Abstract
Exposure to the widely-used phthalate plasticizer di-(2-ethylhexyl)-phthalate (DEHP) has been shown to be closely related to an increased prevalence of allergic diseases in infants and juveniles. Earlier work in our laboratory found that DEHP-related anaphylactic responses could be ascribed to T-follicular helper (Tfh) cell hyperfunction directly. The Tfh cell, a newly identified CD4+ TH cell subset, until recently has been considered as a key player in humoral immunity. Tfh cells can respond to stimulation through various receptors. Signaling lymphocytic activation molecule family member-1 (SLAMF1, CD150) is a surface co-stimulatory receptor that can bind to an intracytoplasmic adaptor signaling lymphocytic activation molecule-associated protein (SAP) to initiate downstream signaling cascades, regulating some events of immune response. The present study explored the role of SLAMF1 in Tfh cell differentiation and cytokine secretion under the condition of DEHP exposure. Using a weanling mice model of DEHP gavage with ovalbumin (OVA) sensitization, it was found that DEHP acted as an immunoadjuvant to elevate SLAMF1 and SAP expression in host Tfh cells. Ex vivo studies of effects from DEHP exposure on Tfh cells from OVA-sensitized hosts showed that DEHP acted in an adjuvant-like manner to promote the expression of adaptor protein SAP, transcription factors Bcl-6 and c-MAF, and cytokines interleukin (IL)-21 and IL-4 in Tfh cells. Transfection of these Tfh cells with Slamf1 small interfering RNA prior to exposure to the DEHP attenuated the over-expression of these molecules that was caused by the DEHP. In conclusion, this study demonstrated that DEHP, via a SLAMF1-mediated pathway, can impact on Tfh cell differentiation and their ability to form select cytokines.
Introduction
Di-(2-ethylhexyl)-phthalate (DEHP) is a commonly-used phthalate plasticizer to impart flexibility to polyvinyl chloride (PVC) products such as food packaging, bottles, toys, building materials, and medical devices. Due to its wide application and stability of its chemical structure, as well as its non-covalently binding to/easily leaching from PVC, DEHP is increasingly being detected in soil, water, and atmospheric dust particles. DEHP is considered an environmental persistent organic pollutant, and its potential impact on human health has been highlighted on a global scale (Erythropel et al. Citation2014; Gao and Wen Citation2016). Human exposure routes to DEHP are multiple, and include inhalation, skin and medical contact, as well as ingestion of contaminated foods, waters, and other materials (Johns et al. Citation2015). In particular, children are more likely to be exposed to DEHP by sucking or chewing on plastic products and so, consequently, they are more susceptible to potential DEHP hazards (Braun et al. Citation2013; Wang et al. Citation2018). Once DEHP is absorbed in the digestive tract, it enters the hepato-enteral circulation and is rapidly metabolized to mono-2-ethylhexyl-phthalate (MEHP) and other secondary products in the liver (Wittassek and Angerer Citation2008).
Recent epidemiology surveys have noted a positive association between DEHP exposure and increased prevalence of anaphylactic diseases, including allergic rhinitis, bronchial asthma, and atopic dermatitis among genetically-stable populations, especially infants and juveniles (North et al. Citation2014; Wang et al. Citation2014; Beko et al. Citation2015; Li et al. Citation2017). Anaphylaxis is a hypersensitivity reaction rooted in an imbalance in host humoral immunity (Galli and Tsai Citation2012; Holgate Citation2012). A previous study from our laboratory showed that gavage with DEHP resulted in improper enhancement of humoral immune responses (re: to ovalbumin [OVA]) among OVA-sensitized weanling mice. DEHP acted as an immunoadjuvant to augment OVA-specific IgE and IgG1 production, amplified germinal center formation in lymphoid nodules, as well as stimulated expansion of T-follicular helper (Tfh) and plasma cells. Based on results of immune adoptive transfusion, that study also found these outcomes were related to intrinsic dysfunction among host Tfh cells (Han et al. Citation2014).
The Tfh cell is a new subset of CD4+ T-helper (TH) cells; until recently, Tfh cells had been identified as the most important subpopulation to potently assist B-cells and so mediate humoral immune responses (Johnston et al. Citation2009; Nurieva et al. Citation2009). Tfh cells retain high levels of expression of the chemokine receptor CXCR5; this functionally directs them toward B-cell follicular areas via chemotactic effects from the specific ligand CXCL13. Tfh cells synthesize characteristic cytokines (such as interleukin [IL]-21 and IL-4) that facilitate antibody production by and class-switch recombination events in B-cells. Over recent years, it has been shown that the expression of typical transcription factors (such as Bcl-6 and c-MAF) – along with their mutual regulation – is of crucial significance for the initial differentiation of, and essential functions thereafter, of Tfh cells (Crotty Citation2014; Qi et al. Citation2014; Butler and Kulu Citation2015; Jogdand et al. Citation2016; Wali et al. Citation2016; Qin et al. Citation2018).
Like most T-cells, Tfh cells can respond to stimulation through various receptors (Webb and Linterman Citation2017). The signaling lymphocytic activation molecule (SLAM) family of receptors belong to a CD2 subgroup of the immunoglobulin (Ig) superfamily, with a series of SLAM isoforms exhibiting diverse cellular distributions and functional properties. Among the isoforms, SLAM family member-1 (SLAMF1, CD150) is the prototype that is constitutively expressed on the surface of T-and B-cells, dendritic cells, monocytes-macrophages, natural killer (NK) cells, and NK-T cells (van Driel et al. Citation2016; Wu and Veillette Citation2016; Dragovich and Mor Citation2018). SLAMF1 possesses one or more copies of an immunoreceptor tyrosine-based switch motif (ITSM) in its cytoplasmic tail, through which it recruits Src homology 2 (SH2) domain-containing signal transduction molecules like SLAM-associated protein (SAP, encoded by gene Sh2d1a) to initiate downstream signaling cascades (Vilar et al. Citation2011; Romero et al. Citation2014). Thus, SLAMF1 may play an important role in regulating co-stimulatory signals, immunological synapse formation, cytokines synthesis, high-affinity antibody production, and other key properties in immune cells (Yusuf et al. Citation2010; Zhao et al. Citation2012; Zhong and Veillette Citation2013a, Citation2013b).
To obtain a better understanding of how DEHP might have induced the earlier-noted effects in Tfh cells, and to better explore any role of SLAMF1 in Tfh cell differentiation and cytokine secretion (under the condition of DEHP exposure), the present study examined the influence of DEHP on SLAMF1-SAP-dependent signal transduction pathways in Tfh cells. It was hoped that the data from this study could provide a new understanding of DEHP immunotoxic effects and the mechanisms underlying such outcomes.
Materials and methods
Chemicals
DEHP (purity ≥99.5%), OVA (purity ≥98%), and dimethyl sulfoxide (DMSO, purity ≥99.5%) were purchased from Sigma (St. Louis, MO). Slamf1 small interfering RNA (Slamf1 siRNA, ID: 71714) and negative control siRNA (NC siRNA) were purchased from Thermo Fisher Scientific (Waltham, MA). Anti-mouse CD3 and anti-CD28 (both functional grade-purified) monoclonal antibodies were bought from eBioscience (San Diego, CA). S9 metabolic activation solution was purchased from Moltox (Boone, NC). RPMI 1640 medium was purchased from HyClone (Logan, UT). Additional sources and vendors of specific reagents, such as antibodies and kits, are indicated as needed below.
Animals
Since children are more susceptible to DEHP hazards, weanling mice were used in this study. Weanling BALB/c mice (both genders, 3–4 weeks of age, 12–16 g) were obtained from the Experimental Animal Center of Nantong University (Nantong, China). Mice were housed (gender separated) in a temperature-controlled (24–26 °C) room at 60 [±5]% humidity under a 12-h light/dark cycle. All mice had ad libitum access to OVA-free rodent chow and filtered distilled water. Mice were quarantined for at least 7 days before study initiation.
All experiments involving mice were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications, revised 2011) and were approved by the Chinese National Committee to the Use of Experimental Animals for Medical Purposes, Jiangsu Province. All efforts were made to minimize the number of mice used with regard for alleviation of suffering.
In vivo exposure and immunization protocols
Since gastrointestinal exposure best reflects actual human exposures, a murine model of DEHP gavage with OVA sensitization was established here. In brief, BALB/c mice were randomly divided into eight groups (8 mice/group; four male and four female): four were comprised of DEHP (30, 300, 3000 μg/kg) or corn oil gavage with OVA sensitization and the other four were DEHP (30, 300, 3000 μg/kg) or corn oil gavage with normal saline [sham-sensitization]. The DEHP gavage dosages were chosen based on current risk assessment parameters and safety limit values.
For the exposure, DEHP was dissolved in laboratory-grade corn oil; the mice were then gavaged with DEHP or corn oil at equivalent volume once a day from Day 1 to Day 28 (28 times) of the study. For the mice that were to be sensitized with OVA, a solution of 100 μg OVA in 50 μl normal saline was injected into two footpads (double subcutaneous injection) on Day 7 and Day 16 of the study. Parallel DEHP-only and vehicle-only control groups were sham-sensitized with 50 μl normal saline on each of those days. On Day 29, all mice were euthanized by all mice were euthanized by cervical dislocation and their spleens harvested at necropsy.
Isolation of splenic CD4+CXCR5+tfh cells
From each spleen, single cell suspensions of splenic lymphocytes were prepared by density gradient centrifugation (to help remove red blood cells, etc.) and the final purified cells were re-suspended in phosphate-buffered saline (PBS, pH 7.4) to 5 × 106 cells/ml. Splenic CD4+ TH cells were then isolated from each preparation using a Mouse CD4+ T-Cell Isolation Kit II (Miltenyi, Bergisch Gladbach, Germany). Splenic CD4+CXCR5+Tfh cells were further purified from all other CD4+ cells present by magnetic separation using phycoerythrin (PE)-conjugated anti-CXCR5 and then anti-PE MicroBeads (Miltenyi) in succession, according to manufacturer protocols. The cells were then re-suspended in PBS and counted using a hemocytometer. The purity of isolated CD4+CXCR5+Tfh cells was subsequently seen to be >95% (flow cytometry). The isolated cells were all found to maintain good viability and be able to undergo proliferation.
SLAMF1 and SAP mRNA expression in the isolated tfh cells
Total RNA was extracted from aliquots of isolated splenic CD4+CXCR5+Tfh cells (106) of each mouse in each group using Trizol (Invitrogen, Carlsbad, CA) per manufacturer instructions. After determining the purity and concentration of each extract using a NanoDrop One micro-volume spectrophotometer (Thermo Fisher Scientific), 2 μg total RNA from each extract was then reverse transcribed into cDNA using oligo-dT primers and an Omniscript RT Kit (Qiagen, Hilden, Germany) according to manufacturer protocols. Real-time qPCR was then done in a Qiagen Roter-Gene 3000 system using a QuantiTect SYBR Green PCR Kit (Qiagen). The primers used were: Slamf1 (F) 5′-ACAGGCGTGCTTATGAAGTAGA-3′ and (R) 5′-CCAC-GGGATCTCTGCTTCATAT-3′; Sh2d1a (F) 5′-GACGCAGTGGCTGTGTATCAT-3′ and (R) 5′-ACTTCTAGCTGAGGACTTCTT-3′; and, Gapdh (F) 5′-GCTTGAAGGTGTTGCCCT-CAG-3′ and (R) 5′-AGAAGCCAGCGTTCACCAGAC-3′. Relative mRNA expression levels in each case were normalized to the Gapdh reference and calculated using the 2-△△Ct method.
SLAMF1 receptor expression on tfh cell surface
Spleen lymphocytes from each group (106) were incubated for 20 min at 4 °C in the dark in a solution containing 2.5 µg/ml fluorescein isothiocyanate (FITC)-anti-mouse CD4, 2.5 µg/ml PE-anti-mouse CXCR5, and 5 µg/ml allophycocyanin (APC)-anti-mouse SLAMF1 (eBioscience). After centrifugation/washing with PBS, the cells were re-suspended in PBS and then underwent flow cytometric analysis in a FACS Calibur instrument (BD Bioscience, San Jose, CA) to assess SLAMF1 expression levels on the cells as a function of host treatment. All data were analyzed using FCS Express software (BD Bioscience). A minimum of 10,000 events/sample was acquired.
SAP protein expression in tfh cells
Whole protein was extracted from each set of splenic CD4+CXCR5+Tfh cells (aliquots of 106 cells) using M-PER mammalian protein extraction reagent (Pierce, Rockford, IL). Total protein concentration in each extract was measured using the NanoDrop One spectrophotometer. Aliquots of total protein (20 μg/lane) were then resolved over 15% gels (SDS-PAGE) and the separated proteins then electrotransferred onto poly-vinylidene-difluoride (PVDF) membranes (Millipore, Boston, MA). Two sets of membranes were prepared for each group. Each membrane was then blocked with 5% fat-free milk in Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBS-T) for 2 h at room temperature (RT), and then incubated at RT for 2 h in a solution of TBS-T containing any one of the following primary rabbit monoclonal antibodies at 0.1 µg/ml: anti-mouse SAP or anti-mouse GAPDH (Abcam, Boston, MA). After rinsing with TBS-T buffer, each membrane was then treated with a solution of TBS-T containing secondary antibody (0.1 µg/ml horseradish peroxidase-conjugated goat anti-rabbit IgG antibody [Abcam]) and incubated at RT for 2 h. After gentle washes with TBS-T to remove unbound secondary antibody, all membranes underwent development with enhanced chemiluminescence reagents (Amersham, Little Chalfont, Buckinghamshire, UK). The intensity of relative protein expression was then measured in a chemiluminescence imaging system (Syngene, Cambridge, Cambridgeshire, UK) using Image J analysis software (National Institutes of Health, Bethesda, MD).
Ex vivo analyses of effects of DEHP on tfh cells
For this study, additional sets of BALB/c mice were sensitized with OVA (100 μg in 50 μl normal saline) by double subcutaneous injection into the footpads on Day 1 and Day 10. At 10 days post-sensitization, all mice were euthanized by cervical dislocation and their spleens harvested at necropsy. Tfh cells from each mouse were then isolated as above.
For these in vitro studies, the Tfh cells were counted and diluted with complete medium (RPMI 1640 containing 10% fetal bovine serum [FBS, South America source], but without any antibiotics) to 5 × 105 cells/ml. The cells were then transferred into 6-well culture plates (2 ml suspension/well) and cultured at 37 °C in a 5% CO2/95% air humidified incubator. After 24 h, the Tfh cells were transiently transfected with Slamf1 siRNA (to silence Slamf1 gene) or with NC siRNA using Lipofectamine 2000 transfection reagent (Invitrogen) according to manufacturer protocols. The mRNA knockdown efficiency was evaluated 48 h post-transfection by qRT-PCR; protein knockdown efficiency was evaluated 72 h post-transfection by Western blot (see above for details of qRT-PCR and Western blot protocols).
Upon completion of the above, these Tfh cells were seeded into 24-well plates (106 cells/ml/well) and randomly delegated into one of six groups: (1) Control (non-transfected), (2) NC siRNA cells, (3) Slamf1 siRNA cells, (4) DEHP-treated control cells, (5) DEHP-treated NC siRNA cells, and (6) DEHP-treated Slamf1 siRNA cells. For the exposures, DEHP solution was dissolved in 0.1% DMSO and added to wells for groups (4), (5), and (6) at a final concentration of 50 μg/ml. Wells for cells in groups (1), (2), and (3) received only 0.1% DMSO at an equivalent volume. After this, S9 metabolic activation mixture (0.4 ml/well) was added to all the wells (specifically, to promote DEHP metabolism in group (4), (5), and (6) wells). Anti-mouse CD3 (100 µl, final level in well of 0.5 μg/ml) and anti-mouse CD28 (100 µl, final level in well of 0.5 μg/ml) antibody were then both added to each well to maintain induction and culture. Doses of all chemicals/antibodies above were chosen based on reference data/preliminary experimental results. The plates were then incubated at 37 °C for 72 h at which point cell culture supernatants were collected and frozen for later analysis. The cells were collected, washed, and prepared for analyses in the various protocols outlined below.
SLAMF1, SAP, bcl-6 and c-MAF mRNA expression in ex vivo-treated tfh cells
Total RNA was extracted from the original Slamf1 siRNA- or NC siRNA-transfected cultures, as well as each Groups (1) – (6) Tfh culture above using Trizol. Thereafter, the isolated RNA was measured and evaluated in real-time qRT-PCR using the same protocols outlined above. Besides primers for Slamf1, Sh2d1a and Gapdh, another other primer pairs were used: Bcl-6 (F) 5′-CCTGAGGGAAGGCAATATCA-3′, (R) 5′-CGGCTGTTCAGGAACTCTTC-3′; c-Maf (F) 5′-GCAGAGACACGTCCTGGAGTCG-3′, (R) 5′-CGAGCTTGGCCCTGCAACTAGC-3′; and, Gapdh (F) 5′-GCTTGAAGGTGTTGCCCTCAG-3′ and (R) 5′-AGAAGCCAGCGTTCACCAGAC-3′. As before, relative mRNA expression levels in each cell set were all normalized to the Gapdh reference and calculated using the 2-△△Ct method.
SLAMF1 and SAP protein expression in ex vivo-treated tfh cells
Whole protein was extracted from the original Slamf1 siRNA- or NC siRNA-transfected cultures, as well as each Group (1)–(6) Tfh cultures above using M-PER mammalian protein extraction reagent. Once isolated and quantified, aliquots of the samples then underwent Western blot analyses as outlined above. In this case, three membranes were generated for each group to permit simultaneous analyses of three individual proteins. For these studies, the primary rabbit monoclonal antibodies used were anti-mouse-SLAMF1, -SAP, or -GAPDH.
Bcl-6 and c-MAF expression in ex vivo-treated tfh cells
Aliquots containing Tfh cells (106) from each of the culture groups were incubated for 20 min at RT in a solution of PBS containing 2.5 µg/ml of anti-mouse CD16/CD32 (eBioscience) to block nonspecific binding. After centrifugation, to permit surface staining, the cells were re-suspended in PBS containing 2.5 µg/ml each of FITC-anti-mouse CD4 and PE-anti-mouse CXCR5 (eBioscience). The cells were then incubated for 30 min at 4 °C in the dark, and then washed three times with PBS. For intracellular staining, the cells were then fixed and permeabilized with Fixation/Permeabilization working solution using a Transcription Factor Staining Buffer Kit (eBioscience) according to manufacturer protocols. The cells were then treated with PBS containing 0.3 µg/ml APC-anti-mouse Bcl-6 or 1.25 µg/ml eFluor 660-anti-mouse c-MAF (eBioscience) for 30 min at 4 °C in the dark, and then washed three times with kit-provided permeabilization buffer. The final post-stained cell pellets were re-suspended in 200 μl PBS, and flow cytometry analysis was performed in the FACS Calibur system. All data were analyzed using FCS Express software; minimum of 10 000 events/sample was acquired.
Quantification of secreted IL-21 and IL-4 from ex vivo-treated tfh cells
The amounts of the Tfh cell characteristic cytokines IL-21 and IL-4 in the supernatants that were collected after 72 h of culturing of the cells in each treatment group were measured using a Mouse IL-21/IL-4 Platinum ELISA Kit (eBioscience), according to manufacturer instructions. Cytokine levels were determined using a spectrometer enzyme-labeled instrument (Bio-Rad, Hercules, CA); all values were extrapolated from standard curves generated from kit-provided mouse IL-21 and IL-4 standards examined in parallel. The lower limits of quantification for IL-21 and IL-4 were 5.0 and 2.0 pg/mL, respectively. All samples were analyzed in triplicate.
Statistical analysis
Results are expressed as means ± SD. Statistical differences between groups were evaluated using an analysis of variance (ANOVA). The criterion for significance in all cases was a p-value < 0.05. Linear correlation analysis was also performed. All data were analyzed using IBM SPSS Statistics 20.0 software (IBM Corp, Armonk, NY).
Results
Effects of DEHP exposure on OVA-sensitized weanling mice spleen Tfh cell surface receptor SLAMF1 expression
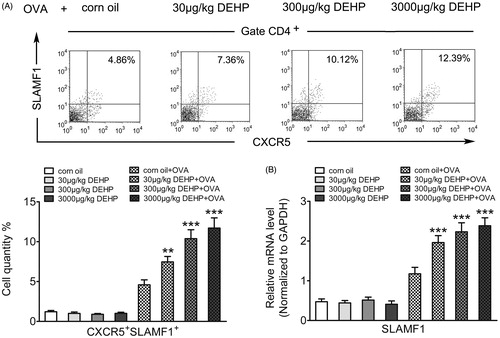
Real-time qRT-PCR and flow cytometric analyses were used to assess SLAMF1 mRNA/protein expression in murine spleen Tfh cells from each group. Relative to OVA sensitization groups, most of the measured values for cells from DEHP-only and vehicle-only control groups without OVA sensitization were quite small, indicating DEHP oral administration alone could not impart an allergen effect. In OVA-sensitized weanling mice, as a result of DEHP exposure, SLAMF1 mRNA expression and the percentage of CXCR5+SLAMF1+ Tfh cells among the entire CD4+ TH cell populations notably increased compared to corresponding values seen with the corn oil control mice (, Table 1 in supplementary material). The effect was apparent across all the levels of DEHP tested (30, 300, 3000 μg/kg) although the effect itself was not dose-dependent.
Figure 1. Effects of DEHP exposure on OVA-sensitized weanling mice spleen Tfh cell surface receptor SLAMF1 expression. (A) Expression percentage of CXCR5+SLAMF1+ Tfh cells gated on CD4+ T-cell populations (%) (flow cytometry). (B) SLAMF1 mRNA expression (normalized to Gapdh; real-time qRT-PCR). Data shown are means ± SD, n = 8 mice/group. **p < 0.01, ***p < 0.001 vs. corn oil + OVA control group.
Effects of DEHP exposure on OVA-sensitized weanling mice spleen Tfh cell adaptor protein SAP expression
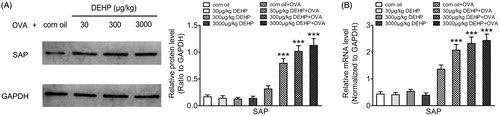
Data from real-time qRT-PCR and Western blots of SAP in isolated Tfh cells showed the majority of values for SAP with cells from DEHP-only and vehicle-only control groups without OVA sensitization were very low, suggesting DEHP intake (orally) alone was not antigenic. In OVA-sensitized weanling mice, SAP mRNA and protein expression levels in isolated cells from mice that had received any of the tested levels of DEHP were significantly higher than in cells from the corn oil control (, Table 2 in supplementary material). The effect was apparent across all DEHP levels tested (30, 300, 3000 μg/kg) though the effect itself was not dose-dependent.
Figure 2. Effects of DEHP exposure on OVA-sensitized weanling mice spleen Tfh cell adaptor protein SAP expression. (A) SAP protein expression (ratio to GAPDH; Western blot). (B) SAP mRNA expression (normalized to Gapdh; real-time qRT-PCR). Data shown are means ± SD, n = 8 mice/group. ***p < 0.001 vs. corn oil + OVA control group.
Influence of Slamf1 siRNA transfection (with DEHP exposure) on Tfh cell SAP expression
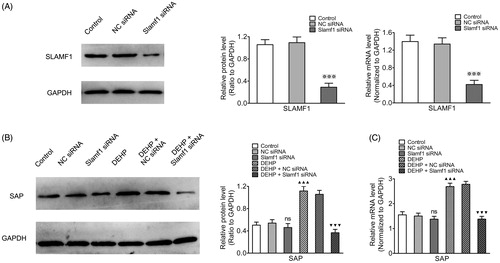
As SLAMF1 has a pivotal role in signal transduction pathways (Sawada Citation2012; Hu et al. Citation2013; Zhong and Veillette Citation2013a, Citation2013b), an siRNA-targeting Slamf1 gene was used to silence Slamf1 expression in the Tfh cells. As seen in , mRNA and protein expression levels of SLAMF1 in the Tfh cells was significantly reduced after transfection, proving knockdown was satisfactory. To explore the influence of DEHP on SLAMF1-SAP signaling pathway in Tfh cells when silencing Slamf1 gene, SAP mRNA/protein expression in Tfh cells from each group were evaluated by real-time qRT-PCR and Western blots. The data showed that SAP mRNA/protein expression levels from Tfh cells from the DEHP exposure (no silencing) group were higher than those of control group Tfh cells. After transfection with Slamf1 siRNA, SAP mRNA/protein expression levels decreased in the DEHP + Slamf1 siRNA group compared to that in cells from the DEHP exposure (no silencing) group or the DEHP + NC siRNA group (, Table 3 in supplementary material).
Figure 3. Influence of Slamf1 siRNA transfection (with DEHP exposure) on Tfh cell adaptor protein SAP expression. (A) SLAMF1 protein (Western blot) and SLAMF1 mRNA expression (real-time qRT-PCR). (B) SAP protein expression (ratio to GAPDH; Western blot). (C) SAP mRNA expression (normalized to Gapdh; real-time qRT-PCR). Data shown are means ± SD, n = 6 samples/group. ※※※p < 0.001 vs. control group or NC siRNA group; ▲▲▲p < 0.001 vs. control group; ▼▼▼p < 0.001 vs. DEHP exposure group or DEHP + NC siRNA group; ns = p > 0.05 vs. control group or NC siRNA group.
Influence of Slamf1 siRNA transfection (with DEHP exposure) on Tfh cell nuclear transcription factor expression
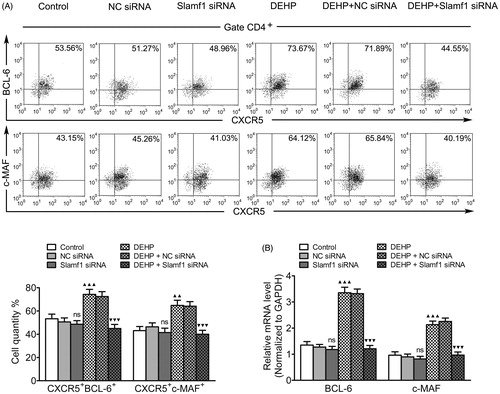
Both Bcl-6 and c-MAF are regarded as intrinsic Tfh cell regulators (Kroenke et al. Citation2012; Liu et al. Citation2012; Andris et al. Citation2017). To clarify the influence of DEHP on Tfh cell transcriptional control, mRNA/protein expression levels of Bcl-6 and c-MAF transcription factors were examined in Tfh cells from each group – with and without Slamf1 gene silencing. Using real-time qRT-PCR and flow cytometry, Bcl-6 and c-MAF mRNA expression levels and the percentage of CXCR5+BCL-6+/CXCR5+c-MAF+ Tfh cells among all CD4+ TH cell populations were evaluated. The results indicated the values associated with the cells from the DEHP exposure (no silencing) group increased compared to those with the cells from the control group. After transfection, both Bcl-6 and c-MAF mRNA levels, as well as levels of CD4+CXCR5+BCL-6+/CD4+CXCR5+c-MAF+Tfh cells, decreased in the DEHP + Slamf1 siRNA cells relative to those in the DEHP exposure (no silencing) or DEHP + NC siRNA cells (, Table 4 in supplementary material).
Figure 4. Influence of Slamf1 siRNA transfection (with DEHP exposure) on Tfh cell nuclear transcription factor expression. (A) Expression percentages of CXCR5+BCL-6+ and CXCR5+c-MAF+ Tfh cells gated on CD4+ T-cell populations (%) (flow cytometry). (B) Bcl-6 and c-MAF mRNA (normalized to Gapdh; real-time qRT-PCR). Data shown are means ± SD, n = 6 samples/group. ▲▲p < 0.01, ▲▲▲p < 0.001 vs. control group; ▼▼▼p < 0.001 vs. DEHP exposure group or DEHP + NC siRNA group; ns = p > 0.05 vs. control group or NC siRNA group.
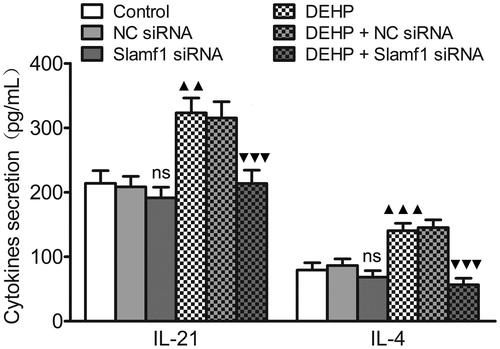
Influence of Slamf1 siRNA transfection (with DEHP exposure) on Tfh cell cytokine secretion
Mature Tfh cells can synthesize some cytokines, especially IL-21 and IL-4 (Luthje et al. Citation2012; Read et al. Citation2016; Sahoo et al. Citation2016). To monitor the influence of DEHP on Tfh cell cytokine secretion when the Slamf1 gene was silenced, secretion of IL-21 and IL-4 by Tfh cell cultures generated from each treatment group was evaluated by ELISA. The secreted amounts of IL-21 and IL-4 from the DEHP exposure (no silencing) group were higher than those from cells from control group Tfh cells. As a result of Slamf1 siRNA transfection, relative to levels produced by Tfh cells from the DEHP exposure (no silencing) group or the DEHP + NC siRNA group, amounts of IL-21 and IL-4 were decreased (, Table 5 in supplementary material).
Figure 5. Influence of Slamf1 siRNA transfection (with DEHP exposure) on Tfh cell cytokine secretion. Levels of IL-21 and IL-4 in Tfh cell culture supernatants as measured by ELISA. Data shown are means (pg/ml) ± SD, n = 6 samples/group. ▲▲p < 0.01, ▲▲▲p < 0.001 vs. control group; ▼▼▼p < 0.001 vs. DEHP exposure group or DEHP + NC siRNA group; ns = p > 0.05 vs. control group or NC siRNA group.
Discussion
Recent epidemiology studies have uncovered a positive association between host exposure to DEHP and increased prevalence of allergic diseases in infants and juveniles (North et al. Citation2014; Wang et al. Citation2014; Beko et al. Citation2015; Li et al. Citation2017). One experiment showed that DEHP exposure-related anaphylactic responses could be ascribed to induction of Tfh cell hyper-function (Han et al. Citation2014). The Tfh cell, a newly confirmed CD4+ TH cell subset, until recently was considered a key player in humoral immunity by assisting B-cells in their capacity to produce antibodies (Johnston et al. Citation2009; Nurieva et al. Citation2009). It has been subsequently shown these cells have an even larger role in host immunocompetence. Specifically, it has been shown that abnormal differentiation and hyperfunction of Tfh cells can cause an immune imbalance in a host (Ma and Deenick Citation2014; Ueno et al. Citation2015; Varricchi et al. Citation2016; Gensous et al. Citation2018).
As with many immune cells, Tfh cells may respond to stimulation through various receptors (Webb and Linterman Citation2017). As the prototypic member of SLAM family, SLAMF1 is a Type I transmembrane glycoprotein widely-expressed on the surface of T-cells. It is rapidly induced after naive T-cell activation (de Calisto et al. Citation2014; Chen et al. Citation2017). SLAMF1 has been viewed as a distinct co-stimulatory molecule used to strengthen and sustain T-cell receptor (TCR) signals that, in turn, allow Tfh cells to selectively provide help to B-cells (Cannons et al. Citation2011; Ma and Deenick Citation2011; Gordiienko et al. Citation2018). As such, it has been proposed that SLAMF1 receptors participate in modulating humoral immune responses, and that differences in subsequent cellular responses depend on the type and intensity of stimuli. In the present study, it was found that DEHP exposure (by oral intake) led to elevated SLAMF1 expression in splenic Tfh cells from OVA-sensitized weanling mice, implying that SLAMF1 receptor expression on the Tfh cell surface might be somehow related to any adjuvant effects/toxicities associated with DEHP exposure.
Its unique functional domain indicates that SLAMF1 is not only a surface receptor but also a signal transduction factor (Wu and Veillette Citation2016; Dragovich and Mor Citation2018). SLAMF1 carries one or more paired ITSM in its cytoplasmic tail; upon external stimulation, SLAMF1 can selectively bind SH2-containing intracellular adaptor molecules, chiefly SAP. SAP is a small adaptor protein that links SLAM family receptors to active signaling molecules like the Src family protein tyrosine kinase Fyn (Dong et al. Citation2012; Proust et al. Citation2012; Zhong and Veillette Citation2013a; Samanta and Mukherjee Citation2017). The present study revealed that exposure to DEHP (by oral intake) led to a boost in SAP adaptor expression inside Tfh cells from OVA-sensitized weanling mice; these changes paralleled those in expression of SLAMF1 receptor on the cells. In addition, ex vivo exposure to DEHP also acted in an adjuvant manner to reinforce SAP expression in Tfh cells.
The studies here also showed that transfection of the host Tfh cells with Slamf1 siRNA reduced the induced-expression of SAP caused by DEHP. These results suggested that a SLAMF1-SAP-dependent pathway might be involved in any DEHP-induced abnormal differentiation and/or hyperfunction of Tfh cells. It was interesting to note that there was no significant down-regulation of SAP expression after Slamf1 gene silencing in cells without any DEHP exposure. It is possible that SAP signaling transduction is controlled by multiple SLAM family members other than SLAMF1 under normal physiological conditions.
To further clarify whether the abnormal differentiation and hyperfunction of Tfh cells due to DEHP exposure was being mediated by SLAMF1 receptors, an ex vivo intervention model was used to investigate DEHP immunotoxicity in Tfh cells. In non-silenced Tfh cells from OVA-sensitized hosts, DEHP displayed an adjuvant effect in promoting expression of transcription factors (Bcl-6 and c-MAF) and cytokines (IL-21 and IL-4). Transfection of Tfh cells with Slamf1 siRNA attenuated these effects. Simply silencing Slamf1 gene without DEHP exposure had no influence on expression of these four proteins. These coordinated changes seen in the activated Tfh cells was not unexpected. It is known that Bcl-6 can induce substantial expression of surface markers and c-MAF can induce synthesis of IL-21 and IL-4 (Kroenke et al. Citation2012; Liu et al. Citation2012; Andris et al. Citation2017). The observed changes in relative expression of Tfh cells among all the splenic TH cells was also likely linked to the above-noted changes in Bcl-6 expression. There is evidence to show the Tfh cell differentiation program is closely dependent upon inter-actions among certain cooperative and antagonistic transcription factors (Lu et al. Citation2011; Cannons et al. Citation2013). Thus, when taken together, the data from the current study indicate that SLAMF1 might be the specific surface receptor for DEHP (and/or its metabolites) during any targeting of host Tfh cells, with any induced changes leading to abnormal differentiation and/or hyper-function.
Conclusions
The present study demonstrated that the environmental pollutant DEHP, via a SLAMF1-mediated pathway, could interferes with Tfh cell differentiation and cytokine secretion. The findings here provide a new theoretical basis to postulate novel mechanisms underlying DEPH immunotoxicity. Research building on these findings could eventually contribute to improving the prevention and/or treatment of DEHP-related allergic diseases.
Supplemental Material
Download MS Word (17 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andris F, Denanglaire S, Anciaux M, Hercor M, Hussein H, Leo O. 2017. The transcription factor c-Maf promotes the differentiation of follicular helper T-cells. Front Immunol. 8:480.
- Beko G, Callesen M, Weschler C, Toftum J, Langer S, Sigsgaard T, Host A, Kold Jensen T, Clausen G. 2015. Phthalate exposure through different pathways and allergic sensitization in pre-school children with asthma, allergic rhinoconjunctivitis and atopic dermatitis. Environ Res. 137:432–439.
- Braun J, Sathyanarayana S, Hauser R. 2013. Phthalate exposure and children's health. Curr Opin Pediatr. 25(2):247–254.
- Butler NS, Kulu DI. 2015. The regulation of T-follicular helper responses during infection. Curr Opin Immunol. 34:68–74.
- Cannons J, Lu K, Schwartzberg P. 2013. T-follicular helper cell diversity and plasticity. Trends Immunol. 34(5):200–207.
- Cannons J, Tangye S, Schwartzberg P. 2011. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 29:665–705.
- Chen S, Cai C, Li Z, Liu G, Wang Y, Blonska M, Li D, Du J, Lin X, Yang M, et al. 2017. Dissection of SAP-dependent and SAP-independent SLAM family signaling in NKT cell development and humoral immunity. J Exp Med. 214(2):475–489.
- Crotty S. 2014. T-follicular helper cell differentiation, function, and roles in disease. Immunity. 41(4):529–542.
- de Calisto J, Wang N, Wang G, Yigit B, Engel P, Terhorst C. 2014. SAP-dependent and -independent regulation of innate T-cell development involving SLAMF receptors. Front Immunol. 5:186.
- Dong Z, Davidson D, Perez-Quintero L, Kurosaki T, Swat W, Veillette A. 2012. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity. 36(6):974–985.
- Dragovich M, Mor A. 2018. The SLAM family receptors: Potential therapeutic targets for inflammatory and autoimmune diseases. Autoimmun Rev. 17(7):674–682.
- Erythropel H, Maric M, Nicell J, Leask R, Yargeau V. 2014. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol. 98(24):9967–9981.
- Galli S, Tsai M. 2012. IgE and mast cells in allergic disease. Nat Med. 18(5):693–704.
- Gao D, Wen Z. 2016. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ. 541:986–1001.
- Gensous N, Charrier M, Duluc D, Contin-Bordes C, Truchetet M, Lazaro E, Duffau P, Blanco P, Richez C. 2018. T-follicular helper cells in autoimmune disorders . Front Immunol. 9:1637.
- Gordiienko I, Shlapatska L, Kovalevska L, Sidorenko S. 2018. SLAMF1/CD150 in hematologic malignancies: Silent marker or active player? Clin Immunol. pii: S1521-6616(18)30437-6.
- Han Y, Wang X, Chen G, Xu G, Liu X, Zhu W, Hu P, Zhang Y, Zhu C, Miao J. 2014. Di-(2-ethylhexyl)-phthalate adjuvantly induces imbalanced humoral immunity in ovalbumin-sensitized BALB/c mice ascribing to T-follicular helper cells hyperfunction. Toxicology. 324:88–97.
- Holgate S. 2012. Innate and adaptive immune responses in asthma. Nat Med. 18(5):673–683.
- Hu J, Havenar-Daughton C, Crotty S. 2013. Modulation of SAP-dependent T:B-cell interactions as a strategy to improve vaccination. Curr Opin Virol. 3(3):363–370.
- Jogdand G, Mohanty S, Devadas S. 2016. Regulators of Tfh cell differentiation. Front Immunol. 7:520.
- Johns L, Cooper G, Galizia A, Meeker J. 2015. Exposure assessment issues in epidemiology studies of phthalates. Environ Intl. 85:27–39.
- Johnston R, Poholek A, DiToro D, Yusuf I, Eto D, Barnett B, Dent A, Craft J, Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T-follicular helper cell differentiation. Science. 325(5943):1006–1010.
- Kroenke M, Eto D, Locci M, Cho M, Davidson T, Haddad E, Crotty S. 2012. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 188(8):3734–3744.
- Li M, Chen C, Guo Y. 2017. Phthalate esters and childhood asthma: A systematic review and congener-specific meta-analysis. Environ Pollut. 229:655–660.
- Liu X, Yan X, Zhong B, Nurieva RI, Wang A, Wang X, Martin-Orozco N, Wang Y, Chang SH, Esplugues E, et al. 2012. Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med. 209(10):1841–1852.
- Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, Anderson SM, Wei L, Sun H, O'Shea JJ, et al. 2011. Functional and epigenetic studies reveal multi-step differentiation and plasticity of in vitro-generated and in vivo-derived follicular T-helper cells. Immunity. 35(4):622–632.
- Luthje K, Kallies A, Shimohakamada Y, Belz G, Light A, Tarlinton D, Nutt S. 2012. The development and fate of follicular helper T-cells defined by an IL-21 reporter mouse. Nat Immunol. 13(5):491–498.
- Ma C, Deenick E. 2011. The role of SAP and SLAM family molecules in the humoral immune response. Ann N Y Acad Sci. 1217:32–44.
- Ma C, Deenick E. 2014. Human T-follicular helper (Tfh) cells and disease. Immunol Cell Biol. 92(1):64–71.
- North M, Takaro T, Diamond M, Ellis A. 2014. Effects of phthalates on the development and expression of allergic disease and asthma. Ann Allergy Asthma Immunol. 112(6):496–502.
- Nurieva R, Chung Y, Martinez G, Yang X, Tanaka S, Matskevitch T, Wang Y, Dong C. 2009. Bcl6 mediates the development of T-follicular helper cells. Science. 325(5943):1001–1005.
- Proust R, Bertoglio J, Gesbert F. 2012. The adaptor protein SAP directly associates with CD3ζ chain and regulates T-cell receptor signaling. PloS One. 7(8):e43200.
- Qi H, Chen X, Chu C, Lu P, Xu H, Yan J. 2014. Follicular T-helper cells: Controlled localization and cellular interactions. Immunol Cell Biol. 92(1):28–33.
- Qin L, Waseem T, Sahoo A, Bieerkehazhi S, Zhou H, Galkina E, Nurieva R. 2018. Insights into the molecular mechanisms of T-follicular helper-mediated immunity and pathology. Front Immunol. 9:1884.
- Read K, Powell M, Oestreich K. 2016. T-follicular helper cell programming by cytokine-mediated events. Immunology. 149(3):253–261.
- Romero X, Sintes J, Engel P. 2014. Role of SLAM family receptors and specific adapter SAP in innate-like lymphocytes. Crit. Rev. Immunol. 34(4):263–299.
- Sahoo A, Wali S, Nurieva R. 2016. T-helper 2 and T-follicular helper cells: Regulation and function of interleukin-4. Cytokine Growth Factor Rev. 30:29–37.
- Samanta S, Mukherjee S. 2017. Microscopic insight into thermodynamics of conformational changes of SAP-SLAM complex in signal transduction cascade. J Chem Phys. 146(16):165103.
- Sawada S. 2012. SLAM-associated protein plays a key role in development of autoimmunity. Autoimmun Rev. 11(11):804–805.
- Ueno H, Banchereau J, Vinuesa C. 2015. Pathophysiology of T-follicular helper cells in humans and mice. Nat Immunol. 16(2):142–152.
- van Driel B, Liao G, Engel P, Terhorst C. 2016. Responses to microbial challenges by SLAMF receptors. Front Immunol. 7:4.
- Varricchi G, Harker J, Borriello F, Marone G, Durham S, Shamji M. 2016. T-follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy. 71(8):1086–1094.
- Vilar M, Frutuoso M, Arruda S, Lima D, Bezerra C, Pompeu M. 2011. The role of the SLAM-SAP signaling pathway in the modulation of CD4+ T-cell responses. Braz J Med Biol Res. 44(4):276–282.
- Wali S, Sahoo A, Puri S, Alekseev A, Nurieva R. 2016. Insights into the development and regulation of T-follicular helper cells. Cytokine. 87:9–19.
- Wang I, Lin C, Lin Y, Hsieh W, Chen P. 2014. Early-life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ Intl. 62:48–54.
- Wang R, Wang Q, Ma C, Li S, Han R. 2018. Phthalates in soft glass (a soft transparent PVC plastic sheet used extensively in household and public place in developing countries in recent years): Implication for oral exposure to young children. Chemosphere. 211:861–866.
- Webb L, Linterman M. 2017. Signals that drive T-follicular helper cell formation. Immunology. 152(2):185–194.
- Wittassek M, Angerer J. 2008. Phthalates: Metabolism and exposure. Int J Androl. 31(2):131–138.
- Wu N, Veillette A. 2016. SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol. 38:45–51.
- Yusuf I, Kageyama R, Monticelli L, Johnston R, Ditoro D, Hansen K, Barnett B, Crotty S. 2010. Germinal center T-follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol. 185(1):190–202.
- Zhao F, Cannons J, Dutta M, Griffiths G, Schwartzberg P. 2012. Positive and negative signaling through SLAM receptors regulate synapse organization and thresholds of cytolysis. Immunity. 36(6):1003–1016.
- Zhong M, Veillette A. 2013a. Critical role of SAP in progression and reactivation but not maintenance of T-cell-dependent humoral immunity. Mol Cell Biol. 33(6):1223–1232.
- Zhong M, Veillette A. 2013b. The adaptor molecule signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) is essential in mechanisms involving the Fyn tyrosine kinase for induction and progression of collagen-induced arthritis. J Biol Chem. 288(44):31423–31436.
