Abstract
Cyclophilin A (CypA), an 18 kDa multi-functional protein with cis–trans isomerase activity, is both a ligand for cyclosporine A and a proinflammatory factor. CypA is also a chemoattractant for hemopoietic stem cells and progenitors of different lineages, and can mediate regenerative processes in an organism. Accumulated experimental data have suggested there are practical applications for this protein in the treatment of several diseases (i.e. neutralization of cyclosporine A side effects, etc.). However, the range of CypA safe doses as well as its toxic effects remain unknown. The study here investigated the acute toxicity of a single intraperitoneal (IP) or subcutaneous (SC) dosing of recombinant human CypA (rhCypA) in both female and male mice and its effect on gene expression of acute phase proteins (APP) in the female mice after IP treatment. The results showed that toxicity of rhCypA was most evident in female and male mice dosed IP with 750 mg/kg, and manifested as kidney injury and increased granulocyte/lymphocyte ratios in the blood. Enhanced expression of Sаа1 and Sаа2 genes was induced with doses of 0.1–2 mg/mouse of rhCypA. Injection of the maximal dose (750 mg/kg) significantly stimulated expression of all the APP genes studied.
Introduction
Cyclophilin A (CypA) is a 18 kDa protein with cis–trans isomerase activity (Fischer et al. Citation1984) that has intracellular and secreted forms. Cytosolic CypA is a multi-functional chaperone that takes part in folding, transport, and assembly of proteins, and in the regulation of cell proliferation (Wang and Heitman Citation2005), and signal transduction from a T-cell receptor (Colgan et al. Citation2004). As CypA is also the main ligand for cyclosporine A (CsA), it thus mediates the action of this immunosuppressive drug (Fruman et al. Citation1994).
There are other manners in which CypA counters the toxicities induced by CsA. For example, nephrotoxicity is one of the most severe side effects of CsA application. It has been shown that over-expression of CypA protects against CsA-induced nephrotoxicity in vivo (Hong et al. Citation2004). In another case, CypA can be applied during transplantation of allogenic myoblasts to prevent both induction of apoptosis and blockage of differentiation caused by CsA (Hong et al. Citation2002). Lastly, while CsA decreases bone mineral density and facilitates osteoporosis development, CypA in contrast stimulates osteoblast differentiation and inhibits osteoclast activity, leading to an anabolic effect. Accordingly, CypA might have a potential application in the development of new drug treatments for osteoporosis (Guo et al. Citation2016).
Secreted CypA is detected in human blood, tissues, and milk (Spik et al. Citation1991; Rama-Chandran et al. Citation2014) and is produced by different cell types in response to infections and various stresses (Sherry et al. Citation1992; Jin et al. Citation2000). Extracellular CypA is a proinflammatory factor that attracts immune cells to the site of inflammation (Xu et al. Citation1992; Arora et al. Citation2005; Damsker et al. Citation2007) and contributes to pathogenesis of several diseases (Seko et al. Citation2004; Nigro et al. Citation2013; Huang et al. Citation2015). At the same time, CypA induces migration of murine bone marrow stem cells as the chemoattractant for progenitors of different lineages (Khromykh et al. Citation2007). This indicates that this protein can take part in regenerative processes. As CypA attracts bone marrow stem cells to sites of ischemia, it has the potential for use in the development of new approaches to treat limb ischemia (Perrucci et al. Citation2016). Immunologic studies have also shown that over-expression of CypA inhibits replication of the influenza virus (Liu et al. Citation2012); as such, CypA could potentially be used in the treatment of influenza.
While the above illustrates the functional diversity of CypA, and vast experimental data suggest there could be a wide practical application for this protein in the treatment of several diseases, to date the range of safe doses of CypA – as well as pathological changes caused by its use at high levels – remains unknown. Thus, the aim of the work here was to determine toxic doses of recombinant human cyclophilin A (rhCypA) as a potential drug. For this, studies were performed to study the toxic effects of rhCypA in murineF1 hybrids (CBA x C57BL/6) after systemic and local administration of the test agent. Further, because CypA is proinflammatory, this study also examined its influence on gene expression of acute phase proteins (APP); the specific APP panel examined was chosen based on studies by Murata et al. (Citation2004).
Materials and methods
Production of rhCypA
The full-length human CypA gene, optimized for expression in Escherichia coli (with the amino acid sequence corresponding to the native protein), was cloned in the pET plasmid. CypA was isolated from the bacterial biomass of E. coli strain BL21(DE3)Gold transformed with the resultant recombinant plasmid pETCYPopti (Khromykh et al. Citation2016). The recombinant protein was purified by ammonium sulfate fractionation followed by tandem anion exchange chromatography on DEAE- and Q-Sepharose columns (GE Healthcare, Chicago, IL). Isolated RhCypA was dissolved in Na-K phosphate-buffered saline (PBS, pH 7.3); purity, as assessed via electrophoresis, was seen to be >95%. Endotoxin (ET) analyses (using LAL-tests, Kinetic Turbidi-metric [KTA] LAL analyses, Charles River, Wilmington, MA) showed ET levels in the rhCypA samples never exceeded 0.038 ng mg−1 protein, a value below permissive limits (Malyala and Singh Citation2008).
Animals
Male and female F1(CBA/Lac x C57BL/6) mice (18–22 g, 6- to 8-week of age) were obtained from the breeding facility of the Federal State Budgetary Institution at the N.N. Blokhin National Medical Research Center of Oncology (N.N. Blokhin NMRCO, Moscow, Russia). This particular strain was chosen for use here based on the recommendations stated in Festing (Citation1979), Mironov (Citation2012), and Padmanabhan (Citation2014). Because of their genetic and phenotypic uniformity and stability, as well as overall hybrid vigor, using the F1(CBA/Lac x C57BL/6) mice in these studies would enforce the value of observations made herein and also allow us to limit the numbers of mice needed in each experimental group. Mice were housed in facilities maintained at 20–24 °C with a 40% relative humidity and a 12-h light-dark cycle. All mice had ad libitum access to standard rodent chow and filtered tap water. All experimental procedures were approved by the Committee on the Ethics of Animal Experiments of the N.N. Blokhin NMRCO.
Toxicity indexes
In an initial set of studies, acute toxicity indexes, LD10 and LD50 values were calculated across the treatment groups using EPA Probit analysis software (V.1.5; http://www.epa.gov/nerleerd/stat2.htm). Lethality in the mice was monitored over a 14-day post-exposure period for each route of injection employed. The average lethal doses were then calculated according to Prozorovskii (Citation2007).
Treatment protocols/RhCypA administration
For the acute toxicity studies, experimental groups each were comprised of 10 mice. RhCypA was administered by a single intraperitoneal (IP) or subcutaneous (SC) injection. Control mice received PBS (vehicle) as a placebo in parallel. Food and water consumption, general condition, and behavior of mice, as well as visible signs of intoxication and animal death were assessed daily thereafter. Toxicology studies were conducted on Days 3, 7, and 14 post-rhCypA injection (according to protocols outlined in Mironov [Citation2012]). These included blood analyses (via collection of blood from the retro-orbital venous sinus and analysis of levels of erythrocytes, thrombocytes, leukocytes, lymphocytes, and granulocytes in a Cell hematology analyzer [Nihon Kohden, Tokyo, Japan]), and pathomorphological analyses of tissues collected at necropsy (see below).
Pathomorphology
On Days 3, 7, and 14 post-rhCypA injection, after collection of their blood samples, all mice in each group were euthanized by cervical dislocation. In any case of animal death before a given timepoint, examination was carried out as close to the time of death. In each case, kidneys, adrenal glands, liver, spleen, thymus, mesenteric lymph nodes, lungs, heart, thyroid, stomach, small and large intestine, and brain were isolated and weighed at necropsy. Thereafter, each organ was analyzed using macroscopic and histopathological methods. For histological evaluation, each organ was fixed in 10% neutral formalin (JLS-Chemical, St. Petersburg, Russia), dehydrated in a gradient of alcohols, and embedded in Histomix (BioVitrum, St. Petersburg). Tissue sections (5 µm) were then prepared using an AccuCut SRM 200 microtome (Sakura, Alphen den Rijn, the Netherlands) and stained with hematoxylin-eosin. All samples were examined using an Eclipse E200 light microscope (Nikon, Melville, NY) (Bikov Citation2002). All evaluations were performed by board-certified pathologists blinded to the animal treatment.
Analysis of expression of APP genes
For these specific studies, only female mice were employed (n = 3 mice/group). Specific-ally, female mice were injected IP with 5, 100, or 750 mg rhCypA/kg (i.e. 0.1, 2.0, or 15.0 mg/mouse, respectively). The IP dose of 750 mg/kg dose approximated the LD50 value in female mice from the trial studies. After 24 h, all mice were euthanized and their livers were isolated, quickly frozen in liquid nitrogen, and then powdered. Total RNA was then isolated using TRI-reagent (TR118, MRC, Cincinnati, OH). After quantitation of total RNA using a P360 nano-photometer (Implen, Munich, Germany), aliquots containing 2-µg total RNA were converted to cDNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA). These, in turn, were used in quantitative polymerase chain reactions (qPCR)to assess amounts of mRNA of various APP, including serum amyloid A (Saa1 and Saa2), serum amyloid P component (Apcs), fibrinogen-α (Fga), haptoglobin (Hp), hemopexin (Hpx), andα-1-acid glycoprotein-1 (Orm1). The specific primers used for the analysis of gene expression of each APP are listed in . The hypoxanthine-guanine phosphoribosyltransferase (hprt) gene was used as a housekeeping gene in all cases. qPCR was performed in three technical repeats on a CFX96system (BioRad, Hercules, CA) using SYBR Green (DNK-Syntez, Moscow). Results were analyzed using CFX Manager software (BioRad). Relative gene expression levels were calculated using the ΔΔCt method, using levels of corresponding APP gene expression in livers of naive mice as reference.
Table 1. Primers used in the current experiments.
Statistical analysis
All data are presented as means ± SD. Mouse survival analyses were performed using alog-rank (Mantel-Cox) test. All other data were compared using a one-way analysis of variance (ANOVA), followed by a Tukey’s multiple comparisons test. A p values < 0.05 was considered significant. All statistical analyses were performed using Prism software (v. 8.1.2, GraphPad, San Diego, CA).
Results
CypA is an ancient, evolutionary conserved protein (Wang and Heitman Citation2005), and human and murine CypA has near-full homology (NCBI Reference Sequences NP_066953.1and NP_032933.1, respectively). As such, acute toxicity studies of rhCypA using mouse models provide valid valuable information of possible side-effects that could be extrapolated to potential effects in a human host.
Survival after administration of rhCypA
Previously (Khromykh et al. Citation2007, Citation2016, and data not shown), effects of rhCypA (via intravenous [IV] injection of 0.03–0.10 mg rhCypA/mouse) on the migration of stem cells within sublethally irradiated mice were assessed [using method of Till and McCulloch (Citation1961)]. Based on these data, doses of 150, 250, 350, 500, and 750 mg rhCypA/kg were used here for the acute toxicity studies. Initial studies were performed on females with the wide range of rhCypA doses. For subsequent experiments with male mice, noninformative doses or with similar toxic effects were excluded, based on the effects observed in the females; only select dosages with different toxicity (i.e. nontoxic, toxic, and highly toxic) were studied.
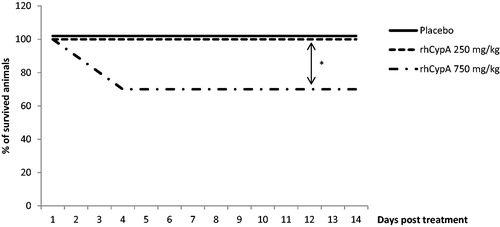
Visible signs of rhCypA intoxication in mice were observed at the 350–750 mg/kg doses. These included adynamia, inertia, ruffled hair, accelerated breathing, or decreased responses to external stimuli. The IP injection of 500 or 750 mg rhCypA/kg caused deaths in ≈40% of the female mice by Day 5 post-injection (). Injection with 350 mg rhCypA/kg led to a final 10–15% death rate within 2–6 days. The lowest dose (i.e. 150 mg rhCypA/kg) had no impact on female survival. In comparison, a single SC injection of 350, 500, or 750 mg rhCypA/kg resulted in no mortality of the female mice (). The lowest dose (i.e. 250 mg rhCypA/kg) had no impact on male mouse mortality after IP administration (). In males injected IP with 750 mg rhCypA/kg, a final mortality rate of 30% was attained by Day 4 ().
Figure 1. Survival dynamics of female mice after single IP rhCypA administration. Female mice were IP-injected with doses of 150–750 mg rhCypA/kg. Survival was monitored for 14 days. *p ˂ 0.05, **p ˂ 0.01. Comparison of survival curves was performed using log-rank (Mantel-Cox) tests. N = 10 mice/group.
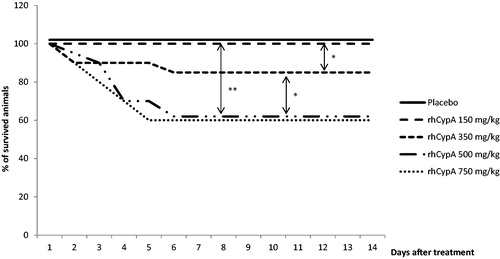
Figure 2. Survival dynamics of female mice after single SC rhCypA administration. Female mice were SC-injected with doses of 350–1500 mg rhCypA/kg. Survival was monitored for 14 days. *p ˂ 0.05. Comparison of survival curves was performed using log-rank (Mantel-Cox) tests. N = 10 mice/group.
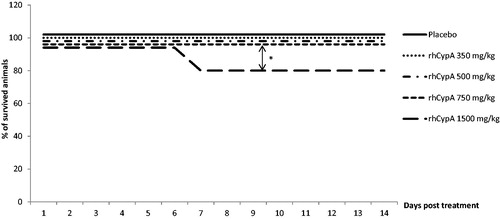
Figure 3. Survival dynamics of male mice after single IP rhCypA administration. Male mice were IP-injected with doses of 250 and 750 mg rhCypA/kg. Survival was monitored for 14 days. *p ˂ 0.05. Comparison of survival curves was performed using log-rank (Mantel-Cox) tests. N = 10 mice/group.
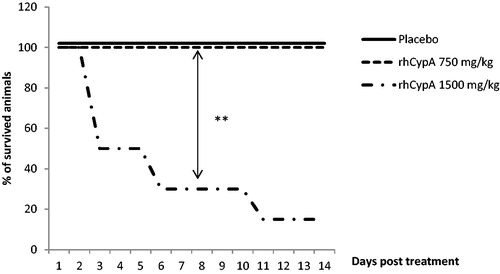
This same dose of the protein had no impact on male mice mortality after SC exposure (). As no animal deaths were observed after SC administration of the maximal dose (i.e. 750 mg/kg), in order to calculate the toxicity indexes the rhCypA dosage was increased up to 1500 mg/kg. This resulted in a 20% mortality among the female mice by Day 7 (). In males, dosing SC with 1500 mg/kg led to 50% mortality by Day 3, 70% by Day 6, and 83% by Day 11 ().
Figure 4. Survival dynamics of male mice after single SC rhCypA administration. Male mice were SC-injected with doses of 750 and 1500 mg rhCypA/kg. Survival was monitored for 14 days. **p ˂ 0.01. Comparison of survival curves was performed using log-rank (Mantel-Cox) tests. N = 10 mice/group.
Based on these data, acute toxicity LD10 and LD50 indices for the male and female mice for both the IP and SC rhCypA administration routes were calculated (). The data showed that female mice exhibited greater sensitivity to systemic rhCypA administration as compared to males (LD50=612 vs. 893 mg/kg, respectively). In contrast, the SC injection LD50for female mice was ≈ 3-fold higher than for males (LD50=3051 vs. 1134 mg/kg, respectively; ).
Table 2. Toxicity indices for rhCypA.
Pathomorphological evaluation of organs after rhCypA administration
All viscera of dosed and control animals were weighed and examined by macroscopic and histological methods. No changes in organ weights (except for kidneys) were observed in the mice irrespective of sex, dosages, or routes of rhCypA administration (data not shown). With regard to the kidneys specifically, a significant 1.7-fold increase in kidney weights was noted by Days 3–4 in male mice that died after IP injection of 750 mg rhCypA/kg (). No changes in kidney weights were noted in males that survived dosing with 250 mg rhCypA/kg. These findings correlated well with the survival dynamics () and reflected the acute phase of rhCypA toxicity. In female mice, by Day 14 after the IP injection of 350, 500, or 750 mg rhCypA/kg, an increase in kidney weights was noted in comparison to in control counterparts; however, these changes in weight were not significant (data not shown). This latter effect could potentially be explained by the considerable time that had elapsed post-rhCypA injection over which there was a decrease in manifestations of the protein’s toxicity.
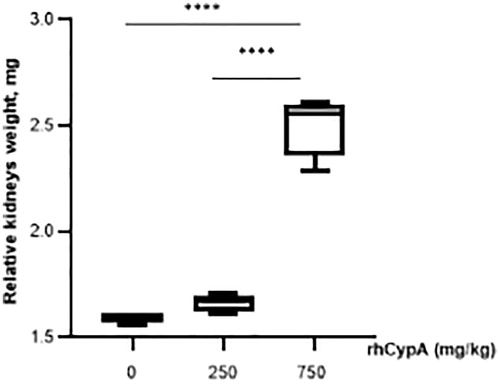
Figure 5. Relative kidney weights of male mice after single IP rhCypA administration. Male mice were IP-injected with 250–750 mg rhCypA/kg. On Days 3–4, relative kidney weights in males that had survived the 250 mg/kg injection or had died from the single 750 mg/kg dose were measured. Data shown are means ± SD (n = 3–5). ****p < 0.0001 (one-way ANOVA, followed by Tukey’s multiple comparisons).
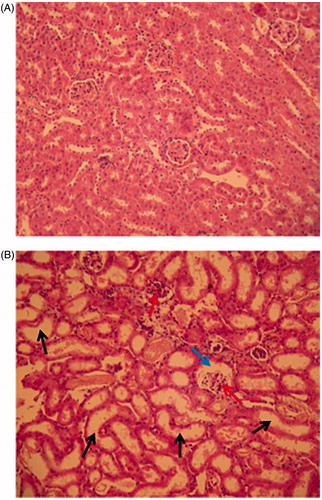
Pathological alterations were found in the kidneys of females that received the 500 or 750 mg/kg doses. These included evidence of renal tubule dilation, renal tubule epithelial cell desquamation, extension of the glomerular cavity, destruction of capillary loops, and dilation of renal collecting tubules. Hyperemia in the zone of the renal collecting tubules was also observed, indicating an impaired secretory function of these kidneys (. Pathological alterations in the kidneys developed in the male mice after IP injection of 750 mg rhCypA/kg, and were the same as in females treated with this dose.
Figure 6. Histological analysis of kidneys of female mice after single IP injection of 750 mg rhCypA/kg. Representative kidney section from control and treatment group are shown. Tissue sections were stained by hematoxylin-eosin; 200× magnification. (A) Control mice. Epithelium of renal tubules, collecting tubules, and glomeruli are not changed. Lumina of collecting tubules are clear and not dilated. (B) Mice IP-treated with 750 mg rhCypA/kg. Renal tubules and collecting tubules are dilated (black arrows), glomerular cavities are expanded (blue arrows), capillary loops are partially destroyed (red arrows).
When the SC route was used to administer rhCypA to the female mice (in 350–750 mg/kg doses), this did not result in any pathological changes in the kidneys (data not shown). These alterations were not observed even by Day 14 in female mice that survived an SC dosing with 1500 mg rhCypA/kg. Interestingly, by Day 14 after the single SC injection of male mice with 750 mg rhCypA/kg, the severity of kidney damage corresponded to that noted in mice IP-injected with the same dose (see above), although this dosage at SC administration had no impact on male mouse mortality. The same abnormalities were noted in male mice dosed SC with 1500 mg rhCypA/kg; these mice either died within 3–6 days of the injection or survived to Day 14 post-rhCypA injection (data not shown).
Taken together, the pathomorphological studies here showed that the severity of kidney damage after a single IP or SC exposure to rhCypA was dose-related (). This damage, moreover, correlated with mortality rates in the mice.
Table 3. Severity of kidney damage in mice after rhCypA administration.
Influence of high doses of rhCypA on mice blood
Analyses of blood samples collected after the single IP injection of rhCypA at any doses (150–750 mg/kg) showed that leucocytes counts remained stable over time (data not shown). However, IP injection of 750 mg rhCypA/kg caused a decrease in blood lymphocyte/granulocyte ratios by Day 3 among all surviving female and male mice (). These changes persisted among survivors until Day 14. The lower doses of rhCypA tested did not influence these ratios (data not shown). The single SC injection of rhCypA did not cause any significant changes in blood counts or in lymphocyte/granulocyte ratios in either the female or male mice at any post-exposure timepoint (data not shown).
Table 4. Blood leukocyte:granulocyte (L:G) ratios in mice after single IP rhCypA injection.
RhCypA effects on hepatic APP gene expression
As CypA is a proinflammatory factor, effects of rhCypA on gene expression of several APP were assessed. Taking into account species-specific differences in APP expression profiles in response to various stimuli – and based on the published data (Cray et al. Citation2009) – the following APP were evaluated in livers harvested 24 h after the single rhCypA injection: serum amyloid A (Saa1and Saa2), serum amyloid P component (Apcs), fibrinogen-α (Fga), haptoglobin (Hp), hemopexin (Hpx), and α-1-acid glycoprotein 1 (Orm1). In these specific studies, only female mice were IP-injected with the rhCypA, as females exhibited greater sensitivity to the protein when given the agent via this route (according to calculated toxicity indexes; ).
The data presented in show that the single IP injection of female mice with 5 or 100 mg rhCypA/kg led to 7.0- and 28.5-fold increases in hepatic Saa1 expression and 2- and 5-fold increases in Saa2 expression, respectively, compared to in livers of control female mice. In contrast, mRNA levels of Fga and Нр genes were decreased on average by 70% and 60%, respectively, irrespective of dose. Expression of Аpcs, Hpx, and Orm1 did not change as compared to the control values regardless of the two doses used.
Figure 7. RhCypA treatment impact on acute phase protein (APP) gene expression. Female mice were IP-injected once with various doses of rhCypA. After 24 h, liver mRNA levels of indicated APP were assessed (qPCR). N = 3 mice/group. Figure shows one of three representative results. *p < 0.05,**p < 0.01, ****p < 0.0001 as compared to the reference (one-way ANOVA).
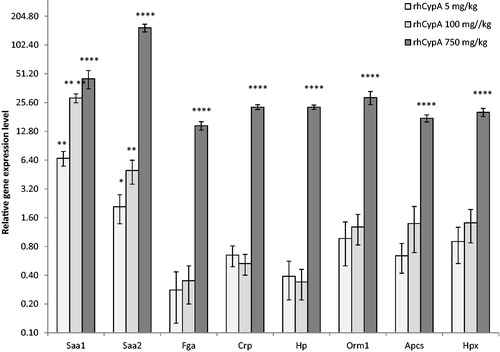
In comparison to effects with those two doses, injection of 750 mg rhCypA/kg stimulated gene expression of each APP studied as compared to levels in the livers of control counterparts. The average increases in APP mRNA levels were, for Saa1 = 45-fold, Saa2 = 155-fold, Fga= 14-fold, Нp= 23-fold, Apcs= 17-fold, Нрх= 20-fold, and Оrm1 = 28-fold.
Discussion
CypA is known as a factor in the pathogeneses of a variety of immune-based and other diseases (Nigro et al. Citation2013). Nevertheless, CypA also has multiple important “positive” functions in a healthy organism (i.e. regulating stem cell migration, as well as helping to support normal hematopoiesis, immune response development, and constant immunosurveillance) (Khromykh et al. Citation2007; Perrucci et al. Citation2016). It was shown in in vivo systems that CypA induced recruitment of bone marrow-derived cells to areas in diseased aortas (Nigro et al. Citation2011) and hypertrophic hearts (Satoh et al. Citation2011). In a mouse model of hind-limb ischemia, CypA levels were seen as up-regulated in adductor muscles (Tirziu et al. Citation2005).
In general, other “positive” aspects of CypA function have been less studied. Still, the accumulating data has allowed for suggestions of potential clinical applications for manufactured forms of CypA such as recombinant human CypA (rhCypA), for example, as a supportive treatment for CsA-based therapies, in treatment for osteoporosis, for stimulation of tissue regeneration after ischemic damage, for neutralization of lymphopenia and recovery of normal hematopoiesis after various stresses, etc.). Accordingly, the current study sought to determine potential toxicities associated with host (i.e. mouse) exposure to a wide range of single rhCypA doses – from safe to lethal – to help to begin to establish doses that might potentially/eventually be used in humans without inducing harmful side-effects. This view was predicated on the fact there is a near-full homology between human and murine CypA; as such, this could allow for extrapolation of any observed effects in mouse models to potential effects in a human host.
The work presented here evaluated selected toxic properties of rhCypA after a single IP or SC injection into female and male F1(СВА/Lac х C57BL/6) mice at doses ranging from 5 to 750 mg/kg. For comparison, normal CypA concentrations in mouse blood plasma are ∼ 20.0 ng mL−1 (Li et al. Citation2014). In our ongoing studies, a single IP or SC injection of 5 mg rhCypA/kg led to a peak concentration (Cmax) in the blood (plasma) of 1100 and 880 ng rhCypA/mL within 1.7 and 2.2 h (Tmax), respectively. The area under the curve (AUC) for those analyses was 9.9 and 7.5 (µg × h)/mL, respectively, for the IP or SC injection (unpublished data). Accordingly, the dosages used herein were selected to thus both approximate and exceed normal physiological concentrations of secreted CypA in mice.
Systemic (IP) injection of high doses of rhCypA yielded significant toxicity in the experimental animals, and females demonstrated higher sensitivity in comparison with males (LD50 = 612 and 893 mg/kg for females and males, respectively). In contrast, male mice appeared to be more sensitive to local (SC) administration of the protein (LD50 =949 mg/kg) in comparison with females (LD50 =1691 mg/kg). This difference could be due to some physiological features of male mice that require additional study.
The pathohistological analysis revealed that mortality among the female and male mice here, using either route of rhCypA injection, was associated with kidney damage that appeared to arise in a dose-related manner. These analyses did not find any morphological changes in other visceral organs of mice treated with rhCypA by either route (data not shown). It is likely that the observed toxic effects were probably not due to any ET accumulation in the rhCypA samples (highest expected content was 1.14 ng ET in a total dosage of 30 mg rhCypA given to mice in the 1500 mg rhCypA/kg SC injection groups). This supposition is supported by additional analyses performed here that showed that a single IP administration of 10 μg E. coli LPS/mouse did not cause any visible toxic effects in F1(CBA/Lac x C57BL/6) mice within a 3-week time frame (data not shown).
A possible explanation for this seemingly select damage to the kidneys in these mice treated with high doses of rhCypA could be amyloidosis-like processes (Westermark et al. Citation2015). Amyloidosis develops due to incomplete dissolution of SAA; as a result, there is an increased risk for accumulation of insoluble fibrillar AA-amyloid in parenchymal compartments of the kidneys (Khalighi et al. Citation2014). In a similar manner, this pathologic process can also lead to the formation of glomerular lesions (Said et al. Citation2013). Although it is more common for amyloidosis to arise in the context of chronic inflammatory conditions, in the studies here, acute kidney damage arose in the short-term. Thus, it is possible that rhCypA – at extremely high doses – could induce amyloidosis-like processes in a host, potentially in the absence of induction of a systemic inflammatory state.
Several cytokines, for example, IL-6, IL-1, and TNFα, are strong inducers of APP synthesis in the liver. Rapid increases in APP levels provide for early defense in an infectious process and precede a full immune response (Suffredini et al. Citation1999). Evaluation of levels of APP levels during some diseases has been shown to be of prognostic significance during subsequent treatments (Abreu et al. Citation2018; Ceron et al. Citation2018). Data presented here indicated that rhCypA induced hepatic expression of the genes of several APP, and that the spectrum and level of expression of the APP genes, were directly dependent on rhCypA concentrations (see ). Both Saa1 and Saa2 gene expression was seen to be the most sensitive to rhCypA administration, and functionally SAA complements the effects of CypA. For example, both CypA and SAA induce EC activation by promoting surface expression of VCAM-1 (Mullan et al. Citation2006; Dong et al. Citation2011). As a chemoattractant, CypA stimulates accumulation of leukocytes and granulocytes (Khromykh et al. Citation2007); SAA is a known chemoattractant for monocytes and polymorphonuclear cells (Badolato et al. Citation1994; Lee et al. Citation2008). In the current study, IP injection of the highest rhCypA dose (i.e. 750 mg/kg) increased granulocyte/lymphocyte ratios in the blood of all the mice. As the high doses of rhCypA here induced production of high levels of SAA (see ), the cumulative effect of the presence of these agents in these hosts likely resulted in a skewing of this toward the granulocytes. That SС injection of rhCypA at all doses did not influence the blood counts of female and male mice (data not shown) could have been due to a slow absorption of rhCypA into the general circulation. In comparison with the effects from IP dosing with the rhCypA, the resulting blood levels of rhCypA and SAA likely did not attain a sufficiently high-enough state to trigger mass attraction of granulocytes into the blood.
Taken together, the treatment of mice with rhCypA here with a single dosage of 5–150 mg/kg did not cause any acute toxic effects in spite of induced enhanced gene expression of Saa1 and Saa2. These findings have, moreover, been confirmed in ongoing chronic toxicity studies in mice, rats, and rabbits in our laboratories. Such patterns suggest that rhCypA has a wide range of safe dosages and thus could potentially be developed as a perspective drug for use in various clinical applications.
Conclusions
The analyses here of acute toxicity of rhCypA in female and male F1(CBA/Lac x C57BL/6) mice after a single IP (0.1–15 mg/mouse) or SC (0.1–30 mg/mouse) injection showed that the highest dosages (i.e. 750–1500 mg/kg) induced toxicity in the form of kidney damage, regardless of host gender or route of dosing. Of particular importance was a finding that rhCypA induced hepatic gene expression of acute phase proteins. Serum amyloid A showed the greatest sensitivity to rhCypA. Nevertheless, rhCypA at 0.1–3.0 mg/mouse (5–150 mg/kg) – by either route – was ultimately considered to be safe here. Thus, for now, it can be surmised that use of a single low dose of rhCypA could have a potential application in various clinical settings. Chronic toxicity studies confirmed that the same degree of safety seen here in the mice was noted when repeated dosing of rhCypA was tested (data not shown).
Disclosure statement
The authors declare no conflict of interest, financial or otherwise. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Abreu D, Monteiro J, Souza C, Karam R, Fernandes R, Lessa T, Fagliari J, Miglino M, Ambrosio C. 2018. Immunophenotyping lymphocyte and acute phase proteins in canine X-linked muscular dystrophy. An Acad Bras Ciênc. 90(3):2977–2990.
- Arora K, Gwinn W, Bower M, Watson A, Okwumabua I, MacDonald H, Bukrinsky M, Constant S. 2005. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 175(1):517–522.
- Badolato R, Wang J, Murphy W, Lloyd A, Michiel D, Bausserman L, Kelvin D, Oppenheim J. 1994. Serum amyloid A is a chemoattractant: Induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 180(1):203–209.
- Bikov V. 2002. Cytology and general histology. St. Petersburg: SOTIS.
- Ceron J, Pardo-Marin L, Caldin M, Furlanello T, Solano-Gallego L, Tecles F, Bernal L, Baneth G, Martinez-Subiela S. 2018. Use of acute phase proteins for the clinical assessment and management of canine leishmaniosis: General recommendations. BMC Vet Res. 14(1):196.
- Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J. 2004. Cyclophilin A regulates TCR signal strength in CD4+ T-cells via a proline-directed conformational switch in Itk. Immunity. 21(2):189–201.
- Cray C, Zaias J, Altman N. 2009. Acute phase response in animals: A review. Comp Med. 59(6):517–526.
- Damsker JM, Bukrinsky MI, Constant SL. 2007. Preferential chemotaxis of activated human CD4+ T-cells by extracellular cyclophilin A. J Leukoc Biol. 82(3):613–618.
- Dong Z, Wu T, Qin W, An C, Wang Z, Zhang M, Zhang Y, Zhang C, An F. 2011. Serum amyloid A directly accelerates progression of atherosclerosis in apolipoprotein Edeficient mice. Mol Med. 17:1357–1364.
- Festing M. 1979. Properties of inbred strains and outbred stocks with special reference to toxicity testing. J Toxicol Environ Health. 5(1):53–68.
- Fischer G, Bang H, Mech C. 1984. Determination of enzymatic catalysis for cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed Biochim Acta. 43(10):1101–1111.
- Fruman D, Burakoff S, Bierer B. 1994. Immunophilins in protein folding and immunosuppression. Faseb J. 8(6):391–400.
- Guo M, James A, Kwak J, Shen J, Yokoyama K, Ting K, Soo C, Chiu R. 2016. Cyclophilin A (CypA) plays dual roles in regulation of bone anabolism and resorption. Sci Rep. 6:22378.
- Hong F, Lee J, Piao Y, Jae Y, Kim Y, Oh C, Seo J, Yun Y, Yang C, Ha J, et al. 2004. Trans-genic mice over-expressing cyclophilin A are resistant to cyclosporin A-induced nephrotoxicity via peptidyl-prolyl cis-trans isomerase activity. Biochem Biophys Res Commun. 316(4):1073–1080.
- Hong F, Lee J, Song J, Lee S, Ahn H, Cho J, Ha J, Kim S. 2002. Cyclosporin A blocks muscle differentiation by inducing oxidative stress and inhibiting the peptidyl-prolyl-cis-trans isomerase activity of cyclophilin A: Cyclophilin A protects myoblasts from cyclosporin A-induced cytotoxicity. Faseb J. 16(12):1633–1635.
- Huang C, Chang C, Kuo C, Huang C, Lin C, Liu C. 2015. Decrease in plasma cyclophilin A concentration at 1-mo after myocardial infarction predicts better left ventricular performance and synchronicity at 6 mo: A pilot study in patients with ST elevation myocardial infarction. Int J Biol Sci. 11(1):38–47.
- Jin Z, Melaragno M, Liao D, Yan C, Haendeler J, Suh Y, Lambeth J, Berk B. 2000. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 87(9):789–796.
- Khalighi M, Wallace W, Palma-Diaz M. 2014. Amyloid nephropathy. Clin Kidney J. 7(2):97–106.
- Khromykh L, Kulikova N, Anfalova T, Muranova T, Abramov V, Vasiliev A, Khlebnikov V, Kazansky D. 2007. Cyclophilin A produced by thymocytes regulates the migration of murine bone marrow cells. Cell Immunol. 249(1):46–53.
- Khromykh L, Kalinina A, Kozir A, Kolesnikov A, Silaeva Y, Kazanskiy D. 2016. Escherichia coli BL21(DE3)GoldpETCYPoptistrain-producer of recombinant human Cyclophilin A. Moscow, Russia, Patent #603283.
- Lee H, Kim S, Shim J, Lee S, Lee H, Cho K, Yun J, Bae Y. 2008. Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J Immunol. 181(6):4332–4339.
- Li Y, Yan J, Wu C, Wang Z, Yuan W, Wang D. 2014. CD137-CD137L interaction regulates atherosclerosis via cyclophilin A in apolipoprotein E-deficient mice. PLoS One. 9(2):e88563.
- Liu X, Zhao Z, Xu C, Sun L, Chen J, Zhang L, Liu W. 2012. Cyclophilin A restricts influenza A virus replication through degradation of the M1 protein. PLoS One. 7(2):e31063.
- Malyala P, Singh M. 2008. Endotoxin limits in formulations for preclinical research. J Pharm Sci. 97(6):2041–2044.
- Mironov A. 2012. Guidelines for preclinical studies of drugs. Guidelines for the study of the general toxic effect of drugs. Griph K. 1:13–25.
- Mullan R, Bresnihan B, Golden-Mason L, Markham T, O'Hara R, Fitzgerald O, Veale D, Fearon U. 2006. Acute-phase serum amyloid A stimulation of angiogenesis, leukocyte recruitment, and matrix degradation in rheumatoid arthritis through an NF-#B-dependent signal trans-duction pathway. Arthritis Rheum. 54:105–114.
- Murata H, Shimada N, Yoshioka M. 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J. 168(1):28–40.
- Nigro P, Pompilio G, Capogrossi M. 2013. Cyclophilin A: a key player for human disease. Cell Death Dis. 4:e888.
- Nigro P, Satoh K, O'Dell M, Soe N, Cui Z, Mohan A, Abe J, Alexis J, Sparks J, Berk B. 2011. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 208(1):53–66.
- Padmanabhan S, editor. 2014. Animal models in pharmacogenomics: Handbook of pharmacogenomics and stratified medicine. New York: Elsevier; p. 73–87, Chapter 5.
- Perrucci G, Straino S, Corliano M, Scopece A, Napolitano M, Berk B, Lombardi F, Pompilio G, Capogrossi M, Nigro P. 2016. Cyclophilin A modulates bone marrow-derived CD117+ cells and enhances ischemia-induced angiogenesis via the SDF-1/CXCR4 axis. Int J Cardiol. 212:324–335.
- Prozorovskii V. 2007. Statistic processing of data of pharmacological investigations. Psycho-Pharmacol Biol Narcol. 7:2090–2120.
- Ramachandran S, Venugopal A, Kutty V, Chitrasree V, Mullassari A, Pratapchandran N, Santosh K, Pillai M, Kartha C. 2014. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc Diabetol.13:38.
- Said S, Sethi S, Valeri A, Leung N, Cornell L, Fidler M, Herrera Hernandez L, Vrana J, Theis J, Quint P, et al. 2013. Renal amyloidosis: Origin and clinico-pathologic correlations of 474 recent cases. Clin J Am Soc Nephrol. 8(9):1515–1523.
- Satoh K, Nigro P, Zeidan A, Soe N, Jaffré F, Oikawa M, O'Dell M, Cui Z, Menon P, Lu Y, et al. 2011. Cyclophilin A promotes cardiac hypertrophy in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 31:116–1123.
- Seko Y, Fujimura T, Taka H, Mineki R, Murayama K, Nagai R. 2004. Hypoxia followed by reoxygenation induces secretion of cyclophilin A from cultured rat cardiac myocytes. Biochem Biophys Res Commun. 317(1):162–168.
- Sherry B, Yarlett N, Strupp A, Cerami A. 1992. Identification of cyclophilin as a pro-inflammatory secretory product of LPS-activated macrophages. Proc Natl Acad Sci USA. 89(8):3511–3515.
- Spik G, Haendler B, Delmas O, Mariller C, Chamoux M, Maes P, Tartar A, Montreuil J, Stedman K, Kocher H. 1991. A novel secreted cyclophilin-like protein (SCYLP). J Biol Chem. 266(17):10735–10738.
- Suffredini A, Fantuzzi G, Badolato R, Oppenheim J, O'Grady N. 1999. New insights into biology of the acute phase response. J Clin Immunol. 19(4):203–214.
- Till J, McCulloch E. 1961. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 14(2):213.
- Tirziu D, Moodie K, Zhuang Z, Singer K, Helisch A, Dunn J, Li W, Singh J, Simons M. 2005. Delayed arteriogenesis in hypercholesterolemic mice. Circulation. 112(16):2501–2509.
- Wang P, Heitman J. 2005. The cyclophilins. Genome Biol. 6(7):226.
- Westermark G, Fändrich M, Westermark P. 2015. AA amyloidosis: Pathogenesis and targeted therapy. Annu Rev Pathol. 10:321–344.
- Xu Q, Leiva M, Fischkoff S, Handschumacher R, Lyttle C. 1992. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 267(17):11968–11971.
