Abstract
Early-life exposure to arsenic (As) increases risks of respiratory diseases/infections in children. However, data on the ability of the innate immune system to combat bacterial infections in the respiratory tracts of As-exposed children are scarce. To evaluate whether persistent low-dose As exposure alters innate immune function among children younger than 5 years-of-age, mothers and participating children (N = 51) that were members of the Health Effects of Arsenic Longitudinal Study (HEALS) cohort in rural Bangladesh were recruited. Household water As, past and concurrent maternal urinary As (U-As) as well as child U-As were all measured at enrollment. In addition, U-As metabolites were evaluated. Innate immune function was examined via measures of cathelicidin LL-37 in plasma, ex vivo monocyte-derived-macrophage (MDM)-mediated killing of Streptococcus pneumoniae (Spn), and serum bactericidal antibody (SBA) responses against Haemophilus influenzae type b (Hib). Cyto-/chemokines produced by isolated peripheral blood mononuclear cells (PBMC) were assayed using a Multiplex system. Multivariable linear regression analyses revealed that maternal (p < 0.01) and child (p = 0.02) U-As were positively associated with plasma LL-37 levels. Decreased MDM-mediated Spn killing (p = 0.05) and SBA responses (p = 0.02) were seen to be each associated with fractions of mono-methylarsonic acid (MMA; a U-As metabolite) in the children. In addition, U-As levels were seen to be negatively associated with PBMC formation of fractalkine and IL-7, and positively associated with that for IL-13, IL-17 and MIP-1α. These findings suggested that early-life As exposure may disrupt the innate host defense pathway in these children. It is possible that such disruptions may have health consequences later in life.
Introduction
Epidemiological studies have shown increased risk of morbidity in children, particularly related to respiratory tract infections (RTI) and diarrheal diseases, due to chronic and early life arsenic (As) exposure (Raqib et al. Citation2009; Sanchez et al. Citation2016; Rahman et al. Citation2017). Emerging evidence indicates that As has multiple harmful effects on immune regulation and its surveil-lance system which potentially amplify susceptibility to many infectious diseases as well as other immune-related health outcomes (Ferrario et al. Citation2016). Arsenic can act as immunostimulant to cause an elevation/perpetuation of inflammatory responses harmful to a host. The immunosuppressive effects of As include modulation of the numbers, functions and survival of immune and hematopoietic cells, and impaired humoral and cell-mediated immunity (Ferrario et al. Citation2016). The majority of these findings are based on in vitro and experimental studies. Limited informa-tion is available on As-induced innate immune modulation in humans, particularly in children.
Macrophages are crucial regulators of innate immunity that bridge the innate and adaptive immune systems. Experimental and in vitro studies have shown that toxic effects of As on macrophages include hindering of differentiation of monocytes into macrophages, as well as of macrophage antigen-presenting capacity and bactericidal\phagocytic abilities (Sengupta and Bishayi Citation2002; Bishayi and Sengupta Citation2003; Lemarie et al. Citation2006). However, studies of macrophage function in humans exposed to As during early life are lacking. Evolutionarily-conserved host defense peptides (HDP) are considered effectors of innate immunity secreted by various cells types including macrophages. There are two major classes in mammals, i.e. defensins and cathelicidins. Humans have only one cathelicidin, LL-37, that has broad-spectrum antibacterial, chemotactic, and immunomodulatory properties (van Harten et al. Citation2018). Both β-defensins and LL-37 play major roles in host responses against pulmonary pathogens related to bronchiectasis, recurrent airways infections, and in the pathogeneses of chronic obstructive pulmonary diseases (COPD) (Persson et al. Citation2017). To date, only a single study has reported an association between As exposure and altered β-defensin-1 levels in humans (Hegedus et al. Citation2008).
Serum bactericidal antibody (SBA) are a major arm of the overall innate immune system. SBA responses measure functional antibody formation against various bacterial pathogens and are used to evaluate immunogenicity of bacterial vaccines as a correlate of protection (Jang et al. Citation2016; Shimanovich et al. Citation2017). In Bangladeshi patients with As-induced skin lesions, levels of complement-mediated SBA and serum concentrations of complement 3 (C3) were found to be significantly lower as compared to in healthy unexposed controls (Islam et al. Citation2012). Higher expression of inflammatory cytokines have been found in individuals chronically-exposed to As (Ahmed et al. Citation2014; Dutta et al. Citation2015), although formation of T-cell cytokines was suppressed (Biswas et al. Citation2008; Martin-Chouly et al. Citation2011; Ahmed et al. Citation2014).
Against this backdrop, it was hypothesized that chronic As exposure modulates the induction of innate immune responses in young children. The study reported here determined the effect of early-life exposures to As on innate immunity in rural Bangladeshi children ≤ 5 yr-of-age who had been repeatedly exposed to As in their drinking water and food. The evaluations performed encompassed analyses of levels of As metabolites in urine, serum LL-37, as well as ex vivo measures of monocyte-derived-macrophage (MDM)-mediated Streptococcus pneumoniae (Spn) killing, serum bactericidal antibody (SBA) responses against Haemophilus influenzae type b (Hib), and cytokines production by isolated peripheral blood mononuclear cells (PBMC). Individuals who excrete relatively higher proportions of MMA and lower proportions of DMA exhibit a higher propensity for As-related adverse health outcomes (Vahter Citation2002) that may be a consequence of altered immune function.
Materials and methods
Study area and subjects
Mothers of participating children were members of the Health Effects of Arsenic Longitudinal Study (HEALS; established in 2000) cohort in Araihazar, Bangladesh. At baseline, the HEALS participants had already been chronically-exposed to a wide range of As levels in their drinking water (Chen et al. Citation2009), though subsequent exposure has declined dramatically since study initiation (Huhmann et al. Citation2019). The purpose of the HEALS was to investigate health outcomes associated with As exposure via drinking water. All participants underwent health assessments and personal interviews every 2–3 years during home visits by trained study personnel. Well water for each household was tested for As at baseline and during each follow-up visit. Blood and urine samples were also collected from participants using the protocols of Chen et al. (Citation2009) and Huhmann et al. (Citation2019).
The current study included 51 healthy children (≤ 5 yr-of-age) and their mothers who were already participants in the HEALS cohort. A total of 60 children were randomly identified from the HEALS central database. There were no unexposed children examined in the current study. Written informed consent was obtained from all mothers of the participating children. Of the original 60, 3 were above the desired age range, 2 were ill, and 2 were unwilling to donate blood. In addition, 2 blood samples were discarded due to insufficient volume or inadequate numbers of isolatable PBMC. Body weight (in light clothes and barefoot) was measured to the nearest 0.1 kg (digital scale) and height was measured (stadiometer). These parameters were converted to height-for-age, weight-for-age, and body mass index-for-age Z-scores (SD scores), using the WHO growth reference for school-aged children and adolescents (de Onis et al. Citation2007). This study was approved by the Bangladesh Medical Research Council (BMRC); Columbia University also provided the ethical clearance for the study.
Water and urine sample collection and arsenic assessment
Household water samples that had been collected in trace element-free polyethylene bottles were analyzed by high-resolution inductively-coupled plasma mass spectrometry (HR ICP-MS) as previously described (van Geen et al. Citation2007). The detection limit of the method was 0.1 μg/L; the standard deviation of a single measurement was conservatively estimated at 4 µg/L.
Spot urine samples were collected in 50-ml acid-washed tubes and stored at −80 °C until shipped to Columbia University on dry ice. Urinary As analyses were performed by a graphite furnace atomic-absorption (GFAA) using a Perkin-Elmer Analyst 600 graphite furnace system. The detection limit was 2 µg U-As/L. Urinary creatinine was analyzed by a colorimetric method based on the Jaffe reaction. To compensate for variations in hydration status, total U-As concentration was adjusted with creatinine and expressed as µg As/g creatinine (µg/g). Maternal U-As collected 2–4 years prior was considered as past exposure and referred to as maternal U-Aspast, and U-As during enrollment in the study as maternal U-Asconcurrent.
Inorganic As (iAs) is metabolized by a series of reduction and methylation reactions producing mono-methylarsonic acid (MMA) and di-methylarsinic acid (DMA). Urinary As metabolites in samples were assayed by ICP-MS-DRC coupled to a high performance liquid chromatography (HPLC) system as previously described (van Geen et al. Citation2007). ICP-MS-DRC was used as a detector for As metabolites that had been chromatographically separated over a PRP-X100 Anion Exchange Column (Hamilton, Reno, NV) using 10 mM ammonium nitrate-ammonium phosphate solution (pH 9.1) as the mobile phase. The system allowed detection of inorganic As (i.e. As+3 and As+5), total MMA, and total DMA. The extent of As methylation efficiency was assessed by calculating relative amounts (%) of the metabolites in each child’s urine. Total arsenic was calculated based on the concentration of the sum of iAs, MMA, and DMA in the urine.
Blood samples
Peripheral blood samples (5 ml) from all the As-exposed children were collected into Li-Heparin-coated tubes (SARSTEDT, Nümbrecht, Germany) and then stored/transferred in cool boxes (within 2–3 h of collection) to the Laboratory of the International Center for Diarrheal Disease, Bangladesh (icddr,b) in Dhaka. Plasma and PBMC were separated from whole blood using Ficoll-Paque Plus density gradient centrifugation (Sigma, St. Louis, MO). Isolated plasma was stored at −80 °C for later use in analyses of LL-37 and bactericidal activity (see below). Isolated PBMC from these samples were cultured for use in evaluations of monocyte-derived-macrophage (MDM) killing activities (see below).
LL-37 measurement
Human cathelicidin host defense peptide LL-37 acts as a first line of defense effector molecule in the innate immune system. Concentrations of LL-37 in the isolated plasma samples were measured by ELISA (Hycult Biotechnology, Uden, Netherlands), according to manufacturer protocols. The lower limit of detection of the kit was 0·14 ng/ml; the intra- and inter-assay CV was 4.96 and 7.89%, respectively. All samples were measured in duplicate.
Monocyte-derived-macrophage (MDM) culture
Measures of bacteriolytic activity against a respiratory pathogen by MDM generated from cells isolated from the As-exposed children were done as described in Gordon et al. (Citation2000). In the present study, Gram-positive Streptococcus pneumoniae (Spn), a major cause of community-acquired pneumonia and meningitis in children (DeAntonio et al. Citation2016), was the test pathogen. In brief, isolated PBMC were washed and suspended in enriched culture medium (RPMI 1640 supplemented with 10% autologous plasma, 1% L-glutamine, 1% pyruvate, 1% penicillin-streptomycin) (all Gibco, Grand Island, NY). Thereafter, cells (5 x 106 cells/well) were plated into 4-well cell culture plates (NUNC, Roskilde, Denmark). After 2 h incubation in 37 °C in a 5% CO2 incubator, the supernatants containing non-adherent cells (mostly lymphocytes) were removed (see below for cytokine analysis), leaving the adherent cells (mostly monocytes) attached to the plastic surface. These cells were cultured for 7 d, and then treated (dose in well = 5 μg/ml) with bacterial LPS (lipopolysaccharide from Escherichia coli Type 011:B4, Sigma). Other matched cells received vehicle only (no stimulant). After 48 h of incubation, the MDM were then washed and incubated with enriched RPMI medium containing an additional 10% (w/v) bovine serum albumin (BSA, Sigma) for 30 min at 37 °C. The supernatant in the well was removed and the MDM washed again with enriched media prior to use in the protocol below.
MDM-mediated killing assay
Type 1 Streptococcus pneumoniae (Spn) (ATCC 49616; Manassas, VA) was grown to mid-log phase in brain heart infusion (BHI) broth with 20% fetal calf serum (ThermoFisher Scientific, Waltham, MA). A suspension of Spn was prepared to a concentration of 5 x 107 colony-forming units (CFU) at an absorbance of 0.6 (at 600 nm) and stored at −80 °C for later use. For the assay, the bacterial cells were opsonized before use for the killing assay. The frozen cells were thawed and pelleted by centrifugation (10 000 rpm, 5 min), washed three times with RPMI and re-pelleted. For opsonization, the bacterial pellet was re-suspended in 10% autologous/pooled plasma or 10% phosphate-buffered saline (PBS, pH 7.4; control) and incubated at 37 °C in a shaker incubator (at 120 rpm) for 30 min. Thereafter, the opsonized bacterial cells were pelleted and washed by centrifugation. To evaluate killing activity, the MDM in the wells described above were infected with Spn at a multiplicity of infection of 100 (100 bacteria/one macrophage) and the plates were then incubated for 1 h at 4 °C. At that point, extracellular fluid (ECF) containing non-ingested bacteria was collected. The now-infected MDM were washed three times with warm RPMI and then further incubated in media with 10% autologous plasma for 20 min at 37 °C. After this, the infected macrophages were lysed by addition of 2% saponin in RPMI to the wells to cause the release of all viable intracellular bacteria. Intracellular fluid (ICF) containing bacteria in the cell lysates in each well was collected with vigorous aspiration and then centrifuged 5 min at 10 000 rpm. Both ECF and ICF were cultured on chocolate agar plates overnight at 37 °C and under 5% CO2; the total colonies (CFU) of the Spn were then counted.
The number of adhered Spn (bound to macrophage surface/ingested by cells) was calculated by subtracting the number of viable Spn in ECF from the number of Spn inoculated/well. The percentage of killing of internalized bacteria by MDM (% killing capacity) was calculated as 100% x ([number adhered\ingested bacteria _ number of viable bacteria in ICF]/number of adhered\ingested bacteria). The “relative CFU count” was calculated for each participant, i.e. ratio of CFU found with ICF of LPS-stimulated cells compared to that in ICF of unstimulated cells, to account for inter-subject variations in the baseline (unstimulated) killing activity of MDM (Gordon et al. Citation2000).
Serum bactericidal antibody (SBA) response
The SBA assay is an important in vitro method for measuring complement-mediated functionality of natural/vaccine-induced antibodies targeting pathogens (Ercoli et al. Citation2015). Gram-negative Haemophilus influenzae type b (Hib) was selected for evaluation of acquired SBA responses as all the study children were vaccinated during infancy with Hib through the Expanded Program on Immunization (EPI) in Bangladesh. Each serum sample was incubated at 56 °C for 30 min to inactivate complement before being used in the assay. The exogenous complement source used in the assay was healthy young rabbit (male, 4–6-wk-of-age; Animal Resource Facility, icddr,b) sera.
Colonies of Hib type B (ATCC 49247) that had been grown on chocolate agar plates at 37 °C after receipt, were inoculated into Brain Heart Infusion (BHI, supplemented with 10 μg hemin/ml and 10 μg NAD+/ml [both Sigma]), and incubated for 2 h at 37 °C. Bacteria in mid-log phase were then harvested by centrifugation (12 000 x g, 4 °C) and washed twice with PBS. The final pellet was suspended in Hanks Buffer Salt Solution (HBSS) containing CaCl2 and MgCl2 and 2% BHI. The optical density of the suspension was adjusted to 0.41 (at 600 nm), a value which corresponded to 2.5 x 108 CFU/ml. The bacterial solution was then opsonized with rabbit complement. After washing, the bacteria were prepared for introduction (at 103 CFU/well) into sera and diluent media-containing wells of MaxiSorp flat bottom microtiter plates (Sigma) kept on ice. The plates were incubated 75 min at 37 °C in a 5% CO2 incubator and then the absorbance in each well was taken at 595 nm. Pooled sera was used as a positive control. As other controls, bacteria without sera, and sera plus complement without bacteria, were used.
Cytokines and chemokines in non-adherent cell culture supernatant
Subsets of cell samples (n = 16) from the As-exposed children were assayed for basal and inducible cyto-/chemokine production. Non-adherent cells (mostly lymphocytes) originally separated from the adherent PBMC cells were counted and plated into 96-well plates (at 106 cells/well) and then treated with 5 μg phytohemagglutinin/ml (PHA; Sigma) or medium only (control). The cells were cultured 48 h at 37 °C in a 5% CO2 incubator before well supernatants were collected for assessment of select cyto-/chemokines secreted by diverse cell types such as monocytes, dendritic cells, neutrophils, NK cells as well as T-helper (TH)-1 and TH2 cells (e.g. Fractalkine, GM-CSF, IFNγ, CXCL11, IL-1β, -2, -4, -5, -6, -7, -8, -10, -12 (p70), -13, -17 A, -21, -23, MIP1α, MIP1β, MIP-3α, TNFα) using a Milliplex MAP 384-Well High Sensitivity Human Cytokine Magnetic Bead Panel (Merck, Darmstadt, Germany). Cytokine data was expressed as the ratio of PHA-stimulated levels to control levels.
Statistical analyses
Statistical Package for Social Science (SPSS) for Windows (v(0).20; IBM, Armonk, NY) and Stata/IC (v(0).13, Stata Corp., Houston, TX) were used for data analysis. Demographic characteristics of the study participants were calculated based on child age, sex, body mass index (BMI), z-scores, and mother’s age and BMI. Urinary arsenic, LL-37, MDM killing was log-transformed since data did not follow the Gaussian distribution. Associations between exposures, outcomes, and covariates were initially evaluated using Spearman’s rank correlation coefficient (for continuous variables), Mann-Whitney U test, analysis of variance, or Kruskal-Wallis test (for categorical variables), as appropriate.
To evaluate As effects on participant LL-37, cytokines, MDM killing capacity, and SBA, multivariate linear regression was applied. The association between %MMA U-As and water As and outcomes (LL-37, SBA and MDM killing) was not linear. To evaluate potential effects of % MMA U-As and water arsenic on LL-37, SBA and MDM killing, the multivariate-adjusted regression analysis was restricted to the lowest tertile of As metabolites (i.e. reference group) and comparisons made with the second or third tertiles. The associations between %MMA and LL-37, MDM killing and SBA were measured by using multivariate linear regression. All the regression models were adjusted for covariates that were significantly associated with both exposure and outcomes or changed the effect estimate by 5% or more. These covariates were age, sex, weight for age z-score and mother’s BMI. A p values < 0.05 was considered significant.
Results
Study subjects
The mean age of the children was 4.15 [± 0.54] years, with an almost equal number of boys and girls (26 and 25, respectively). The children were mildly-malnourished (). Compared to the World Health Organization (WHO) reference standard, about 24% (n = 12) and 30% (n = 15) children were underweight and stunted, respectively. All children were examined by a physician and found to be healthy without any recent history of illness during enrollment. There were no unexposed children examined in the current study.
Table 1. Demographic and exposure characteristics of study children and mothers (N = 51).
The average age of the mothers at time of enrollment was 24 years. The median BMI of the mothers was 20.2 (15.9, 32.3) kg/m2, with 27% being undernourished and 16% overweight and/or obese. About 10% of the mothers completed at least 5 years of school, 65% had a high school level education, and 14% were illiterate.
Relationship of arsenic (as) concentration in water and urine
The median household W-As concentration (43.0 µg/L) found was below the Bangladesh maximum contaminant level of 50 µg/L; the range varied from 0.6 – 326 µg/L. About 55% (n = 28) of the household W-As levels were ≤ 50 µg/L and 45% (n = 23) were > 50 µg/L. Household W-As levels were seen to be strongly associated with maternal U-Asconcurrent (r = 0.51, p = 0.0001), U-Aspast (r = 0.56, p = 0.000), and child U-As (r = 0.37, p = 0.006) values. This indicated that the drinking water was the major source of As for the mothers and to a lesser extent for the children in the study. Urinary-As levels varied from relatively low to moderate in exposed children (U-As, median 55.0, IQR [40.0, 99.0]) µg/L) as well as in their mothers (present = median 61.0 [IQR 47.0, 138.0] µg/L and past = median 178.0 [IQR 64.0, 340.2] µg/L).
Maternal U-Aspast was as high as 197 μg/g (median) and declined to a median concentration of 114.3 μg/g at the time of the present study (p < 0.001). Concurrent U-As concentrations decreased in a majority of the mothers (79%), while the concentration increased in ≈ 21% of the mothers. Child U-As concentration (median with interquartile range, 39 (16, 102) μg/g) was significantly lower than maternal U-Asconcurrent (p = 0.01) and U-Aspast (p < 0.001) which reflected considerably reduced extent of As exposure among children under 5-yr-of-age.
Association of arsenic exposure with cathelicidin LL-37 in plasma
The mean plasma LL-37 concentration in children was 25.6 [± 14.2] ng/ml. A multi-variable adjusted linear regression analysis showed that maternal U-Asconcurrent (β-coefficient = 0.20; 95% confidence interval [CI] = 0.03, 0.37; p = 0.02), U-Aspast (β = 0.27; 95% CI = 0.06, 0.49; p = 0.01), and child U-As (β = 0.17; 95% CI = 0.02, 0.32; p = 0.02) values were positively associated with child plasma LL-37 levels. In stratified analyses by sex, height, and weight, the association was found to be stronger among boys, particularly in children of normal weight and height (). Household W-As of children was also positively-associated with plasma LL-37.
Table 2. Multivariable adjusted linear regression analysis of the association between urinary/water As and plasma LL-37, stratified by sex and anthropometric Z-score.
Table 3. Association of As exposure with culture supernatant cyto-/chemokine levels (n = 16).
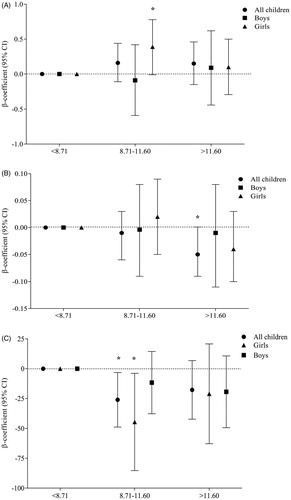
To evaluate the influence of child As metabolites in urine (iAs, MMA, or DMA) with plasma LL-37 levels, each metabolite was categorized into tertiles. Compared to the lowest tertile (i.e. the reference group) of %MMA, plasma LL-37 concentrations in the second tertile increased significantly mainly in girls (p = 0.05) (). This suggested a possible non-monotonic relationship between As and the %MMA.
Figure 1. Association of tertiles of %MMA with (A) plasma LL-37, (B) MDM killing capacity, and (C) serum bactericidal antibody (SBA) response, stratified by sex. “p” was determined by multi-variate-adjusted regression analysis and the model was adjusted by child age, sex, WAZ (weight for age z-score), and mother BMI (body mass index). Plasma LL-37 concentrations in the second tertile of %MMA was significantly higher than the lowest tertile (reference group) in girls (*p = 0.05). MDM-mediated killing capacity was significantly lower (*p = 0.05) in the highest fraction of MMA compared to the lowest. SBA responses in the second tertile of %MMA was significantly lower than the first tertile in all children (*p = 0.02) and in girls (*p = 0.03). MMA: mono-methylarsonic acid; MDM: monocyte-derived macrophage; SBA: serum bactericidal antibody response.
Monocyte-derived macrophage (MDM) mediated spn killing
No association was found between MDM-mediated Spn killing and As exposure (W-As, maternal U-As, child U-As). There was no influence of nutritional status (stunting or under-weight) or sex in the association between As exposure and MDM-mediated killing. However, when the association by child %MMA was stratified in tertiles, MDM-mediated Spn killing capacity was significantly reduced (p = 0.05) in the highest %MMA fraction compared to the lowest ().
Serum bactericidal antibody (SBA) response against hib
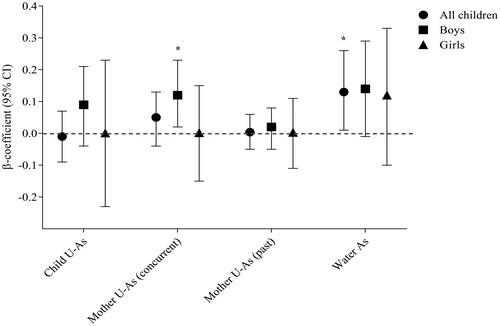
In the multivariable adjusted linear regression analysis, a significant positive association was obtained between child SBA response and household W-As (p = 0.04) and between SBA in boys and maternal U-Asconcurrent (p = 0.02). No association was found between SBA and current As exposure of children (). However, a positive association between W-As and SBA was pronounced among boys and in children of normal weight. When the associations between As metabolites and SBA were stratified by child %MMA in tertiles, SBA responses in the second %MMA tertile decreased considerably compared to the first (β = −26.05, 95% CI = −48.83, −3.26; p = 0.02) (); the impact was stronger in the girls (β = −44.60, 95% CI = −85.34, −3.86; p = 0.03).
Figure 2. Association of arsenic exposure (child U-As, mother’s concurrent U-As, mother’s past U-As, and household water arsenic) with SBA response. Analysis was performed stratifying by sex. “p” was determined by multivariate-adjusted regression analysis and the model was adjusted by child age, sex, WAZ (weight for age z-score), and mother BMI (body mass index). Significant positive associations were obtained between household W-As and SBA response in all children (*p = 0.04) and between maternal U-Asconcurrent and SBA response in boys (*p = 0.02).
Associations of as exposure with ex vivo lymphocyte/PBMC cytokine/chemokine formation
PHA stimulation of isolated lymphocytes (predominantly non-adherent cells as described above) from the As-exposed children led to significant induction of IL-10, IL-13, IL-17A, IL-2, IL-21, IL-5 and MIP-1α compared to unstimulated counterpart controls from the same hosts (data not shown).
Multivariable regression analysis of As exposure and culture supernatant cyto-/chemo-kine levels showed there were negative associations between child U-As and concentrations of fractalkine (CX3CL) (β = −0.47; 95% CI = −0.88, −0.05; p = 0.03) as well as tumor necrosis factor (TNF)-α (β = −3.30, 95% CI = −7.20, 0.43; p = 0.08). Inverse associations were also detected between household W-As or maternal U-Aspast and IL-7 concentrations associated with the children’s cells (). On the other hand, strong positive associations were observed between maternal U-Asconcurrent and children’s cell IL-13, IL-17A, and MIP-1α levels. No significant associations were noted between As exposure and the other cytokines in the panel.
Discussion
The findings of this study suggest that early childhood As exposure dysregulates fundamental processes of innate immunity in these children. Serum bactericidal antibody responses and LL-37 levels increased with increasing As exposure, but without any apparent change in macrophage-mediated killing activities. Expression of some cytokines was also impacted by the As exposure. In addition, poor As methylation efficiency was seen to be associated with reduced innate immune function in these children.
A recent long-term follow-up study conducted in rural Bangladesh with widespread As contamination of groundwater reported higher risk of deaths due to respiratory, cardiovascular, and cerebrovascular diseases (Rahman et al. Citation2019). Accumulating evidence suggests that As exposure disrupts immune function and contributes to chronic lung diseases (Parvez et al. Citation2008; Ahmed et al. Citation2017). Inflammation orchestrated by inflammatory and other immune cells is a crucial component of pulmonary diseases such as asthma, COPD, tuberculosis, and lung cancers (Coussens and Werb Citation2002; Leepiyasakulchai et al. Citation2012; Górska et al. Citation2017). In turn, each of these pathologies can be exacerbated by As-induced inflammatory responses (Olivas-Calderon et al. Citation2015).
An important role for the defense peptide LL-37 in the pathophysiology of chronic lung diseases is gradually being recognized. Recent studies have linked changes in LL-37 expression with increased risk of airway inflammation, lung infections, and acute exacerbation of COPD (Jiang et al. Citation2012; Persson et al. Citation2017). Recent studies suggested LL-37 could promote release of inflammatory IL-8 and proliferation of non-specifically-activated CD4+ T-cells, actions that may ultimately induce apoptosis of bronchial and alveolar epithelial cells (Jiang et al. Citation2012; Thomi et al. Citation2018). These findings are relevant in the context of inflammation-mediated chronic lung diseases. In contrast, another study reported high risk of frequent exacerbations in COPD patients with low plasma LL-37 levels (Yang et al. Citation2015).
In the study, plasma LL-37 levels increased with increasing As exposure, predominantly in children of normal weight and height. It is likely that with chronic As exposure, LL-37 levels may remain elevated in the systemic circulation owing to its role in innate immune surveillance and chemoattractant activity. Elevated LL-37 levels, in turn, may promote inflammation and have long-lasting consequences for decreased lung function (Jiang et al. Citation2012) or chronic lung disease later in life (Sanchez et al. Citation2016; Rahman et al. Citation2017). The stimulating effects of As exposure on LL-37 levels may be masked by multiple stress factors in growth-retarded children, thus hindering the optimum response. However, this hypothesis needs to be tested in larger cohorts of children with both a wider age range and As exposure levels.
The SBA is as an innate host defense mechanism against pathogens; however, recently it is being increasingly used as a tool to assess functional antibody responses against infection or vaccination (Rahman et al. Citation2005; Jang et al. Citation2016; Shimanovich et al. Citation2017). An earlier study from our group showed that there was a reduced antibody response to live attenuated mumps vaccination among children with high vs. low levels of As exposure (Raqib et al. Citation2017). In a NHANES study among adults, chronic As exposure was associated with lower protective humoral immunity to herpes zoster (Cardenas et al. Citation2015). However, another NHANES study showed a positive association between U-As and total anti-hepatitis A virus seroprevalence (Cardenas et al. Citation2016). Again, no significant difference was noted in SBA responses against oral cholera vaccine in 2-5-yr-old Bangladeshi children with high As exposure (mean U-As, 292 µg/L) compared to those with low exposure (mean U-As, 6.60 µg/L) (Saha et al. Citation2013). However, in these same children, diphtheria and tetanus vaccine-specific IgG titers were higher in the high As exposure group. The current findings are not directly comparable with other studies as those were mostly used for vaccine efficacy and so there were methodological differences. However, it is conceivable that to compensate for suppressed cellular immunity as a result of chronic As exposure (Ahmed et al. Citation2014; Mannan et al. Citation2018; Yu et al. Citation2018), humoral immunity is disproportionately activated, as in HIV-infected individuals (Shearer et al. Citation2000).
Experimental studies revealed that low-to-moderate levels of As influenced monocyte differentiation, compromised antigen presentation, reduced phagocytic efficiency, and altered the chemotactic indices of macrophages (Sengupta and Bishayi Citation2002; Lemarie et al. Citation2006; Ferrario et al. Citation2016). There are limited studies on effects of in vivo As exposure on human macrophage function (Banerjee et al. Citation2009), especially intracellular killing capacity. Inefficient As metabolism has been associated with As-related adverse health effects in adults and altered immune function in children (Tseng Citation2009; Ahmed et al. Citation2017; Mannan et al. Citation2018). Consistent with this, the present study also found As methylation efficiency was related to reduced MDM-mediated Spn killing capacity and SBA responses in 2-5-yr-old children.
The findings of imbalanced CX3CL expression due to chronic As exposure may reflect perturbed immunostasis and impaired recruitment or survival of immune/inflammatory cells to counteract an infection or toxic insult (Liu et al. Citation2016). Reduced plasma IL-7 levels in relation to As exposure was seen previously in individuals living in rural areas with As-contaminated drinking water (Raqib et al. Citation2009). The IL-7 results are supported by other studies showing toxic effects of MMA+3 and As+3 in suppressing early T-cell development in the thymus through inhibition of IL-7 signaling pathways especially at low-to-moderate levels of As (Xu et al. Citation2016).
A central role of IL-13 in mediating airway hyper-reactivity and mucus expression in asthma is well known (Newcomb et al. 2012). Studies have also suggested a link between increases in IL-17A production and severe asthma as well as lung infections; it is believed IL-17A contributes to asthma pathophysiology by increasing the capacity of IL-13 to activate intra-cellular signaling pathways (Hall et al. Citation2017). Our recent work (Parvez et al. Citation2019) has found a similar effect of As on IL-17A among adults from the same study area in Bangladesh. Whether increased expression of IL-13 and IL-17A in response to high As exposure also contributes to respiratory illness in the As-exposed children remain to be determined. Lastly, MIP-1α is also important in the pathogeneses of various inflammatory diseases (Serody et al. Citation2000; Bhavsar et al. Citation2015). Accordingly, chronic As exposure may lead to a state of persistent inflammation through imbalanced cytokine expression by various cell types and contribute to the pathogenesis of chronic lung diseases. However, no changes in TH1 and TH2 cytokines in relation to As exposure were noted in the present study.
The current study has few limitations. Due to its pilot nature, the sample size was small and so inferring a conclusion is not feasible. However, the findings do suggest directions for further research. One limitation was that in utero exposure data of children was not available, though data from the mothers’ past exposures (2–4 years back) suggested a continued persistent exposure of their children to As. In addition, the range of As exposures was relatively small (median 39 µg/g) compared to that in other as well as our own studies (>79 µg/L) (Islam et al. Citation2012; Saha et al. Citation2013; Ahmed et al. Citation2014, Citation2017; Olivas-Calderon et al. Citation2015; Raqib et al. Citation2017).
In summary, the present findings suggest that early-life exposure to As disrupts innate host defense pathway in children ≤ 5 yr-of-age by affecting SBA responses and plasma LL-37 levels. These findings, albeit preliminary, indicate that children exposed to As may face long-term respiratory health consequences. Poor As methylation efficiency in the children was also found to adversely affect SBA and macrophage-mediated killing of respiratory pathogens. The study also indicated that persistent As exposure may compromise host defenses and innate immune surveillance, including the formation of key peptides needed for use against infections. Findings from this study warrant further studies of respiratory infections and chronic lung diseases in As-exposed populations.
Acknowledgements
The authors would like to thank all the HEALS staff and field-workers for their ongoing commitment to the study.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Ahmed S, Akhtar E, Roy A, von Ehrenstein O, Vahter M, Wagatsuma Y, Raqib R. 2017. Arsenic exposure alters lung function and airway inflammation in children: A cohort study in rural Bangladesh. Environ Intl. 101:108–116.
- Ahmed S, Moore S, Kippler M, Gardner R, Hawlader M, Wagatsuma Y, Raqib R, Vahter M. 2014. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicol Sci. 141(1):166–175.
- Banerjee N, Banerjee S, Sen R, Bandyopadhyay A, Sarma N, Majumder P, Das J, Chatterjee M, Kabir S, Giri A. 2009. Chronic arsenic exposure impairs macrophage functions in the exposed individuals. J Clin Immunol. 29(5):582–594.
- Bhavsar I, Miller C, Sabbagh M. 2015. Macrophage inflammatory protein (MIP)-1α/CCL3 as a biomarker. In Preedy V, and Patel V, editors. General methods in biomarker research and their applications. Biomarkers in disease: Methods, discoveries and applications. Dordrecht, Germany: Springer; p. 223–249.
- Bishayi B, Sengupta M. 2003. Intracellular survival of Staphylococcus aureus due to alteration of cellular activity in arsenic- and lead-intoxicated mature Swiss albino mice. Toxicology. 184(1):31–39.
- Biswas R, Ghosh P, Banerjee N, Das J, Sau T, Banerjee A, Roy S, Ganguly S, Chatterjee M, Mukherjee A. 2008. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum Exp Toxicol. 27(5):381–386.
- Cardenas A, Smit E, Bethel J, Houseman E, Kile M. 2016. Arsenic exposure and the seropreva-lence of total hepatitis A antibodies in the US population: NHANES, 2003-2012. Epidemiol Infect. 144(8):1641–1651.
- Cardenas A, Smit E, Houseman E, Kerkvliet N, Bethel J, Kile M. 2015. Arsenic exposure and prevalence of the varicella zoster virus in the United States: NHANES (2003-2004 and 2009-2010). Environ Health Perspect. 123(6):590–596.
- Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, Graziano J, Ahsan H. 2009. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: Review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 239:184–192.
- Coussens L, Werb Z. 2002. Inflammation and cancer. Nature. 420(6917):860–867.
- de Onis M, Onyango A, Borghi E, Siyam A, Nishida C, Siekmann J. 2007. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 85(9):660–667.
- DeAntonio R, Yarzabal J-P, Cruz JP, Schmidt JE, Kleijnen J. 2016. Epidemiology of community-acquired pneumonia and implications for vaccination of children living in developing and newly industrialized countries: A systematic literature review. Hum Vaccin Immunother. 12(9):2422–2440.
- Dutta K, Prasad P, Sinha D. 2015. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Intl J Hyg Environ Health. 218(6):564–574.
- Ercoli G, Baddal B, Alessandra G, Marchi S, Petracca R, Arico B, Pizza M, Soriani M, Rossi-Paccani S. 2015. Development of a serological assay to predict antibody bactericidal activity against non-typeable Haemophilus influenzae. BMC Microbiol. 15:87.
- Ferrario D, Gribaldo L, Hartung T. 2016. Arsenic exposure and immunotoxicity: A review including the possible influence of age and sex. Curr Environ Health Rep. 3(1):1–12.
- Gordon S, Irving G, Lawson R, Lee M, Read R. 2000. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 68(4):2286–2293.
- Górska K, Paplińska-Goryca M, Nejman-Gryz P, Goryca K, Krenke R. 2017. Eosinophilic and neutrophilic airway inflammation in the phenotyping of mild-to-moderate asthma and chronic obstructive pulmonary disease. Copd. 14(2):181–189.
- Hall SL, Baker T, Lajoie S, Richgels PK, Yang Y, McAlees JW, van Lier A, Wills-Karp M, Sivaprasad U, Acciani TH, et al. 2017. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription-6 activation. J Allergy Clin Immunol. 139(2):462–471.
- Hegedus CM, Skibola CF, Warner M, Skibola DR, Alexander D, Lim S, Dangleben NL, Zhang L, Clark M, Pfeiffer RM, et al. 2008. Decreased urinary β-defensin-1 expression as a biomarker of response to arsenic. Toxicol Sci. 106(1):74–82.
- Huhmann B, Harvey C, Navas-Acien A, Graziano J, Parvez F, Chen Y, Argos M, Ahmed A, Hasan A, Ahsan H, et al. 2019. Changes in arsenic exposure in Araihazar, Bangladesh from 2001 through 2015 following a blanket well testing and education campaign. Environ Int. 125:82–89.
- Islam L, Zahid M, Nabi A, Hossain M. 2012. Function of serum complement in drinking water arsenic toxicity. J Toxicol. 2012:302817.
- Jang M, Sahastrabuddhe S, Yun C, Han S, Yang J. 2016. Serum bactericidal assay for evaluation of typhoid vaccine using a semi-automated colony-counting method. Microb Pathogen. 97:19–26.
- Jiang Y, Xiao W, Zhu M, Yang Z, Pan X, Zhang Y, Sun C, Xing Y. 2012. The effect of human antibacterial peptide LL-37 in the pathogenesis of chronic obstructive pulmonary disease. Respir Med. 106(12):1680–1689.
- Leepiyasakulchai C, Ignatowicz L, Pawlowski A, Kallenius G, Skold M. 2012. Failure to recruit anti-inflammatory CD103+ dendritic cells and a diminished CD4+Foxp3+ regulatory T-cell pool in mice that display excessive lung inflammation and increased susceptibility to Mycobac-terium tuberculosis. Infect Immun. 80(3):1128–1139.
- Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L. 2006. Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol. 177(5):3019–3027.
- Liu W, Jiang L, Bian C, Liang Y, Xing R, Yishakea M, Dong J. 2016. Role of CX3CL1 in diseases. Arch Immunol Ther Exp. 64(5):371–383.
- Mannan T, Ahmed S, Akhtar E, Ahsan K, Haq A, Kippler M, Vahter M, Raqib R. 2018. Associations of arsenic exposure with telomere length and naïve T-cells in childhood-A birth cohort study. Toxicol Sci. 164(2):539–549.
- Martin-Chouly C, Morzadec C, Bonvalet M, Galibert M, Fardel O, Vernhet L. 2011. Inorganic arsenic alters expression of immune and stress response genes in activated primary human T-lymphocytes. Mol Immunol. 48(6–7):956–965.
- Newcomb D, Boswell M, Huckabee M, Goleniewska K, Dulek D, Reiss S, Lukacs N, Kolls J, Peebles R. 2012. IL-13 regulates TH17 secretion of IL-17A in an IL-10-dependent manner. J Immunol. 188(3):1027–1035.
- Olivas-Calderon E, Recio-Vega R, Gandolfi A, Lantz R, Gonzalez-Cortes T, Gonzalez-De Alba C, Froines J, Espinosa-Fematt J. 2015. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol Appl Pharmacol. 287(2):161–167.
- Parvez F, Chen Y, Brandt-Rauf PW, Bernard A, Dumont X, Slavkovich V, Argos M, D’Armiento J, Foronjy R, Hasan MR, et al. 2008. Non-malignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect. 116(2):190–195.
- Parvez F, Lauer F, Factor-Litvak P, Liu X, Santella R, Islam T, Eunus M, Alam N, Sarwar G, Rahman M, et al. 2019. Assessment of arsenic and polycyclic aromatic hydrocarbon (PAH) exposures on immune function among males in Bangladesh. PLoS One. 14(5):e0216662
- Persson L, Anerud M, Hardie J, Miodini Nilsen R, Bakke P, Eagan T, Hiemstra P. 2017. Anti-microbial peptide levels are linked to airway inflammation, bacterial colonisation and exacerbations in chronic obstructive pulmonary disease. Eur Respir J. 49:3.
- Rahman A, Granberg C, Persson L. 2017. Early life arsenic exposure, infant and child growth, and morbidity: A systematic review . Arch Toxicol. 91(11):3459–3467.
- Rahman M, Sarker P, Roy S, Ahmad S, Chisti J, Azim T, Mathan M, Sack D, Andersson J, Raqib R. 2005. Effects of zinc supplementation as adjunct therapy on the systemic immune responses in shigellosis. Am J Clin Nutr. 81(2):495–502.
- Rahman M, Sohel N, Yunus F, Alam N, Nahar Q, Streatfield P, Yunus M. 2019. Arsenic exposure and young adult's mortality risk: A 13-year follow-up study in Matlab, Bangladesh. Environ Int. 123:358–367.
- Raqib R, Ahmed S, Ahsan K, Kippler M, Akhtar E, Roy A, Lu Y, Arifeen S, Wagatsuma Y, Vahter M. 2017. Humoral immunity in arsenic-exposed children in rural Bangladesh: Total immunoglobulins and vaccine-specific antibodies. Environ Health Perspect. 125(6):067006.
- Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque A, Nermell B, Yunus M, Roy S, Persson L, et al. 2009. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett. 185(3):197–202.
- Saha A, Chowdhury M, Nazim M, Alam M, Ahmed T, Hossain M, Hore S, Sultana G, Svennerholm A, Qadri F. 2013. Vaccine-specific immune response to an inactivated oral cholera vaccine and EPI vaccines in a high and low arsenic area in Bangladeshi children. Vaccine. 31(4):647–652.
- Sanchez T, Perzanowski M, Graziano J. 2016. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environ Res. 147:537–555.
- Sengupta M, Bishayi B. 2002. Effect of lead and arsenic on murine macrophage response. Drug Chem Toxicol. 25(4):459–472.
- Serody J, Burkett S, Panoskaltsis-Mortari A, Ng-Cashin J, McMahon E, Matsushima G, Lira S, Cook D, Blazar B. 2000. T-Lymphocyte production of macrophage inflammatory protein-1α is critical to the recruitment of CD8+ T-cells to the liver, lung, and spleen during graft-vs-host disease. Blood. 96(9):2973–2980.
- Shearer W, Easley K, Goldfarb J, Jenson H, Rosenblatt H, Kovacs A, McIntosh K, Group PCHS. 2000. Evaluation of immune survival factors in pediatric HIV-1 infection. Ann NY Acad Sci. 918:298–312.
- Shimanovich A, Buskirk A, Heine S, Blackwelder W, Wahid R, Kotloff K, Pasetti M. 2017. Functional and antigen-specific serum antibody levels as correlates of protection against shigellosis in a controlled human challenge study. Clin Vaccine Immunol. 24(2):e00412–16.
- Thomi R, Schlapbach C, Yawalkar N, Simon D, Yerly D, Hunger R. 2018. Elevated levels of the anti-microbial peptide LL-37 in hidradenitis suppurativa are associated with a TH1/TH17 immune response. Exp Dermatol. 27(2):172–177.
- Tseng C. 2009. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 235(3):338–350.
- Vahter M. 2002. Mechanisms of arsenic biotransformation. Toxicology. 181:211–217.
- van Geen A, Cheng Z, Jia Q, Seddique A, Rahman M, Rahman M, Ahmed K. 2007. Monitoring 51 community wells in Araihazar, Bangladesh, for up to 5 years: Implications for arsenic mitigation. J Environ Sci Health A. 42(12):1729–1740.
- van Harten R, van Woudenbergh E, van Dijk A, Haagsman H. 2018. Cathelicidins: Immunomodulatory anti-microbials. Vaccines. 6(3):63.
- Xu H, Lauer F, Liu K, Hudson L, Burchiel S. 2016. Environmentally relevant concentrations of arsenite and monomethylarsonous acid inhibit IL-7/STAT5 cytokine signaling pathways in mouse CD3+CD4-CD8- double negative thymus cells. Toxicol Lett. 247:62–68.
- Yang Y, Guo Y, Zhang H, Sun T. 2015. Anti-microbial peptide LL-37 circulating levels in chronic obstructive pulmonary disease patients with high risk of frequent exacerbations. J Thorac Dis. 7:740–745.
- Yu S, Liao W, Lee C, Chai C, Yu C, Yu H. 2018. Immunological dysfunction in chronic arsenic exposure: From subclinical condition to skin cancer. J Dermatol. 45(11):1271–1277.
