Abstract
Endosulfan is a DDT-era organochlorine pesticide. Due to past and current environmental contamination, investigation of endosulfan exposure is of current importance. Acute high dose exposure precipitates neural/endocrine system damage, but the effects on the immune system and of lower doses are not well-characterized. Two relatively low concentrations of endosulfan (i.e. 0.1 and 17 µM ENDO) were investigated in an in vitro study using human peripheral blood mononuclear cells (PBMC) to understand effects of relatively low doses (0.1–25.0 µM [≈0.04–10 ppm/40–10,000 ppb]) of ENDO upon normal human T- and B-lymphocytes and NK cells. The study here found that 17 µM ENDO inhibited phytohemagglutinin-M (PHA)-induced human PBMC proliferation. It was also seen that senescence and apoptosis among non-stimulated cells was increased, specifically within CD8 and NK populations, and that CD4:CD8 ratios also were increased. Treatment of non-stimulated PBMC with ENDO led to overall increases in production of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-2, -4, and -6, and decreased production of anti-inflammatory IL-10, suggesting an immunosenescence secretory phenotype. Interestingly, when the cells were pre-stimulated with mitogen (PHA), ENDO became inhibitory against the mitogen-induced proliferation and cytokine formation – with the exception of that of TNFα and IL-6, suggesting differential effects of ENDO on activated cells. Thus, at the organismal level, ENDO might also display differential effects during states of autoimmune disease or chronic viral infection in the exposed host.
Introduction
Organochlorine pesticides (OCP) are synthetic organic agents used worldwide as insecticides, herbicides, fungicides, and for agricultural/household purposes. Endosulfan (hexachloro-hexahydro-methano-benzodioxathiepinoxide) is a cyclodiene organochlorine (OC) insecticide. Despite life threatening toxic effects, endosulfan (ENDO) continues to be widely-used in agricultural pesticide in developing countries, due to its high efficacy, low cost and environmental stability (Menezes et al. Citation2017).
In recent years, as the dangers and secondary effects of pesticides have become known, efforts have been made to control the use of these agents. Endosulfan was defined as a persistent organic pollutant (POP) by the Stockholm Convention on Persistent Organic Pollutants in 2011. While ENDO has now been listed for 7 years – with specific exemptions – in Annex A of this agreement, meaning that “Parties must take measures to eliminate the production and use of the chemical,” the continuing impacts of this pesticide on the environment and human health should not be ignored. Different countries and different regions have had varied success in controlling the use of ENDO, and ENDO might affect the environment and human health for a long time due to its characteristic environmental behavior including persistence, bioconcentration and high biological toxicity (Desalegn et al. Citation2011; Stockholm Convention on Persistent Organic Pollutants Citation2017). The wide spectrum of effects that pesticides have on health involves acute and persistent damage to the nervous system, to the respiratory system and to reproductive organs, as well as contributing to the dysfunction of the immunological and endocrine systems (Martínez-Valenzuela et al. Citation2009).
In Mexico, there is a current problem with pollution from ENDO (Wong et al. Citation2010). Runoff from agricultural fields/discharge during the manufacture of the pesticide is responsible for a majority of ENDO contamination of the air, water, and soil in many parts of the world (Guo et al. Citation2015), as in Mexico. It is thus not surprising that ENDO residues have been detected in various fruits, vegetables, nuts, grains, and fish/seafoods around the world (Canlet et al. Citation2013). Among the latter, concentrations have been found to range from 0.5 to 13.0 ppb in fish and 0.2 to 1.7 ppb in various seafoods.
Studies have shown that > 90% of human exposure to ENDO is via the oral route, i.e. food/liquid intake (Campoy et al. Citation2001; Silva and Carr Citation2010). In countries where ENDO is still commonly used in agriculture, detectable levels have been found in bio-fluids obtained from “non-exposed” general populations, i.e. in maternal milk (0.6–27.9 mg/mL [ppb]) (Campoy et al. Citation2001), in neonatal blood (9.2 mg/mL) (Schaalan et al. Citation2012), and in adult serum (0.2–3.0 mg/mL) (Wade et al. Citation2002; Dar et al. Citation2012; Pi et al. Citation2016). In some parts of the world, levels are even higher; in Spain, healthy young men were found to have mean total serum ENDO levels (including metabolites) ranging from ≈26 to 145 mg/mL (Carreño et al. Citation2007). A recent study in Pakistan showed much higher endemic levels of ENDO, with levels of 166 ppb found in the serum of healthy controls (Attaullah et al. Citation2018). That study also reported slightly higher levels in lymph fluid (207 ppb) and serum (214 ppb) samples of cancer patients. With respect to acute exposures, ENDO has been found at levels up to 590 ng/ml in the blood of agricultural workers after application of the agent in fields in South Africa (Dalvie et al. Citation2009).
Exposure to ENDO and other OCP has been associated with increases in the incidence of breast, ovarian, prostate, leukemia, and other cancers (Rau et al. Citation2012; Yang, et al. Citation2015; Shah et al. Citation2018). Relatedly, ENDO has also been associated with endocrine disruption as ENDO can also bind estrogen receptors (ER); this has led to it being classified as a xenoestrogen (Bulayeva and Watson Citation2004). ENDO has been shown to bind and act as an agonist to ERα (Lemaire et al. Citation2006). At this time, it is not clear if ENDO binds to ER receptors on immune cells (such as lymphocytes).
While effects from acute exposure to toxic doses of ENDO are well documented, effects of low-dose ENDO are not as well characterized. The importance of chronic exposure to low doses of ENDO, such as may occur in agricultural regions where it is still in use or had been used in the past, is an important public health question. An early study in our laboratory investigated the effects of acute low-dose (7 µg/mL) ENDO on Nile Tilapia, a common fish found in reservoirs and fish farms in agricultural regions (Tellez-Bañuelos et al. Citation2009). The findings were of note enough that this work was cited in the 2009 Stockholm Convention Draft Risk Profile (Stockholm Convention on Persistent Organic Pollutants. United Nations Environmental Program (UNEP) Citation2009). Even when use of ENDO was curtailed, it lingered in the environment and so gave rise to ongoing chronic low-dose exposures to hosts who often come into contact with the contaminated materials. Endosulfan residues have been reported in water sources years after use of the agent had stopped. While this would have directly impacted upon exposed fish, because ENDO can bioaccumulate in fatty tissues, populations distant from these sites of contamination often became contaminated via consumption of these ENDO-tainted fish (Mrema et al. Citation2013).
How a specific dose translates to concentrations in body tissues/blood and so affect individual cells is difficult to calculate and can vary greatly. For this reason, the present study relied in part on previous studies in our laboratory to guide the experimental concentrations ultimately used herein. The doses were also based on findings by others that studied effects of in vitro doses of ENDO in differing cell culture models (see Discussion). Some of these studies suggested that doses in the range of 100–200 µM and below should be considered non-cytotoxic (Lu et al. Citation2000; Antherieu et al. Citation2007). Based on those studies, and the concentrations observed in the earlier tilapia studies, the in vitro study here was undertaken to understand effects of relatively low doses (0.1–25.0 µM [≈0.04–10 ppm/40–10 000 ppb]) of ENDO on normal human T- and B-lymphocytes and NK cells within a parent population of peripheral blood mononuclear cells (PBMC).
Materials and methods
Chemicals
Both the α and β isomers of ENDO (99.3% purity) were purchased from Chem Service (West Chester, PA). Stock solutions (25 mM) of ENDO were diluted in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) and stored at 4 °C protected from light. Immediately prior to use in each experiment, ENDO was mixed at a 7:3 ratio of α:β. Working dilutions were then made using RPMI 1640 medium (supplemented with 10% fetal bovine serum and 1% Pen/Strep [10 000 IU penicillin/mL, 10 000 μg streptomycin/mL]; all Gibco, Carlsbad, CA), as a diluent to generate solutions that deliver the test agent at six final levels ranging from 0.1 to 25.0 µM.
While earlier results from proliferation studies were used as a benchmark to establish the appropriate range of concentrations to use here, it is important to note inhibitory effects of ENDO were observed previously. It is also important to note that in those experiments and the work here, ENDO had to be dissolved in DMSO (the latter is known to impart significant effects on the immune system/immune cells). As other studies reported no effect of DMSO when diluted to between ≤ 0.1 and 1.0% [v/v] (El-Shenawy Citation2010; Enhui et al. Citation2016; Ghosh et al. Citation2018), in all the experiments here, the final DMSO level presented to cells ranged from 0.0004% to 0.07% [v/v].
Isolation of peripheral blood lymphocytes
Human PBMC were isolated from peripheral venous blood obtained from 68 healthy donors recruited from the University student population (age 19–26 years; split evenly between male and female donors). Donors self-identified as not recovering from infections, and signed letters of informed consent, according to institutional guidelines, at the time of donation. The samples were not pooled, i.e. cells from each sample were isolated and evaluated as individual experimental subjects for each experiment in the study. For the study, blood from each subject was drawn into two or three (depending on the volume needed for each experiment) 3 mL EDTA-coated Vacutainer tubes (BD Franklin Labs, NJ) and then diluted 1:1 in phosphate-buffered saline (PBS, pH 7.4). The diluted blood was then overlaid atop 2 mL Lymphoprep solution (ρ = 1.077 g/mL; Nycomed Pharma, Oslo, Norway) and centrifuged for 30 min (1500 rpm, 20 °C). The PBMC that aligned at the interface were isolated, transferred to a fresh centrifuge tube, then washed with medium by centrifugation. The final PBMC pellet was suspended in complete medium (RPMI 1640 with 10% FBS and 1% Pen/Strep; RPMI-S), counted in a hemocytometer, and aliquots of the cells were then plated into Costar culture plates of various formats (i.e. from 24- to 96-well, depending on experiment) (Corning Inc., Corning, NY).
Cell proliferation assay
In brief, PBMC were placed in 96-well flat bottom culture plates (at 105 cells/well) and then provided a range of ENDO at concentrations ranging from 0 (media) to 0.1–25.0 μM. After 2 h of incubation with the ENDO, all cells then received phytohemagglutinin-M (PHA; 10 µg/mL final concentration in wells; Gibco); the ENDO remained in the wells and was not removed. The cells were then incubated for a further 24, 48, or 72 h (dedicated plate for each timepoint) at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. At the end of each period, each well in the specified plate received 10 μL WST-1 reagent (Biovision, Milpitas, CA) and the plate was incubated a further 2 h at 37 °C. The plate was then gently shaken for 1 min to mix the well contents, and absorbance in each well was measured using a OPsysMR microtiter plate reader (Dynex Corp., Chantilly, VA) at 420 nm (with 650 nm reference wave-length). All well conditions were evaluated in duplicate. Data were reported as the OD values “normalized” to the OD values for cells incubated with medium alone (i.e. normalizing for proliferation observed with controls for each cell sample.)
Measurement of cell populations and apoptosis via cytometry
In brief, cells were cultured in 24-well plates at 5 × 105 cells/mL (1 mL/well). In this set of experiments, the cells were cultured in RPMI-S alone, RPMI-S with 0.1 μM ENDO, or RPMI-S with 17 μM ENDO for 24 or 48 h; no PHA was added at any time. Etoposide, a topoisomerase inhibitor and a common chemotherapeutic, was used on parallel sets of control cells as a positive control for induction of apoptosis. Cell populations were then evaluated using multi-color flow cytometry. The following conjugated mouse anti-human monoclonal antibodies were used to label cells: phycoerythrin (PE)-anti-CD19 (HD237; Beckman Coulter, Fullerton, CA), PE-Cy7-anti-CD8 (HIT8A) and allophycocyanin (APC)-Fire700-anti-CD3 (UCHT1; both Biolegend, San Diego, CA), and APC-anti-CD56 (B159; BD Pharmingen, San Jose, CA). This first panel was used for analysis of CD4 (CD3+CD8−) and CD8 (CD3+CD8+) T-cells, NK cells (CD3−CD56+), and B-cells (CD3−CD19+) in the cultures.
Apoptosis among the cultured cells was evaluated by flow cytometry using an Annexin V-Fluorescein (FITC) kit (eBioscience, San Diego, CA). After staining of the PBMC, the cells were incubated with Annexin V-FITC according to kit instructions. In brief, PBMC were centrifuged and washed with PBS, and re-suspended in 200 μL kit-provided buffer; thereafter, 5 μL Annexin V-FITC solution was added and the cells incubated for 20 min at room temperature, protected from light. Each sample was then washed once (by centrifugation) with kit buffer and the pellet was re-suspended in 500 μL kit binding buffer. Each tube then immediately received 5 μL of kit-provided vital dye/fluorescent DNA intercalator 7-aminoactinomycin D (7-AAD). Samples were then immediately analyzed in an Attune flow cytometer (Applied Biosystems, Foster City, CA) and analyzed using FlowJo software (v10.0.8; Tree Star, Inc., Ashland, OR). At least 50 000 events were acquired/sample. Appropriate negative and Fluorescence Minus One (FMO) controls were utilized to adjust for background fluorescence in all assays. Results are presented as percentage cells in apoptosis.
Cytokine determination by multiplex immunoassay
A Bio-Plex Pro Human Cytokine 8-plex Assay (BioRad, Hercules, CA) was used for determination of cytokine profiles. In this set of experiments, the cells were cultured for 18 h with RPMI-S alone, RPMI-S + PHA (see above for dose), RPMI-S + 0.1 μM ENDO, RPMI-S + 17 μM ENDO, RPMI-S + 0.1 μM ENDO + PHA, and RPMI-S + 17 μM + ENDO + PHA. At the end of the culture period, supernatant from each well was collected and stored at −20 °C for later analyses. All samples were processed in duplicate. At analysis, the assay plates received each supernatant sample and were processed based on manufacturer protocols. Ultimately, each plate was evaluated using a BioPlex MAGPIX Multiplex Reader (BioRad). All results were reported as fold-change with respect to supernatants from “normalized” control cell wells.
Measurement of intracellular cytokines by flow cytometry
For these experiments, cells were cultured in 24-well culture plates at 5 × 105 cells/well. Here, PBMC were cultured for 18 h in RPMI-S alone, RPMI-S + 0.1 μM ENDO, or RPMI-S + 17 μM ENDO; no PHA was used. After 1 h of incubation at 37 °C, Brefeldin A (Biolegend) and Monensin (Sigma) were added to the wells (final well concentrations of 5 µg/mL and 2 µM, respectively), and the cells were allowed to continue incubating. At the end of the 18 h period, the cells were washed by centrifugation using PBS (each time, 350 ×g, 5 min, 4 °C). The final pellets were each re-suspended in cell-staining buffer (Biolegend) and the cells were counted in a hemocytometer. Aliquots (one for each intracellular cytokine of interest) were taken and the cells in each were stained with a conjugated mouse anti-human antibody panel containing AF488-anti-CD3, PE-Cy7-anti-CD8 (both Biolegend) and APC-anti-CD56 (BD Pharmingen) for 1 h at room temperature in the dark. Thereafter, the cells were washed again by centrifugation using PBS (each time, 350 ×g, 5 min, 4 °C) and each final cell pellet was re-suspended in 250 µL fixation buffer (Biolegend). After 20 min incubation at room temperature, the cells were washed with permeabilization solution (Biolegend) and each tube received a specific “intracellular” anti-body, i.e. PE-anti-IFNγ, PE-anti-IL-4, or PE-anti-IL-10 (all e-Biosciences). The cells were then incubated in the dark for 30 min at room temperature, washed by centrifugation using PBS, fixed with 1% paraformaldehyde, and then immediately analyzed by flow cytometry in the Attune flow cytometer as above.
In all of these studies, as a positive control for cytokine secretion, control wells that were not treated with ENDO received 20 mg/mL phorbol 12-myristate 13-acetate (PMA) + ionomycin (1 μg/mL) (both Sigma). As before, at least 50 000 events were acquired/sample. Appropriate negative and Fluorescence Minus One (FMO) controls were utilized to adjust for background fluorescence in all flow assays. Results are presented as the percentage of each gated population of cells judged positive (i.e. with a signal stronger than for the isotype control) by histogram analysis of mean fluorescence intensity (MFI).
Senescence determination
As above, in these experiments, cells were cultured in 24-well plates at 5 × 105 cells/mL (1 mL/well). Here, the PBMC were cultured in RPMI-S alone, RPMI-S + 0.1 μM ENDO, or RPMI-S + 17 μM ENDO for 72 h. Determination of senescence was then performed via measures of β-galactosidase activity (senescence-associated β-gal, SA-β-gal) in the cells. In brief, at the end of the exposure period, the PBMC were stained with the first antibody panel (see ‘Measurement of cell populations and apoptosis via cytometry’ section) as noted earlier. Afterwards, each pellet was re-suspended in 200 μl RPMI-S containing 100 nM Bafilomycin A1 (Sigma). The cells were then incubated 1 h at 37 °C before an aliquot of medium containing C12FDG (fluorogenic glycosidase substrate; Invitrogen, Carlsbad, CA) at a level yielding a final 10 µM concentration was added. The cells/solutions were mixed gently and left to incubate for 15 min at 37 °C. The cells were then washed once by centrifugation using cold PBS, and re-suspended in 500 μL PBS. A viability dye (5 µL of kit-provided 7-AAD) was added to each tube to permit exclusion of dead cells during flow cytometric analyses. In all these studies, ethanol (at 50 µL/mL) was added to control cells as a positive control for senescence induction. All cells were then analyzed in the flow cytometry system here, with 50 000 events/sample being acquired. All results are presented as the percentage of the gated cell populations, excluding the 7-AAD+ cells, in senescence.
Statistical analysis
All values shown are means ± SD. All statistical analyses were performed using Prism 7 software (GraphPad, San Diego, CA). A Shapiro-Wilk test was first used to determine normality. For normal data ( only), the repeated measures were analyzed using a one-way analysis of variance (ANOVA; compared against control group), with a Geisser-Greenhouse correction and Dunnet’s post- hoc multiple comparison. For the remaining experiments containing a mix of normal and non-normal data, a non-parametric Friedman test with a Dunn’s multiple compari-sons post-hoc test was used. Differences were considered significant at p values < 0.05.
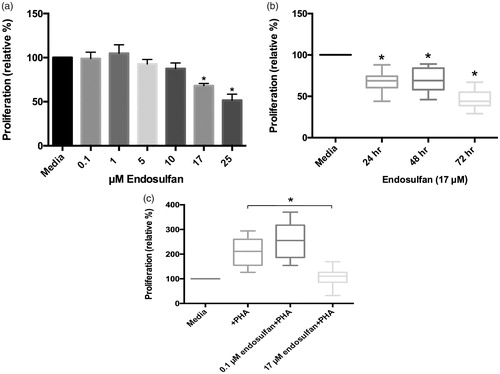
Figure 1. Effects of ENDO on PBMC proliferation. (a) Effects of ENDO (0–25 μM) used to treat PBMC for 24 h. All values for each PBMC population were normalized to proliferation by untreated (PBMC + media) cells from the same corresponding donor. Data reflect cells plated in duplicate/condition from 22 different donors. (b) Effects of 17 μM ENDO after 24, 48, and 72 h. All values normalized to proliferation by untreated (PBMC + media) cells: for 24 h, N = 22 donors/treatment; for 48 h, N = 7 donors/treatment; and for 72 h, N = 18 donors/treatment. (c) Effects of 0.1 and 17 μM ENDO on proliferation of PHA-stimulated PBMC after 72 h. All values normalized to proliferation by untreated (PBMC + media) cells. N = 16 donors/treatment. *Value significantly different from cells treated with PHA (p < 0.05).
Results
The current studies analyzed effects of a range of ENDO concentrations on proliferation among human PBMC. The presence of ENDO gave rise to dose-related effects on proliferation (), with a significant decrease in proliferation with 17 and 25 µM ENDO. It was also noted that increasing the period of exposure also resulted in decreased proliferation, i.e. culturing up to 72 h resulted in more decreased proliferation. In addition, cells stimulated with PHA proliferated in the presence of very low-dose (0.1 µM), but not low dose (17 µM), ENDO.
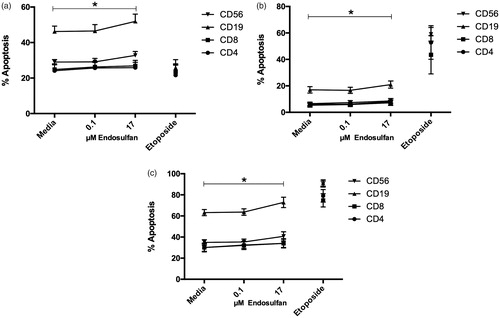
The next question this study addressed was which cell populations, in particular, among the PBMC might have been most affected by the ENDO. As ENDO has been reported to induce apoptosis, using flow cytometry, the present study focused on analysis of apoptosis among NK cells (CD56+), B-cells (CD19+), T-helper cells (CD4+), and cytotoxic T-cells (CD8+) within the treated PBMC. As seen in , 17 µM ENDO induced significant (total) apoptosis in all four cell types after 48 h (see . While significant, this level of apoptosis was far lower than that observed when the cells were treated with the cytostatic control etoposide. Significant early and late increases in apoptosis were also noted in all four cell types (. With respect to basal apoptosis and apoptosis levels in treated cells, NK and B-cells showed the greatest percentage increase in early and total apoptosis after ENDO exposure; cytotoxic T-cells showed the greatest relative increase in induced late apoptosis (.
Figure 2. Apoptosis induced by 0.1 and 17 μM ENDO treatment of PBMC for 48 h. (a) Early; (b) late; and (c) total apoptosis (sum of values of early and late apoptosis) in four cell populations. Etoposide used as a positive control for induction of apoptosis. Data reflect cells from 10 different donors. Values for each parent PBMC population were compared to untreated (PBMC + media) cells from the same corresponding donor. Values shown are means ± SEM. *Value was significantly increased from untreated cells (PBMC + media only) in each indicated population as a result of 48-h exposure to 17 μM ENDO (p < 0.05).
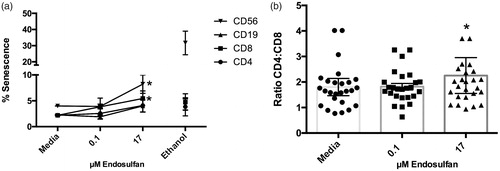
The effect observed in these apoptosis studies was relatively small and did not seem to explain the more notable losses of proliferation observed here. Accordingly, the study next evaluated senescence. The data shown in illustrates that there was a significant large effect on the NK and cytotoxic T-cell populations (an approximate doubling of cells in sense-cence with exposure to 17 µM ENDO). To gain a better understanding of the effects of ENDO on the immune system, CD4:CD8 ratios in the cultures were also calculated (. The results indicated there was a small but significant increase in the ratio; unexposed samples exhibited average values of 1.80 while 0.1 µM ENDO-treated cells had a ratio of 1.82 and 17 µM ENDO-exposed samples a value of 2.25.
Figure 3. Secondary consequences of ENDO exposure. (a) Senescence induced by 0.1 or 17 μM ENDO treatment of PBMC for 72 h. Data reflect cells from 10 different donors. Ethanol used as positive control for induction of senescence. (b) CD4:CD8 T-cell ratios measured after 18, 24, and 48 h of culture with and without ENDO. Data reflect cells from 30 different donors. Values for each parent PBMC population were compared to untreated (PBMC + media) cells from the same corresponding donor. *Value significantly different from untreated cells (PBMC + media only) (p < 0.05).
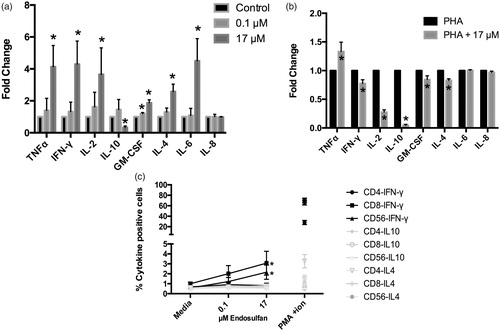
The data also indicated ENDO affected cytokine formation by the various cell types (). While 0.1 µM ENDO caused some increase in cell cytokine production, the effect was significant only with 17 µM ENDO. With this higher dose, levels of pro-inflammatory cytokines (e.g. TNFα, IFNγ, IL-2, -4, and -6) were increased an average of up to four-fold from control cell levels (. Levels of anti-inflammatory IL-10 were significantly decreased in tandem. Interestingly, this effect was mostly lost when cells were pre-stimulated with PHA, with only TNFα still being significantly increased due to the ENDO, with IL-6 only still slightly increased. Levels of IL-2, IFNγ, IL-4, GM-CSF, and IL-10 were all now being significantly decreased (. Analysis of intracellular cytokine formation showed that among IL-4, IL-10, and IFNγ, 17 µM ENDO significantly increased only IFNγ production by exposed NK and cytotoxic T-cells, but not by other cell types present (. Intracellular IL-4 and -10 levels were unaffected.
Figure 4. Effects of ENDO on PBMC cytokine production. Samples of culture medium were collected after 18 h of ENDO treatment and then analyzed using BioPlex system. Values for each parent PBMC population were compared to untreated (PBMC + media) cells from the same corresponding donor. (a) Mean (± SD) results from indicated treatments reported as fold-change with respect to normalized controls. Data reflect cells plated in duplicate/condition from nine different donors. (b) Mean (± SD) results from indicated treatments reported as fold-change with respect to normalized controls. N = 9 sets of donors/treatment. (c) Intracellular cytokine production. N = 10 sets of donors/treatment. PMA + ionomycin was used as positive control for induction of intracellular cytokine production. Values reported as percentage of gated cells of each population positive for intracellular staining of given cytokines; conditions in gray were not considered to have had effects at any concentration *Value significantly different from untreated cells (PBMC + media only) (p < 0.05).
Discussion
The goal of studies like the current one is to better understand potential effects that ENDO exposure may have on human immune system cells. Given that there is a great difference between acute and chronic environmental exposures, routes of ingestion, and lengths of exposure, the concentrations used here (or in any study) are critical. In the present study, earlier work with fish immune cells and in vitro studies of other groups provided key information for selection of doses. It is also important to note that in cell cultures, ENDO has been described as having potentially differing mechanisms of action at low doses (i.e. from 10 to 100 µM) vs. at high doses (from 200 to 1000 µM) (Kannan et al. Citation2000). Other studies suggested doses in the range of <100–200 µM should be considered non-cytotoxic (Lu et al. Citation2000; Antherieu et al. Citation2007).
In preliminary proliferation experiments done here with human PBMC, it was noted that ENDO imparted dose-related inhibitory effects, with significant decreases in proliferation noted with 17 and 25 µM ENDO. Because the goal of the present study was to evaluate effects of low-dose ENDO exposure, the remainder of the studies used just two concentrations, i.e. 0.1 µM or 17 µM ENDO. The 17 µM level was the lowest dose at which there had been a significant decrease in proliferation, and the 0.1 µM dose (lowest used) was the one that showed a tendency towards having an effect (albeit not significant) on cell proliferation.
It is well-known that ENDO can cause apoptosis in different cell types in/from a variety of hosts. Kannan et al. (Citation2000) noted dose-related increases in apoptosis among Jurkat T-cells after 48 h. Those authors reported that 10 µM ENDO caused 13% of the cells to be apoptotic at 48 h, while 50 µM ENDO resulted in 90% apoptosis in the same timeframe. These outcomes are similar to the current observations of ≈ 35–70% of the T, B, and NK cells being apoptotic at 48 h among all the cells exposed to 17 µM ENDO. Another paper (Ahmed et al. Citation2008) showed that ENDO – at what those authors considered low concentrations (5 and 10 µM) – did not cause significantly increased early apoptosis after 6 and 12 h incubations, but did so at 24 h. However, when higher concentrations (20, 50, 100 µM ENDO) were tested, apoptosis was increased significantly starting at 6 h and continuing out to 24 h. Such results are also in line with the current findings that after 48 h, significant apoptosis (early, late, and total) was attained with 17 µM, but not 0.1 µM, ENDO. To explain these results, one model (as discussed by Kannan et al [Citation2000]) is that oxidative stress triggered by ENDO and/or its metabolites may lead to increased production of reactive oxygen species (ROS) among the exposed cells and that this agent in turn might trigger apoptosis. This triggering of apoptosis may be rapid (at higher concentrations) or delayed (at lower concentrations) and dependent on an inhibition of expression of anti-apoptosis molecules or release of apoptogenic proteases to the cytosol (Vaux Citation2011; Zhang et al. Citation2017; Xu et al. Citation2018).
The principal mechanism of acute toxicity through which ENDO acts in the body is by binding to, and blocking, the Cl-channel linked to the inhibitory g-aminobutyric acid (GABA)-receptor. The most common GABA receptors are the GABAA class, which are fast acting ligand gated ion channels. These channels are present on neurons but also on other cell types including some peripheral blood mononuclear cells (Alam et al. Citation2006; Dionisio et al. Citation2011). In mouse models, GABA signaling has been shown to be important for the inhibition of cell mediated autoimmunity (at least in a murine model of diabetes, Tian et al. Citation2004) and for a decrease in proliferation of encephalitogenic (pathogenic) T-cells entering the brain (Bjurstöm et al. Citation2008). Thus, antagonism of the GABA signal might be expected to lead to overactive immune cells in some models. Here, we surmise it does not occur when evaluating the low level effects of ENDO on proliferation and apoptosis; however, ENDO might have other effects on other mediators of the immune system, namely the production of cytokines and chemokines.
In the present experiments, substantial significant increases in pro-inflammatory cytokine formation (and a decrease in anti-inflammatory IL-10) were observed as a result of the exposure of the PBMC to 17 µM ENDO for 18 h. The findings here also revealed that there was significant IFNγ production by both NK and cytotoxic T-cells within this population. These latter findings are all the more interesting in that the study here also showed that ENDO imparted a strong effect on cytotoxic T-cells and that ENDO decreased the relative percentages of CD8 cells among all the PBMC. This outcome is akin to that with other OCP that have been found to also lead to increases in CD4:CD8 ratios by skewing of the denominator populations (Svensson et al. Citation1994; Kłuciński et al. Citation1996; Vine et al. Citation2000; Wade et al. Citation2002; Reed et al. Citation2004).
While the present study also did observe significant apoptosis among B-cells in the parent PBMC populations, some of this deleterious effect might have been balanced out by the presence of the now pro-inflammatory cytokine environment – specifically increases induced in formation of cytokines like IL-4 and IL-6 by other cells within these same populations being treated. By this, both IL-4 and IL-6 stimulate B-cell proliferation and differentiation into plasma cells, thereby countering losses of B-cells to apoptosis induced by the ENDO itself. Further, IL-4 (via Stat6 signaling and up-regulation of Bcl-xl expression) has a strong anti-apoptotic effect on B-cells (Wurster et al. Citation2002). Likewise, IL-6 has multiple targets, and has been found to up-regulate Bcl-2 expression in pro-B cells (and many tumor models) (Fukada et al. Citation1996; Lin et al. Citation2001). Together, these observations lead us to suspect that at an organismal level, the humoral response might not be as seriously impacted by ENDO exposure as would immune responses dependent upon a presence and functionality of NK and/or cytotoxic T-cells.
It is possible that the changes in cytokines seen here may be important clues with respect to any long-term induction of chronic inflammation or autoimmunity in situ following ENDO exposures. Indeed, exposure to DDT (related OCP) has been found to be associated with induction of autoimmunity in a murine model of systemic lupus erythematosus (Li and McMurray Citation2009). With respect to induction of pro-inflammatory cytokines, ENDO has been shown to participate in the MAPK pathway, inducing the formation of reactive oxygen species (ROS), events that induce NF-κB and COX-2 expression (Kim et al. Citation2015). It had been seen that fish splenocytes exposed in vitro to ENDO demonstrated increased ERK/MAPK activity, i.e. increased levels of phosphorylated ERK (p-ERK [Tellez-Bañuelos et al. Citation2011]). Han et al. (Citation2007) showed that ENDO could activate macrophage ERK/MAPK pathways, leading to increased TNFα, IL-6, and IL-2 formation. Further, signaling though estrogen receptors (ER) and activation of AKT/mtTor pathways, has been shown to induce ROS production as well as formation of IFNγ, NO, and TNFα by lymphocytes (Pratap et al. Citation2015). That many of these same changes were noted here in the ENDO-treated PBMC might lead one to suspect that a xenoestrogen like ENDO might be, in addition to interfering with GABA signaling, binding ER receptors on these cells.
It is important to note that when ENDO was used on PBMC pretreated with PHA, a strong (≈3-fold) loss in inducible IL-2 formation, as well as that of other cytokines, was noted. These results suggested to us that ENDO had a different deleterious effect on these already-activated cells. Using DDT, it was found that PHA + DDT abrogated PHA-inducible pro-inflammatory cytokine production by PBMC (Dar et al. Citation2012). Terry et al. (Citation2018) also showed that ENDO decreased NO production by a mouse macrophage cell line (RAW 264.7) that had been stimulated with LPS + IFNγ. Interestingly, in that particular study, ENDO treatment also led to slightly increased formation of TNFα, similar to the current results with PHA. These outcomes led to the possible suggestion that ENDO had a capacity to alter inflammatory responses differentially, particularly depending on cell activation status. The NF-κB pathway is greatly induced by PHA stimulation of the T-cell receptor (Oh and Ghosh Citation2013). Similar to the way that excessive concentrations of PHA can lead to cell death in cultures, one could suspect over-stimulation of the NF-κB pathway caused by the combination of PHA and ENDO and/or the likely additional oxidative stress/DNA damage caused by ENDO alone, results in a saturation of the response and rapid induction of senescence/apoptosis. This seems to be borne out by the present results, wherein ENDO did not seem to impart its expected pro-inflammatory effect when it was used in combination with PHA (or specifically, in already stimulated cells). This also might tend to explain the non-significant effects from the lowest concentrations of ENDO, i.e. it did appear to have a low-level proliferative effect, which would be consistent with the idea of providing low-level NF-κB MAP kinase stimuli.
Long-term effects from ENDO-induced increased inflammatory cytokine formation could prove to be significant. In a rat model, prolonged exposure to ENDO led to increased indications of systemic inflammation (i.e. serum IL-6 and TNFα levels increased 15- and 4-fold from control rat levels) (Téllez-Bañuelos et al. Citation2016). Chronic inflammation may also lead to (or, as discussed below, also be a by-product of) senescence among immune cells. It has been observed that ENDO induces senescence in fish splenocytes (Tellez-Bañuelos et al. Citation2011) and that oxidative stress plays a critical role in the induction of senescence (Carnero et al. Citation2015). In the current work, it was seen that ENDO induced senescence among PBMC treated with 17 µM, but not at 0.1 µM, ENDO. Senescence locks cells at risk for malignant transformation (typically fibroblasts and epithelial cells) into cell cycle arrest, thereby preventing damage from progress-ing beyond the current cell generation (Campisi and d'Adda di Fagagna Citation2007). Accordingly, senescence may prove to be a protective mechanism for any cell stressed by exposure to ENDO. However, it is not completely correct to think of senescent cells as totally quiescent. Such cells can be characterized by a senescence-associated secretory phenotype (SASP), wherein there is increased secretions of a variety of factors (such as IL-6, IL-1, TNFα, GM-CSF, and matrix metalloproteases). This SASP transformation has been linked to aberrant p38/MAPK and NF-κB activation (Freund et al. Citation2011). In the short-term, this shift to SASP may be protective for a host but could, ultimately, be found to negatively influence long-term health (Lecot et al. Citation2016).
In this study, both an increase in cytokine production and senescence was noted among ENDO-treated PBMC. While the original goal here was to discern if low-dose ENDO induced over-activation of different immune cells, the increased pro-inflammatory phenotype in addition to the increase in senescence caused us to wonder if these observations may be linked. Similar to the SASP observed with fibroblasts, immunosenescent T-cells displayed inhibited proliferation, yet retained an ability to produce/up-regulate their production of pro-inflammatory agents. While the SASP model remains to be specifically proven in T-cells, it has been suggested by others that senescent T-cells do exhibit some aspects of SASP (Xu and Larbi Citation2017).
Conclusions
This study showed that ENDO imparted significant effects on PBMC proliferation, senescence, and apoptosis, particularly among CD8 and NK cells. These cells showed both increased apoptosis and senescence, as well as increased cytokine production. Such outcomes suggested ENDO caused a continuum of responses, with those cells not in senescence perhaps being over-stimulated (resulting in apoptosis). The similarity of the changed cytokine profile to the senescence associated secretory phenotype and increases in senescence argue that ENDO may play an important role in immunosenescence and chronic health conditions like autoimmunity, at concentrations far below the cytotoxic level. This could be of particular importance to workers exposed to ENDO and populations facing ongoing environmental ENDO contamination. Thus, this work shows that chronic low-dose exposures should be taken into account when considering the long term environmental effects of ENDO and to give a scientific basis for new norms and regulations regarding its use and danger. Perhaps such considerations should be made in particular during any reevaluation of NOEL and LOAEL for ENDO in various test organisms.
Acknowledgements
The Authors gratefully acknowledge Miguel Enrique Magaña Virgen (MS) for the invaluable professional assistance, Maria Fernanda Rios Perez, (BS) for participation in the proliferation and cytometry studies, and Galina Petrovna Zaitseva (PhD) for the critical reading of this manuscript, and long leadership in this field and our research group.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Additional information
Funding
References
- Ahmed T, Tripathi A, Ahmed R, Das S, Suke S, Pathak R, Chakraboti A, Banerjee B. 2008. Endosulfan-induced apoptosis and glutathione depletion in human peripheral blood mononuclear cells: Attenuation by N-acetylcysteine. J Biochem Mol Toxicol. 22(5):299–304.
- Alam S, Laughton D, Walding A, Wolstenholme A. 2006. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol. 43(9):1432–1442.
- Antherieu S, Ledirac N, Luzy A, Lenormand P, Caron J, Rahmani R. 2007. Endosulfan decreases cell growth and apoptosis in human HaCaT keratinocytes: Partial ROS-dependent ERK1/2 mechanism. J Cell Physiol. 213(1):177–186.
- Attaullah M, Yousuf MJ, Shaukat S, Anjum SI, Ansari MJ, Buneri ID, Tahir M, Amin M, Ahmad N, Khan SU. 2018. Serum organochlorine pesticides residues and risk of cancer: A case-control study. Saudi J Biol Sci. 25(7):1284–1290.
- Bjurstöm H, Wang J, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. 2008. GABA, a natural immunomodulator of T-lymphocytes. J Neuroimmunol. 205(1–2):44–50.
- Bulayeva N, Watson C. 2004. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect. 112(15):1481–1487.
- Campisi J, d'Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Bio 8:729–740.
- Campoy C, Jimenez M, Olea-Serrano M, Moreno Frias M, Cañabate F, Olea N, Bayés R, Molina-Font J. 2001. Analysis of organochlorine pesticides in human milk: Preliminary results. Early Human Dev. 65:183–190.
- Canlet C, Tremblay-Franco M, Gautier R, Molina J, Métais B, Estrada F, Gamet-Payrastre L. 2013. Specific metabolic fingerprint of a dietary exposure to a very low dose of endosulfan. J. Toxicol. 2013:1.
- Carnero A, Blanco-Aparicio C, Kondoh H, Lleonart ME, Martinez-Leal JF, Mondello C, Scovassi AI, Bisson WH, Amedei A, Roy R, et al. 2015. Disruptive chemicals, senescence and immortality. Carcinogenesis. 36(Suppl 1):S19–S37.
- Carreño J, Rivas A, Granada A, Lopez-Espinosa J, Mariscal M, Olea N, Olea-Serrano F. 2007. Exposure of young men to organochlorine pesticides in Southern Spain. Environ Res. 103(1):55–61.
- Dalvie M, Africa A, Solomons A, London L, Brouwer D, Kromhout H. 2009. Pesticide exposure and blood endosulfan levels after first season spray amongst farm workers in the Western Cape, South Africa. J Environ Sci Health. 44(3):271–277.
- Dar S, Das S, Ramachandran V, Bhattacharya S, Mustafa M, Banerjee B, Verma P. 2012. Alterations in T-lymphocyte subset profiles/cytokine secretion by PBMC of systemic lupus erythematosus patients upon in vitro exposure to organochlorine pesticides. J Immunotoxicol. 9(1):85–95.
- Desalegn B, Takasuga T, Harada K, Hitomi T, Fujii Y, Yang H, Wang P, Senevirathna S, Koizumi A. 2011. Historical trends in human dietary intakes of endosulfan and toxaphene in China, Korea and Japan. Chemosphere. 83(10):1398–1405.
- Dionisio L, José De Rosa M, Bouzat C, Esandi M. 2011. An intrinsic GABAergic system in human lymphocytes. Neuropharmacology. 60(2–3):513–519.
- El-Shenawy N. 2010. Effects of insecticides fenitrothion, endosulfan and abamectin on anti-oxidant parameters of isolated rat hepatocytes. Toxicol in Vitro. 24(4):1148–1157.
- Enhui Z, Na C, Meng L, Jia L, Dan L, Yongsheng Y, Ying Z, Fu H. 2016. Isomers and their metabolites of endosulfan induced cytotoxicity and oxidative damage in SH‐SY5Y cells. Environ Toxicol. 31(4):496–504.
- Freund A, Patil C, Campisi J. 2011. P38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30(8):1536–1548.
- Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. 1996. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: Involvement of STAT3 in anti-apoptosis. Immunity. 5(5):449–460.
- Ghosh K, Chatterjee B, Geetha J, Kanade S. 2018. The persistent organochlorine pesticide endo-sulfan modulates multiple epigenetic regulators with oncogenic potential in MCF-7 cells. Sci Total Environ. 624:1612–1622.
- Guo F, Zhang L, Wei J, Li Y, Shi Z, Yang Y, Zhou X, Sun Z. 2015. Endosulfan induced the arrest of the cell cycle through inhibiting the signal pathway mediated by PKCα and damaging the cytoskeleton in spermatogonial cells of mice in vitro. Toxicol Res. 4(2):508–518.
- Han EH, Hwang YP, Kim HG, Jeong HG. 2007. Inflammatory effect of endosulfan via NF-κB activation in macrophages. Biochem Bioph Res Co 355:860–865.
- Integrated Risk Information System (IRIS) Chemical, and Agency, U.S.E.P.A. 1994. Endosulfan (CASRN 115-29-7). USA. Retrieved from https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0235_summary.pdf#nameddest=rfd.
- Kannan K, Holcombe R, Jain S, Alvarez-Hernandez X, Chervenak R, Wolf R, Glass J. 2000. Evidence for the induction of apoptosis by endosulfan in a human T-cell leukemic line. Mol Cell Biochem. 205(1–2):53–66.
- Kim H, Kim Y, Park J, Khanal T, Choi J, Do M, Jin S, Han E, Chung Y, Jeong H. 2015. Endosulfan induces COX-2 expression via NADPH oxidase and the ROS, MAPK, and Akt pathways. Arch Toxicol. 89(11):2039–2050.
- Kłuciński P, Hrycek A, Stasiura-Zielińska H, Kossmann S, Tustanowski J, Friedek D, Kamińska-Kołodziej B. 1996. Humoral and cellular immunity rates in chemical plant workers employed in the production of liquid pesticides. Intl J Occup Med Environ Health. 9:103–110.
- Lecot P, Alimirah F, Desprez P, Campisi J, Wiley C. 2016. Context-dependent effects of cellular senescence in cancer development. Br J Cancer. 114(11):1180–1184.
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. 2006. Activation of α- and β-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 79(12):1160–1169.
- Li J, McMurray R. 2009. Effects of chronic exposure to DDT and TCDD on disease activity in murine systemic lupus erythematosus. Lupus. 18(11):941–949.
- Lin M, Juan C, Chang K, Chen W, Kuo M. 2001. IL-6 inhibits apoptosis and retains oxidative DNA lesions in human gastric cancer AGS cells through up-regulation of anti-apoptotic gene mcl-1. Carcinogenesis. 22(12):1947–1953.
- Lu Y, Morimoto K, Takeshita T, Takeuchi T, Saito T. 2000. Genotoxic effects of alpha-endosulfan and beta-endosulfan on human HepG2 cells. Environ Health Perspect. 108(6):559–561.
- Martínez-Valenzuela C, Gómez-Arroyo S, Villalobos-Pietrini R, Waliszewski S, Calderón-Segura M, Félix-Gastélum R, Álvarez-Torres A. 2009. Genotoxic biomonitoring of agricultural workers exposed to pesticides in north of Sinaloa State. Mexico Environ Intl. 35(8):1155–1159.
- Menezes R, Qadir T, Moin A, Fatima H, Hussain S, Madadin M, Pasha S, Al Rubaish F, Senthilkumaran S. 2017. Endosulfan poisoning: An overview. J Forensic Leg Med. 51:27–33.
- Mrema E, Rubino F, Brambilla G, Moretto A, Tsatsakis A, Colosio C. 2013. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 307:74–88.
- Oh H, Ghosh S. 2013. NF-κB: Roles and regulation in different CD4+ T-cell subsets. Immunol Rev. 252(1):41–51.
- Pi N, Eng S, Nam C, Kelly B. 2016. Associations of serum organo-halogen levels and prostate cancer risk: Results from a case control study in Singapore. Chemosphere. 144:1505–1512.
- Pratap U, Sharma H, Mohanty A, Kale P, Gopinath S, Hima L, Priyanka H, Thyaga Rajan S. 2015. Estrogen up-regulates inflammatory signals through NF-κB, IFNγ, and nitric oxide via Akt/mTOR pathway in lymph node lymphocytes of middle-aged female rats. Intl. Immunopharmacol. 29(2):591–598.
- Rau A, Coutinho A, Avabratha K, Rau A, Warrier R. 2012. Pesticide (endosulfan) levels in the bone marrow of children with hematological malignancies. Indian Pediatr. 49(2):113–117.
- Reed A, Dzon L, Loganathan B, Whalen M. 2004. Immunomodulation of human natural killer cell cytotoxic function by organochlorine pesticides. Hum Exp Toxicol. 23(10):463–471.
- Schaalan M, Abdelraouf S, Mohamed W, Hassanein F. 2012. Correlation between maternal milk and infant serum levels of chlorinated pesticides (CP) and the impact of elevated CP on bleeding tendency and immune status in some infants in Egypt. J Immunotoxicol. 9(1):15–24.
- Shah H, Bhat M, Sharma T, Banerjee B, Guleria K. 2018. Delineating potential transcriptomic association with organochlorine pesticides in the etiology of epithelial ovarian cancer. Open Biochem J. 12:16–28.
- Silva M, Carr W. 2010. Human health risk assessment of endosulfan. II. Dietary exposure assessment. Regul Toxicol Pharm. 56(1):18–27.
- Stockholm Convention on Persistent Organic Pollutants. United Nations Environmental Program (UNEP). 2009. Draft risk profile: Endosulfan Stockholm Convention on Persistent Organic Pollutants. Geneva. http://chm.pops.int/Default.aspx?tabid=592%0A
- Stockholm Convention on Persistent Organic Pollutants. POPs Review Committee (POPRC). 2017. The 16 New POPs. Geneva, Switzerland. http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx
- Svensson B-G, Hallberg T, Nilsson A, SchüTz A, Hagmar L. 1994. Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds. Int Arch Occup Environ Health. 65(6):351–358.
- Téllez-Bañuelos M, Haramati J, Franco-Topete K, Peregrina-Sandoval J, Franco-Topete R, Zaitseva G. 2016. Chronic exposure to endosulfan induces inflammation in murine colon via β-catenin expression and IL-6 production . J Immunotoxicol. 13(6):842–849.
- Tellez-Bañuelos M, Ortiz-Lazareno P, Santerre A, Casas-Solis J, Bravo-Cuellar A, Zaitseva G. 2011. Effects of low concentration of endosulfan on proliferation, ERK1/2 pathway, apoptosis and senescence in Nile tilapia (Oreochromis niloticus) splenocytes. Fish Shellfish Immunol. 31(6):1291–1296.
- Tellez-Bañuelos M, Santerre A, Casas-Solis J, Bravo-Cuellar A, Zaitseva G. 2009. Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol. 27(2):105–111.
- Terry A, Benitez-Kruidenier S, DeKrey G. 2018. Effects of endosulfan isomers on cytokine and nitric oxide production by differentially activated RAW 264.7 cells. Toxicol Rep. 5:396–400.
- Tian J, Lu Y, Zhang H, Chau C, Dang H, Kaufman D. 2004. γ-Aminobutyric acid inhibits T-cell autoimmunity and development of inflammatory responses in a mouse Type 1 diabetes model. J Immunol. 173(8):5298–5304.
- Vaux D. 2011. Apoptogenic factors released from mitochondria. Biochim Biophys Acta. 1813(4):546–550.
- Vine M, Stein L, Weigle K, Schroeder J, Degnan D, Tse C, Hanchette C, Backer L. 2000. Effects on immune system associated with living near a pesticide dump site. Environ Health Perspect. 108(12):1113–1124.
- Wade M, Foster W, Younglai E, McMahon A, Leingartner K, Yagminas A, Blakey D, Fournier M, Desaulniers D, Hughes C. 2002. Effects of subchronic exposure to a complex mixture of persistent contaminants in male rats: Systemic, immune, and reproductive effects. Toxicol Sci. 67(1):131–143.
- Wong F, Alegria H, Bidleman T. 2010. Organochlorine pesticides in soils of Mexico and the potential for soil-air exchange. Environ Pollut. 158(3):749–755.
- Wurster A, Rodgers V, White M, Rothstein T, Grusby M. 2002. Interleukin-4-mediated protection of primary B-cells from apoptosis through Stat6-dependent up-regulation of Bcl-xL . J Biol Chem. 277(30):27169–27175.
- Xu D, Liang D, Guo Y, Sun Y. 2018. Endosulfan causes the alterations of DNA damage response through ATM-p53 signaling pathway in human leukemia cells . Environ Pollut. 238:1048–1055.
- Xu W, Larbi A. 2017. Markers of T-cell senescence in humans. Intl J Mol Sci. 18:1–13.
- Yang J, Wang Z, Ma L, Shen X, Sun Y, Hu D, Sun L. 2015. The organochlorine pesticides residues in the invasive ductal breast cancer patients. Environ Toxicol Pharmacol. 40(3):698–703.
- Zhang L, Wei J, Ren L, Zhang J, Yang M, Jing L, Wang J, Sun Z, Zhou X. 2017. Endosulfan inducing apoptosis and necroptosis through activation RIPK signaling pathway in human umbilical vascular endothelial cells. Environ Sci Pollut Res. 24(1):215–225.
