD8+ T-cells, leading to degranulation of CD107a and production of IFNγ while inducing target cell cytotoxicity. Throughout the studies, T-cell specific readouts were followed and it was noted that the magnitude of CTL activation was greater in BiTE®-mediated responses than with other mitogenic stimuli such as anti-CD3/CD28 antibodies, PHA, or SEB. The BiTE®-induced CTL activity was consistent within a range for each individual animal in a longitudinal study. Dexamethasone-induced immunosuppression on CTL was also successfully measured in this assay. Here, CD107a degranulation was chosen as the primary endpoint to follow activity in the assay due to its direct relationship with cytotoxicity and the robustness of response seen using this readout.
BiTE® is a robust stimulator of Cynomolgus macaque T-cells, mimicking antigen-specific T-cell activation. BiTE® molecules comprise two arms of single-chain antibodies to activate polyclonal T-cells and redirect cytotoxic T-cells against target-expressing cells. The presence of target cells allows a physiologically relevant CTL activation through BiTE® even in the presence of low T-cell numbers. Because total PBMC are used in the assay, target cell cytotoxicity is an aggregated effort of CTL, CD4+ T-cells, and NK cell-mediated responses. For this reason, a CTL-specific response cannot be ascertained from following target cell cytotoxicity and we recommend assessing the degranulation of CD107a on CD8+ T-cells as the primary BiTE®-mediated CTL activity endpoint.
This assay was primarily designed to assess immunosuppression of drug candidates. The suppression of CTL activity can be either due to impaired T-cell function or reduced T-cell absolute numbers. Immunophenotyping, including absolute count changes of the CD8+ T-cell compartment, is frequently performed in studies and can be coordinated with CTL functional assays. Because the amount of effector cells in the culture is determined based on total PBMC counts, there was a concern that an increase or reduction of CD8+ T-cell counts may directly affect the E:T ratio and indirectly affect the interpretation of the results. The present data demonstrated similar CD107a measurements throughout a wide range of E:T ratios from 2.5:1 to 20:1, suggesting changes to the amount of CD8+ T-cells in PBMC will not affect the test result. This could be explained by the saturation of BiTE®-mediated T-cell cytotoxicity mechanism throughout this range. At E:T ratios >20:1, the high density of cells in co-culture could explain the observed slight increase in CD107a degranulation. Assessment of data on the CD8+ T-cell compartment in totality, including absolute CD8+ T-cell counts and function, would contribute to a clearer interpretation of the assay results.
The assay presented here involves robust T-cell activation. One can postulate that the strong stimulation may reduce the ability to detect weak immunosuppression. When various methods of stimulation for CD8+ T-cell activation () were compared, a consistent BiTE®-mediated CD107a and IFNγ response (up to 30% and 15% of T-cells, respectively) across multiple donors was observed. In contrast, the other T-cell stimulation methods generated a response in <5% of T-cells – except for one donor with the anti-CD3/CD28 stimulation. It was demonstrated here that a consistent response of BiTE® stimulation resulted in a greater assay window when compared to other methods of stimulation; this should increase the confidence of detecting a small reduction in CTL activity. Furthermore, dexamethasone-induced reduction of CTL activity was dose-dependent (). The immunosuppressive effect first manifested at 10 nM and achieved statistical significance at 100 nM. This range of effect is similar to that in studies using anti-CD3/CD28-coated beads (Gutsol et al. Citation2015). Lastly, the unique mechanism of action of a BiTE® may bypass certain T-cell regulatory mechanisms and become less suitable for some weakly immunosuppressive agents. It has been reported that blocking PD-1/PD-L1 co-inhibitory pathways can further enhance BiTE®-mediated T-cell activation (Laszlo et al. Citation2015). This suggested to us that the assay as designed was sensitive to immunomodulatory mechanisms such as PD-1/PDL-1 interactions. Overall, the BiTE®-mediated assay may have the sensitivity to detect moderate immunosuppressive effects. Testing additional immunosuppressive agents of varying strengths will provide further insight into this matter.
A possible concern of using total PBMC in this assay is the immunosuppressive potential of Treg cells on CTL function in vitro. Our data revealed that the presence of Treg cells in the co-culture does not suppress CTL function. There could be various explanations for this observation. First, the stimulation by BiTE® and target cells is strong enough to overcome the Treg cell suppression, which is supported by previous work demonstrating that only suboptimal anti-CD3 stimulation can reveal Treg cell suppression effects on human CD8+ T-cells (Abraham et al. Citation2008; Jana et al. Citation2010). Second, the BiTE®-mediated stimulation was optimally observed within a short time period. The current time-course study displayed measurable CTL activation as early as 24 h after co-culture and peak response at 48 h. Often, Treg cell suppression is measured after 72 h of stimulation in human in vitro cell cultures (Jana et al. Citation2010; Tran Citation2013). Finally, CD4+ Treg cells have the potential to become cytotoxic CD4+ T-cells themselves following BiTE®-mediated stimulation. It has been shown that purified human Treg cells can be redirected by BiTE® to become effector T-cells with target cell killing potential (Choi et al. Citation2013). Data have also revealed that Cynomolgus macaque FoxP3+ Treg cells degranulated CD107a upon BiTE®-mediated activation and caused target cell cytotoxicity (data not shown). Therefore, a drug-mediated possible effect on regulatory T-cell counts/functions would not likely impact the BiTE®-mediated CTL function, unless the intrinsic function of CTL had also been affected.
During PBMC purification, any free drug circulating in blood is washed away. If the immunosuppression effect requires the presence of the drug during T-cell activation, this method may underestimate the drug influence as it may no longer be present in the assay culture using the purified PBMC. For example, both cyclosporine A and dexamethasone can rapidly reduce T-cell activation upon in vitro stimulation (Furue and Ishibashi Citation1991). Thus, it is possible for the predicted CTL immunosuppression observed in vivo after administration of either cyclosporine A or dexamethasone to be discordant with in vitro findings due to the washing off effect associated with ex vivo PBMC isolation. Taking this into consideration, it is critical to implement a mechanism-based design and interpretation of alterations observed in this ex vivo functional assay. It is our recommendation that there be inclusion of valuable controls, such as in vitro inclusion of test articles that are representative of the serum exposures observed throughout the course of the in vivo study. Such in vitro assessments of test articles would complement interpretation of the ex vivo results. Additional modifications to re-format the existing PBMC-based assay to a whole blood ex vivo assay are currently under consideration.
Conclusions
A Cynomolgus macaque PBMC-based ex vivo co-culture assay to evaluate CTL function throughout in vivo studies was developed utilizing a human EGFR BiTE® molecule in the presence of human EGFR target-expressing HCT-116 colon cancer cells. In this assay, BiTE®-mediated CTL activity mounted a robust response while engaging target cells. CTL-specific CD107a degranulation was chosen as the primary assay parameter and indicator of CTL function. Additionally, IFNγ production is considered to supplement the CD107a response. From the current results, it can be stated that this assay can consistently examine CTL activity at different timepoints of the same animal, and also be sensitive to measure chemical-induced immunosuppression of CD8+ T-cells. It is quite probable this assay can be transformed to similar assays with other bispecific T-cell engager antibodies and suitable target cells, so as to be applied in a wider industrial setting. Application of this assay to in vivo Cynomolgus macaque toxicity studies will be a valuable addition to the immunotoxicology testing of candidate drugs/chemicals used to modulate immune cells. It will also be of great help in allowing one to address important concerns regarding drug-induced immunosuppression/impairment of antiviral defense and cancer immunosurveillance.
Supplemental_Figures.pdf
Download PDF (841.3 KB)Acknowledgements
The authors would like to thank Dr. Katsu Ishida for helpful discussion and Katie Hsu for technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abraham M, Karni A, Dembinsky A, Miller A, Gandhi R, Anderson D, Weiner L. 2008. In vitro induction of regulatory T-cells by anti-CD3 antibody in humans. J Autoimmun. 30:21–28.
- Aktas E, Kucuksezer U, Bilgic S, Erten G, Deniz G. 2009. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 254:149–154.
- Axelsson S, Faresjo M, Hedman M, Ludvigsson J, Casas R. 2008. Cryopreserved peripheral blood mononuclear cells are suitable for the assessment of immunological markers in Type 1 diabetic children. Cryobiology. 57:201–208.
- Betts M, Brenchley J, Price D, de Rosa S, Douek D, Roederer M, Koup R. 2003. Sensitive and viable identification of antigen-specific CD8+ T-cells by a flow cytometric assay for degranulation. J Immunol Methods. 281:65–78.
- Betts M, Koup R. 2004. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 75:497–512.
- Brennan F, Morton L, Spindeldreher S, Kiessling A, Allenspach R, Hey A, Muller P, Frings W, Sims J. 2010. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. mAbs. 2:233–255.
- Bull M, Lee D, Stucky J, Chiu Y, Rubin A, Horton H, McElrath M. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 322:57–69.
- Burkett M, Shafer-Weaver K, Strobl S, Baseler M, Malyguine A. 2005. A novel flow cytometric assay for evaluating cell-mediated cytotoxicity. J Immunother. 28:396–402.
- Ceuppens J, Baroja M, Lorre K, van Damme J, Billiau A. 1988. Human T-cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 141:3868–3874.
- Choi B, Gedeon P, Herndon J, Archer G, Reap E, Sanchez-Perez L, Mitchell D, Bigner D, Sampson J. 2013. Human regulatory T-cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol Res. 1:163–167.
- Frauwirth K, Thompson C. 2002. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 109:295–299.
- Furue M, Ishibashi Y. 1991. Differential regulation by dexamethasone and cyclosporine of human T-cells activated by various stimuli . Transplantation. 52:522–526.
- Gutsol AA, Sokhonevich NA, Yurova KA, Khaziakhmatova OG, Shupletsova VV, Litvinova LS. 2015. Dose-dependent effects of dexamethasone on functional activity of T-lymphocytes at different grade of differentiation. Molek Biol. 49:130–157.
- ICH. 2005. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Immunotoxicity Studies for Human Pharmaceuticals S8. (Recommended for Adoption at Step 4 of ICH Process, 9/15/2005).
- Jana S, Campbell H, Woodliff J, Waukau J, Jailwala P, Ghorai J, Ghosh S, Glisic S. 2010. The type of responder T-cell has a significant impact in a human in vitro suppression assay. PloS One. 5:e15154.
- Jeurink P, Vissers Y, Rappard B, Savelkoul H. 2008. T-cell responses in fresh and cryopreserved peripheral blood mononuclear cells: Kinetics of cell viability, cellular subsets, proliferation, and cytokine production. Cryobiology. 57:91–103.
- Kamperschroer C, O’Donnell LM, Schneider PA, Li D, Roy M, Coskran TM, Kawabata TT. 2014. Measuring T-cell responses against LCV and CMV in Cynomolgus macaques using ELISPOT: Potential application to non-clinical testing of immunomodulatory therapeutics. J Immunotoxicol. 11:35–43.
- Kay J. 1991. Mechanisms of T-lymphocyte activation. Immunol Lett. 29:51–54.
- Klenerman P, Oxenius A. 2016. T-cell responses to cytomegalovirus. Nat Rev Immunol. 16:367–377.
- Lamers C, van de Griend R, Braakman E, Ronteltap C, Benard J, Stoter G, Gratama J, Bolhuis R. 1992. Optimization of culture conditions for activation and large-scale expansion of human T-lymphocytes for bispecific antibody-directed cellular immunotherapy. Int J Cancer. 51:973–979.
- Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. 2015. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer J. 5:e340.
- Lebrec H, Brennan F, Haggerty H, Herzyk D, Kamperschroer C, Maier C, Ponce R, Preston B, Weinstock D, Mellon R. 2016. HESI/FDA workshop on immunomodulators and cancer risk assessment: Building blocks for a weight-of-evidence approach. Regul Toxicol Pharmacol. 75:72–80.
- Levine BL, Mosca JD, Riley JL, Carroll RG, Vahey MT, Jagodzinski LL, Wagner KF, Mayers DL, Burke DS, Weislow OS, et al. 1996. Antiviral effect and ex vivo CD4+ T-cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 272:1939–1943.
- Lutterbuese R, Raum T, Kischel R, Hoffmann P, Mangold S, Rattel B, Friedrich M, Thomas O, Lorenczewski G, Rau D, et al. 2010. T-cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci USA. 107:12605–12610.
- Mallone R, Mannering S, Brooks-Worrell B, Durinovic-Bello I, Cilio C, Wong F, Schloot N, T-Cell Workshop Committee IoDS. 2011. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T-cell responses: Position statement of T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 163:33–49.
- Mazur P. 1984. Freezing of living cells: Mechanisms and implications. Am J Physiol. 247:C125–C142.
- McLeod J, Walker L, Patel Y, Boulougouris G, Sansom D. 1998. Activation of human T-cells with superantigen (Staphylococcal enterotoxin B) and CD28 confers resistance to apoptosis via CD95. J Immunol. 160:2072–2079.
- O’Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O’Connor DH. 2007. Comprehensive characterization of MHC Class II haplotypes in Mauritian Cynomolgus macaques. Immunogenetics. 59:449–462.
- Ross S, Sherman M, McElroy P, Lofgren J, Moody G, Baeuerle P, Coxon A, Arvedson T. 2017. Bi-specific T-cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing. PloS One. 12:e0183390.
- Rubio V, Stuge T, Singh N, Betts M, Weber J, Roederer M, Lee P. 2003. Ex vivo identification, isolation and analysis of tumor-cytolytic T-cells. Nat Med. 9:1377.
- Shi Y, Wu W, Wan T, Liu Y, Peng G, Chen Z, Zhu H. 2013. Impact of polyclonal anti-CD3/CD28-coated magnetic bead expansion methods on T-cell proliferation, differentiation and function. Int Immunopharmacol. 15:129–137.
- Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, Oyejide A, Kirshner JR, Canova L, Menon J, et al. 2016. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and Cynomolgus molgus monkeys. Sci Reports. 5:17943.
- Teschner D, Wenzel G, Distler E, Schnurer E, Theobald M, Neurauter A, Schjetne K, Herr W. 2011. In vitro stimulation and expansion of human tumour-reactive CD8+ cytotoxic T-lymphocytes by anti-CD3/CD28/CD137 magnetic beads. Scand J Immunol. 74:155–164.
- Tran D. 2013. In vitro suppression assay for functional assessment of human regulatory T-cells. In: Snow A, Lenardo M, editors. Immune homeostasis: Methods and protocols. Totowa, NJ: Humana Press; p. 199–212.
- Trickett A, Kwan Y. 2003. T-cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 275:251–255.
- Turner S, Cross R, Xie W, Doherty P. 2001. Concurrent naive and memory CD8+ T-cell responses to an influenza A virus. J Immunol. 167:2753–2758.
- Walsh C, Zydowsky L, McKeon F. 1992. Cyclosporin A, the cyclophilin class of peptidylprolyl isomerases, and blockade of T-cell signal transduction. J Biol Chem. 267:13115–13118.
