Abstract
FMX101 4% contains 4% micronized minocycline (as an HCl) formulated in a lipophilic foam vehicle for topical administration. FMX101 4% has been shown to be an effective and well-tolerated treatment for moderate-to-severe acne in three Phase 3 pivotal studies, however, skin sensitization and toxicity potential remains to be fully evaluated. Four single-center, randomized, controlled, within-subject comparison studies were conducted to evaluate the potential for phototoxicity, photoallergy, skin sensitization, and cumulative skin irritation with topical administration of FMX101 4% and the corresponding vehicle. Across the four studies, healthy male and non-pregnant female volunteers (age ≥18 years) were randomized to FMX101 4%, vehicle, or other controls. In the phototoxicity study, treated skin was irradiated at 3 and 24 hr post-application, and local tolerability was assessed pre- and post-irradiation. In the photoallergy study, the skin was treated and irradiated (post-24 hr) twice weekly for 3 wk (induction phase), rested for 10–17 d, and naive skin sites were treated and irradiated (challenge phase); skin reactions were assessed after patch removal and post-irradiation. In the sensitization study, the skin was treated for 3 wk (induction phase), then rested for 10–14 d, and naive skin sites were treated for 48 hr (challenge phase); contact sensitization was assessed for both phases. In the cumulative irritation study, treatment and vehicle were applied daily for 21 d; skin irritation was assessed after each application. In all studies, standard safety assessments were conducted. A total of 32, 56, 233, and 42 subjects were enrolled in the phototoxicity, photoallergy, sensitization, and skin irritation studies, respectively. There was no evidence of phototoxicity, photoallergy, skin sensitization, or skin irritation potential with FMX101 4%. Few adverse events, mostly mild to moderate, were reported. In conclusion, FMX101 4% appeared to be well tolerated and non-irritating, and was considered to be non-sensitizing, non-phototoxic, and non-photoallergic.
Introduction
Acne vulgaris (AV) is one of the most common diseases, affecting almost everyone at some point in their life (Zaenglein et al. Citation2016). Minocycline, a semi-synthetic, second-generation tetracycline, is a mainstay of acne treatment and has shown proven effectiveness when dosed orally in treating moderate-to-severe AV (Garner et al. Citation2012; Zaenglein et al. Citation2016). However, the risks of systemic, and oftentimes serious, side effects associated with the absorption of minocycline, such as lupus-like syndrome, autoimmune hepatitis, renal or hepatic failure, and neurologic adverse events, e.g. dizziness, mood alteration, somnolence, and headaches, remain a significant concern (Solodyn® [Package Insert] Citation2016; Zaenglein et al. Citation2016). In some patients, cutaneous side effects, such as hyperpigmentation, skin hypersensitivity reactions, and photosensitivity, may also occur with oral minocycline (Zaenglein et al. Citation2016). Topical antibiotics thus represent an important therapeutic approach for dermatologic conditions, given the lower risk of systemic toxicity expected with their limited absorption (Ochsendorf Citation2010; Zaenglein et al. Citation2016). Nevertheless, skin-related side effects can also occur with the various topical agents used for AV, and these effects can limit their use and effectiveness (Zaenglein et al. Citation2016).
FMX101 4% is a topical formulation that contains 4% micronized minocycline (as an HCl) in a lipophilic foam vehicle. The vehicle is hydrophobic and is expected to dissolve sebum upon contact with the skin, allowing targeted delivery of minocycline to the pilosebaceous unit, where the target organism, Cutibacterium acnes, mostly resides, while minimizing systemic exposure. Preclinical studies have previously demonstrated favorable characteristics with the foam formulation of minocycline (unpublished data). A pharmacokinetic study of once-daily application of 4 g FMX101 4% for 21 d in acne patients ≥18 years of age demonstrated very low systemic exposure and accumulation of minocycline in comparison with a single dose of ≈1 mg/kg of oral minocycline (extended release) (Jones et al. Citation2017). The efficacy, safety, and tolerability of FMX101 4% has previously been demonstrated in Phase 3 clinical studies (Raoof et al. Citation2018; Gold et al. Citation2019). These studies showed a once-daily application of FMX101 4% for 12 wk to be significantly more effective than vehicle in reducing inflammatory and non-inflammatory lesions, and in the achievement of higher rates of treatment success, as measured by an Investigator’s Global Assessment (IGA) score of “clear” or “almost clear” skin, with at least a 2-point reduction in score. The onset of efficacy of FMX101 4% is rapid, producing a significant reduction in inflammatory lesion count as early as Treatment Week 3. FMX101 4% also appears to be well tolerated, even with prolonged use, as indicated by reports of very few systemic treatment-emergent adverse events (TEAE) in the additional 40 wk of open-label treatment phase (Gold et al. Citation2018). In these clinical studies, a large majority of study subjects reported no, or only mild, dermal application-site TEAE. Nevertheless, the true potential for skin sensitization and toxicity issues with this product have not been fully evaluated.
To better understand the pharmacotoxicity of the topical application of FMX101 4%, as well as to align with the institutional guidelines and best practices on providing adequate safety information for a drug in development (US FDA Citation2018), we conducted four separate Phase 1 studies to evaluate the potential for phototoxicity, photoallergic potential, skin sensitization, and cumulative skin irritation with FMX101 4%, as compared with vehicle and/or relevant controls as appropriate.
Materials and methods
Study designs
Four separate, single-center, randomized, controlled, within-subject comparison studies were conducted to evaluate the potential for (1) phototoxicity (Study 06), (2) photoallergy (Study 09), (3) skin sensitization (Study 07), and (4) cumulative skin irritation (Study 08) with topical administration of FMX101 4%, as compared with vehicle, in healthy volunteers. Across the four studies, subjects were eligible for inclusion if they were healthy males or non-pregnant females ≥18 years of age and without any systemic or dermatologic disorder that might increase the risk of an adverse event (AE) or interfere with the study results. Subjects of any Fitzpatrick skin type or race were allowed to be enrolled in the skin sensitization and cumulative skin irritation studies, while only subjects who had Fitzpatrick skin type I, II, or III were allowed in the phototoxicity and photoallergy studies (Note: Fitzpatrick skin types are defined as follows: type I, always burns easily, never tans; type II, always burns easily, tans minimally; type III, burns moderately, tans gradually; type IV, burns minimally, always tans well; type V, rarely burns, tans very well; and, type VI, never burns, deeply pigmented).
Subjects had to refrain from using either topical or systemic non-steroidal anti-inflammatory drugs known to have sensitizing potential (e.g. aspirin, Aleve®, Motrin®, Advil®, or Nuprin®) for 72 hr prior to and during the study. Other restricted medications included the use of systemic or topical corticosteroids within 3 wk prior to and/or during the study and systemic or topical antihistamines 72 hr prior to and/or during the study. All study protocols were approved by an institutional review board or an independent ethics committee at each site, and all subjects provided written informed consent.
Phototoxicity study
The phototoxicity study was designed to detect the ability of FMX101 4% and vehicle to cause dermal irritation under ultraviolet (UV) irradiation. All subjects were first tested to establish their minimal erythemal dose (MED) within 7 d prior to application of the products. FMX101 4% and vehicle were applied under occlusive patch conditions to a total of four pairs of designated sites on the infrascapular area of the back (≈2 cm × 2 cm): one pair of sites was irradiated at approximately 3 hr (±15 min) post-application (Group A), and a second pair of sites was irradiated at ≈24 hr post-application (Group B). The remaining two pairs of sites were not irradiated and served as controls. In addition, two untreated, non-occlusive sites (≈1 cm × 1 cm) were irradiated as irradiated controls. Irradiation was set at 16 J/cm2 of ultraviolet A (UVA) followed by half the MED of ultraviolet B (UVB)/UVA exposure.
Local tolerability assessments were conducted immediately before irradiation and at 21, 45, 69, and 93 hr after irradiation for Group A, and at 24, 48, and 72 hr after irradiation for Group B. The degree of dermal response was assessed as the sum of scores for erythema, edema, and other signs of cutaneous irritation. Erythema was scored on a scale of 0 to 3.0 (“no reaction” to “marked/severe erythema”), while edema was graded on a scale of 0 to 1.5 (“no edema” to “definite edema with erosion/vesiculation”), with the maximum feasible score being 4.5 (). The comparison between FMX101 4% and vehicle was conducted using the average numerical score from the analysis of variance using Fisher’s least significant differences.
Table 1. Grading of response for erythema and edema for phototoxicity and photoallergic reaction studies.
Photoallergy study
The photoallergy study was designed to detect the ability of FMX101 4% and vehicle to cause photoallergic reactions in UV-irradiated skin. All subjects were first tested to establish the MED at screening, using full-spectrum UV light (UVB/UVA exposure).
Induction phase
FMX101 4% and vehicle were applied under occlusive patch conditions to a total of four designated application sites on the infrascapular area of the back; two sites were irradiated at 24 [±4] hr post-application, and two sites were not irradiated. Irradiation was set at twice the subject’s MED using the full Xenon lamp spectrum. These procedures were performed twice weekly for 3 wk (six applications/irradiations), and the sites were evaluated three to four times during each week immediately after patch removal. After completion of the induction phase, subjects entered a rest period of 10 to 17 d (study Weeks 4 and 5).
Challenge phase
FMX101 4% and vehicle were each applied to two previously untreated, non-occlusive sites, with one to be irradiated at 24 [±4] hr post-application at 6 J/cm2 of UVA radiation followed by half the MED of UVA/UVB radiation, and the other to remain nonirradiated. All sites were then examined for dermal reactions before and at ≈24, 48, and 72 hr after irradiation. Dermal reactions at the test sites were evaluated using a visual scale that rated the degree of erythema, edema, and other signs of cutaneous irritation (). The degree of photoallergic response was assessed as the mean numerical equivalent score of erythema (on a scale of 0–3.0) and edema (on a scale of 0–1.5), with the maximum feasible score being 4.5 (). Other signs of cutaneous irritation were also reported (). The diagnosis of a photosensitization response was to be made by the investigator based on review of the observed responses after challenge. For local tolerability assessments, selected pairwise comparisons of the mean numerical equivalent scores were performed using the analysis of variance, with main effects of subject and product, using Fisher’s least significant differences.
A rechallenge could be performed if a cutaneous response observed during the challenge phase indicated possible photosensitization or at the discretion of the investigator. If it was determined by the investigator that a rechallenge should be performed, the rechallenge patches were applied as soon as challenge reactions resolved. The study material was to be applied to naive sites on the back, using appropriate patches to further discriminate a photosensitization reaction from an irritation reaction.
Skin sensitization study
For the determination of skin sensitization, four randomly assigned application sites (≈2 cm × 2 cm) were designated on the infrascapular area of the back for FMX101 4%, vehicle, 0.9% saline (negative control), and 0.2% sodium lauryl sulfate (SLS; positive control). A total of nine applications of treatment and controls were administered under occlusive patch conditions for a total of three times per week over a period of 3 wk in the induction phase. Subjects then entered a 10- to 14-d rest period, followed by a challenge phase, in which subjects received single, 48-hr patch applications of the test products to naive (untreated) sites. Observations at the naive sites during the challenge phase and the patterns of reactivity during the induction phase provided a basis for an interpretation of contact sensitization. If a cutaneous response observed in the challenge phase indicated possible sensitization, or at the discretion of the investigator, a rechallenge phase would occur. Dermal reactions at the application sites were assessed clinically using a visual scale that rated the degree of erythema, edema, and other signs of cutaneous irritation (). Degree of sensitization was determined from the combination of the numerical equivalent scores for dermal response and effects on the skin (). Pairwise comparisons of mean response scores were performed from the analysis of variance using Fisher’s least significant differences.
Table 2. Grading of response for skin sensitization and cumulative skin irritation studies.
Cumulative skin irritation study
Cumulative skin irritation was evaluated using four randomly assigned application sites designated on the infrascapular area of the back for FMX101 4%, vehicle, 0.9% saline (negative control), and 0.2% SLS (positive control). Application of treatment and controls at each of these sites was repeated under occlusive patch conditions over a study period of 22 days. Patch sites were assessed once at baseline and then 21 times after each patch removal/re-application. Dermal reactions at the application sites were assessed clinically using a visual scale that rated the degree of erythema, edema, and other signs of cutaneous irritation (). The parameter for cumulative skin irritation was the mean cumulative irritation score for Days 2 through 22; the grading was the combination of the numerical equivalent score for dermal response and effects on the skin (). The primary variable was the mean cumulative irritation score, which was tested pairwise for differences using Fisher’s protected least significant differences with a two-way analysis of variance (ANOVA).
Safety assessments
Safety assessments were standard safety measures in clinical trials, which included physical examination and any AE, and vital signs in the phototoxicity study only.
Results
Subject disposition and baseline demographics/characteristics
A total of 445 subjects were screened, and 363 subjects were randomized across the four studies; the majority of subjects completed the study (). Across the four studies, the majority of the subjects were female and the mean age ranged between 49 and 59 years (). Varying Fitzpatrick skin types were represented, with types II and III being predominant in the phototoxicity and photoallergic reaction studies, and skin types IV and V more common in the sensitization and cumulative irritation studies.
Table 3. Subject disposition.
Table 4. Baseline demographics and characteristics.
Phototoxicity study
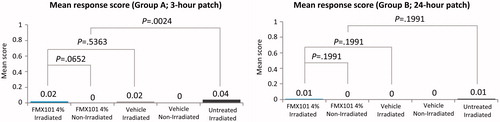
A total of 40 subjects were screened, and 32 subjects were randomized in this study. The mean age was 58.6 years. The majority of the subjects were female (65.6%) and not Hispanic or Latino (81.3%), and had Fitzpatrick skin type III (71.9%) (). The mean response scores were minimal and not significantly different, regardless of whether the sites were irradiated at 3 or 24 hr after application with FMX101 4% (0.02 and 0.01, respectively) or vehicle (0.02 and 0.00, respectively) (). There was minimal dermal response (maximum response score was 1) after irradiation for both FMX101 4% and vehicle in Group A (3 hr [±15 min] post-application) and Group B (24 hr post-application) (Supporting Information Table 1). Minimal dermal response was also observed at the irradiated untreated site and at nonirradiated treated sites, although the irradiated untreated sites had statistically significantly more irritation than the nonirradiated sites treated with either FMX101 4% or vehicle in Group A. There were no AE reported in the study. Overall, the study findings suggested no evidence of phototoxicity with FMX101 4% or vehicle.
Photoallergic reaction study
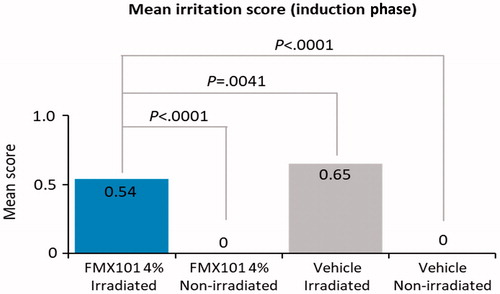
A total of 69 subjects were screened, and 56 subjects were randomized in the study. The mean age was 47.8 years. The majority of the subjects were female (78.6%), white (96.4%), and not Hispanic or Latino (82.1%), and had Fitzpatrick skin type II (30.4%) or III (66.1%) (). In the induction phase, the mean response scores were, as expected, higher for irradiated sites versus nonirradiated sites, regardless of whether they were treated with FMX101 4% (0.54 vs. 0.00; p < 0.0001) or vehicle (0.65 vs. 0.00; p < 0.0001) (). Sites treated with FMX101 4% yielded a significantly lower response score than sites treated with vehicle when subjected to irradiation (0.54 vs. 0.65; p = 0.0041). In the challenge phase, the maximum dermal response score was 1 for sites treated with both FMX101 4% and vehicle at 24 and 48 hr after irradiation (Supporting Information Table 2). Overall, the scores for both FMX101 4% and vehicle were considered lower than the criteria for establishing photosensitizing potential. Therefore, neither treatment was considered to be photoallergic to subjects. No erythema or edema reactions were reported for nonirradiated treated sites. Five subjects experienced AE; three events were moderate (stiff neck, paresthesia symptoms, cold urticaria) and two were mild (cold symptoms). None was related to treatment. Overall, the study findings suggested no evidence of photoallergic reaction with FMX101 4% or vehicle.
Skin sensitization study
A total of 283 subjects were screened, and 233 subjects were randomized in the study. The mean age was 50.0 years. The majority of the subjects were female (78.5%), black or African-American (79.0%), and not Hispanic or Latino (88.8%), and had Fitzpatrick skin type IV (25.3%) or V (57.5%) (). During the induction phase, both the mean and total irritation scores for FMX101 4% were not significantly different from those for the vehicle (0.01 vs. 0.05, p = 0.213; 0.11 vs. 0.46, p = 0.212, respectively); however, they were significantly lower than those for both 0.9% saline (0.01 vs. 0.09, p = 0.008; 0.11 vs. 0.85, p = 0.007, respectively) and 0.2% SLS (0.01 vs. 1.63, p < 0.0001; 0.11 vs. 14.63, p < 0.0001, respectively) (). At any one time, only 1 (0.5%) FMX101 4% subject had a total irritation score of 2 or greater, vs. 4 (1.8%), 11 (5.0%), and 209 (95.9%) subjects for vehicle, 0.9% saline, and 0.2% SLS, respectively (Supporting Information Table 3). In the challenge phase, no subject had a dermal response score more than 1 in any treatment group in the study; only 1 subject had a score of 1 (minimal erythema, barely perceptible, and presented separately) with FMX101 4% and vehicle, as compared with three subjects treated with 0.9% saline and 45 subjects treated with 0.2% SLS (data not shown). Since the challenge showed no response indicative of sensitization to any of the test products, rechallenge was not performed. Thus, FMX101 4% was considered non-sensitizing. A total of 16 TEAE were reported in 13 subjects; most were mild in severity, and none was related to treatment (). One subject discontinued the study due to a syncopal episode; the TEAE was moderate and resolved. Overall, the study findings suggested no evidence of skin sensitization with FMX101 4% or vehicle.
Figure 3. Mean and total irritation score for FMX101 4%, vehicle, 0.9% saline (negative control), and 0.2% SLS (positive control) (skin sensitization study).
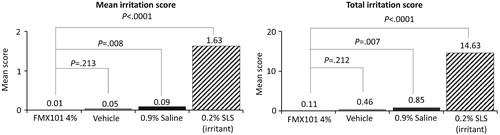
Table 5. Summary of adverse events (AE).
Cumulative skin irritation study
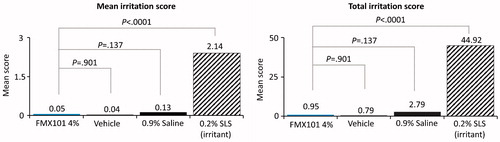
A total of 53 subjects were screened, and 42 subjects were randomized in the study. The mean age was 50.5 years. The majority of the subjects were female (73.8%), black or African-American (78.6%), and not Hispanic or Latino (95.2%), and had Fitzpatrick skin type IV (21.4%) or V (54.8%) (). The mean irritation score from baseline through Day 22 was 0.05 for FMX101 4%, 0.04 for vehicle, 0.13 for 0.9% saline, and 2.14 for 0.2% SLS (). There were no statistically significant differences in the mean scores for FMX101 4%, vehicle, and 0.9% saline; however, the mean score was significantly greater for 0.2% SLS versus all other treatments (p < 0.0001). Similarly, the total irritation score was significantly higher for 0.2% SLS versus FMX101 4%, vehicle, and 0.9% saline (p < 0.0001) (, Supporting Information Table 4). Based on the normalized total score, FMX101 4% and vehicle were classified as indicating no significant irritation. Limiting irritation (Grade 3 or greater) led to only one patch removal for FMX101 4% at Day 15 versus 38 patch discontinuations with 0.2% SLS, which occurred as early as Day 6; no patch removals were reported for vehicle or 0.9% saline throughout the study. As expected, the discontinuation rate was significantly higher for 0.2% SLS versus all other treatments (p < 0.0001), whereas there was no significant difference between FMX101 4%, vehicle, and 0.9% saline. Mild fatigue was reported in three subjects and resolved on its own; this AE was considered to be unlikely related to the treatment. Overall, the study findings suggested no evidence of cumulative irritation with FMX101 4% or vehicle.
Discussion
Oral minocycline is a mainstay of treatment for acne despite the seriousness of some of its systemic side effects, which may include various gastrointestinal, neurologic, and hepatic toxicities. Cutaneous AEs, such as pruritus, hyperpigmentation, maculopapular and erythematous rashes, and photosensitivity, can also occur (Zaenglein et al. Citation2016). Topical therapy for dermatologic conditions can thus be a safer means of drug delivery, and, with the recent introduction of topical foams, also a more convenient and easy-to-use option (Tamarkin et al. Citation2006). However, topical therapies have their own profile of adverse effects, characteristically local irritation, photosensitivity, hypersensitivity reactions, contact sensitization reactions, erythema, dryness, itching, and burning, depending on the active agent (Zaenglein et al. Citation2016).
In the current investigations, the topical application of FMX101 4% demonstrated no evidence for potentially clinically significant phototoxicity, photoallergy, sensitization, or cumulative irritation potential. Foam is becoming a prominent delivery system for topical drugs, exhibiting key advantages of usability, safety, and drug delivery over other formulations, including creams and ointments (Tamarkin et al. Citation2006). Patient evaluations have rated foams as having superior characteristics in relation to ease of application, absorption, and greasy feeling when compared to ointments and creams. For the topical treatment of psoriasis, foam was considered the preferred delivery vehicle (Housman et al. Citation2002; Tamarkin et al. Citation2006). These findings and insights further support the favorable safety profile of the lipophilic foam platform for delivery of an active agent for treating dermatologic conditions.
FMX101 4% was developed for its bacteriostatic and anti-inflammatory activity in a formulation designed to more effectively engage the target microbe, C. acnes, and to minimize systemic exposure to minocycline. Previous pharmacokinetic assessments of once-daily FMX101 4% applied for up to 21 d demonstrated minimal systemic exposure and no accumulation of minocycline (Jones et al. Citation2017). In the current studies, very few AEs were reported, and most were considered mild-to-moderate, overall suggesting that the safety profile for FMX101 4% appeared to be favorable. This was consistent with the safety profile for FMX101 4% reported in previous Phase 3 clinical trials, where there were few reported cutaneous TEAE, with most being mild in nature and transient (Gold et al. Citation2019).
Taken together with the favorable efficacy and safety profiles of FMX101 4% demonstrated in the Phase 3 clinical trials, these results support the use of FMX101 4% as a treatment option for moderate-to-severe AV.
Supplemental Material
Download MS Word (20.5 KB)Acknowledgements
Editorial support was provided by p-value communications.
Disclosure statement
Dr. Dosik served as the principal investigator on these studies. Dr. Stuart is an employee and stockholder of Foamix Pharmaceuticals. Dr. Ellman is a former employee and stockholder of Foamix Pharmaceuticals.
Additional information
Funding
References
- Garner S, Eady A, Bennett C, Newton J, Thomas K, Popescu C. 2012. Minocycline for acne vulgaris: Efficacy and safety. Cochrane Database Syst Rev. 8:CD002086.
- Gold L, Dhawan S, Weiss J, Draelos Z, Ellman H. 2018. FMX101 4% minocycline foam for the treatment of acne vulgaris: Safety and patient satisfaction from the open-label extension of two Phase 3 studies. Presented at: Winter Clinical Dermatology Conference; January 12–17; Maui, HI.
- Gold L, Dhawan S, Weiss J, Draelos Z, Ellman H, Stuart I. 2019. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: Results of 2 randomized, double-blind, Phase 3 studies. J Am Acad Dermatol. 80:168–177.
- Housman T, Mellen B, Rapp S, Fleischer A, Feldman S. 2002. Patients with psoriasis prefer solution and foam vehicles: A quantitative assessment of vehicle preference. Cutis. 70:327–332.
- Jones T, Ellman H, deVries T. 2017. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 16:1022–1028.
- Ochsendorf F. 2010. Minocycline in acne vulgaris: Benefits and risks. Am J Clin Dermatol. 11:327–341.
- Raoof J, Hooper D, Moore A, Zaiac M, Sullivan T, Kircik L, Lain E, Jankicevik J, Stuart I. 2018. FMX101 4% topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: Efficacy and safety from a Phase 3 randomized, double-blind, vehicle-controlled study. Presented at: Fall Clinical Dermatology Conference; October 18–21; Las Vegas, NV.
- Solodyn® [package insert]. 2016. Bridgewater (NJ): Valeant Pharmaceuticals North America LLC; [accessed 2019 Apr 11]. http://www.solodyn.com.
- Tamarkin D, Friedman D, Shemer A. 2006. Emollient foam in topical drug delivery. Expert Opin Drug Deliv. 3:799–807.
- U.S. Food and Drug Administration (UD FDA). 2018. Guidance, compliance, and regulatory information. Updated 2018 Dec 11. https://www.fda.gov/Drugs/GuidanceCompliance Regulatory Information/default.htm. Accessed 2019 Apr 11.
- Zaenglein A, Pathy A, Schlosser B, Alikhan A, Baldwin H, Berson D, Bowe W, Graber E, Harper J, Kang S, et al. 2016. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 74:945–973.