Abstract
The objective of this research was to evaluate consequences to the immune system of long-term exposure to waste anesthetic gases (WAG) by medical theater personnel. Two groups were recruited: (i) 60 healthy male controls; (ii) 120 medical professionals exposed to WAG, subdivided according to theater role, i.e. surgeons, surgical assistants (SA), anesthetists, anesthetic assistants (AA), nurses, and workers. Serum levels of fluoride, hexafluoroisopropanol (HFIP), total lymphocyte counts, as well as of CD3, CD4, and CD8 cells, CD4/CD8 ratios, and immunoglobulins IgA, IgG, IgM, and IgE were assayed. The results showed that fluoride and HFIP titers were significantly increased in anesthetists and AA compared with the other exposed groups. All exposed groups demonstrated significant elevation in lymphocyte count, CD4+ cell levels, CD4/CD8 ratios, as well as levels of IgE, IgM and IgG compared with the controls. With regard to the latter outcomes, a significant increase in IgE was seen in the surgeon, nurse, and worker groups compared with the other professions. Surgeons, anesthetists and AA exhibited higher IgM titers compared with their colleagues. Significantly higher IgG levels were identified in the SA, anesthetists, AA, and workers than in their nurses and surgeon coworkers. Of the six sub-groups, only the anesthetists and their assistants (AA) displayed a significant increase in CD4+ cells and CD4/CD8 ratios and a decrease of CD8+ cells compared with the controls. This spectrum of results suggests that variation exists in immunomodulatory responses to WAG exposure amongst hospital personnel.
Introduction
It is well documented in the literature that long-term exposure to volatile anesthetic compounds has been associated with adverse clinical consequences (Lucchini et al. Citation1995; Vouriot et al. Citation2005). Following inhalation, ≈80% of the gas is expelled by exhalation; however assays of breakdown products have shown the remaining fifth is metabolized and then carried through the blood to other organs and the periphery (Casale et al. Citation2014). According to the Occupational Safety and Health Administration (Citation2010) and the Canadian Center for Occupational Health and Safety (Citation2020), over 200,000 medical personnel persistently subjected to inhalational anesthetics may be at risk of associated occupational diseases. Manifestations could include hematologic, hepatic, immune, neurologic, renal, and reproductive pathologies (Miller et al. Citation2020).
Manufactured within the plasma cells, immunoglobulins (Igs) are an integral part of the immune system. Their production is initiated by foreign antigenic material, e.g. bacteria, viruses, allergens, tumor markers, or chemicals (Burton Citation1990). After secretion into the circulation, Igs then contribute to several immunological functions, e.g. triggering bacterial phagocytosis, and allergic and anti-tumor responses (Engels and Wienands Citation2018). Clinical assays of serum immunoglobulin (Ig) titers can be performed after exposure to any type of toxicant to provide insight into potential effects on circulating antibody status. Diminished Ig detection may suggest the presence of immunodeficiency syndromes (Buckley Citation1986). A presence of elevated Ig levels has been documented to occur in association with a variety of pathologies including those of hepatic, chronic inflammatory, haematological, infectious and neoplastic origins (Dispenzieri et al. Citation2001). Further, a presence of specific antibodies or Ig patterns can act as useful adjunctive diagnostic information, e.g. are useful to verify chronic liver diseases (Yamamoto et al. Citation2003).
The potential for effects from exposure to waste anesthetic gas (WAG) on host immune function has been described in a variety of studies. Analyses of the effect of WAG exposure on the lymphocytic arm of the immune system revealed diminished proportions of total T-cells and CD3 and CD4 (T-helper; TH) cells whereas natural killer (NK) cell populations (CD16+, CD3−) were increased (Bargellini et al. Citation2001). Koutsogiannaki et al. (Citation2016) demonstrated impairment of neutrophil phagocytosis after exposures to volatile anesthetics; this dysfunction was associated with an enhanced risk of bacterial sepsis. Braz et al. (Citation2020) reported an elevation in serum levels of IL-17A in medical residents after exposure to WAG; this is important in that IL-17A has an important role in both host innate and adaptive immunity, and is a potent pro-inflammatory cytokine whose elevation is associated with the development of respiratory problems. Chaoul et al. (Citation2015) noted that there were significant increases in serum levels of pro-inflammatory IL-8 in young physicians incidentally exposed to waste anesthetic gases.
Sevoflurane, a highly fluorinated methyl isopropyl ether, is a popular gaseous anesthetic owing to its cardiorespiratory safety profile (Delgado-Herrera et al. Citation2001). Once entrained and transported from the lungs, it is broken down in the liver to form inorganic fluoride and hexa-fluoro-isopropanol (HFIP) metabolites. Consequently, serum levels of these compounds can be detected rapidly after inhalation (Kharasch Citation1995). In theory, medical personnel using inhalation anesthesia like Sevoflurane are at risk of WAG inhalation and vulnerable to any potential immunomodulating properties of the agents.
The aim of this research was to discern any adverse effects on the immune system (for example, changes in host Ig titers, lymphocyte counts – and specifically in levels of CD3+, CD4+, and CD8+ subtypes) arising from long-term repeated incidental WAG exposures by clinical staff practicing at medical institutions located throughout the Qassim area of Saudi Arabia. To further narrow how the exposure-related effects might be related to proximity to/repetitive exposures to WAG, potential differential effects on various types of medical professionals (according to theater role), including surgeons, surgical assistants (SA), anesthetists, anesthetic assistants (AA), nurses, and workers, were evaluated.
Materials and methods
Study populations
This research was performed over a 5-month period (October 2018–January 2019) in a group of medical institutions located in the Qassim area of South Arabia. A cross-sectional comparative study design was employed. Two subject cohorts were recruited: (i) control group (G1), consisting of 60 healthy males with no history of WAG exposure and (ii) an exposure group, i.e. 120 male operating theater personnel with likely persistent exposure to WAG. The exposure group was composed of: surgeons (G2), surgical assistants (G3), anesthetists (G4), anesthetic assistants (G5), nurses (G6) and workers (G7). All study subjects were matched to controls for age, socio-demographic status, and body mass index (BMI). Individuals who were actively smoking, had less than 1 year employment history in the institution, or had any factors that might influence their immune system, e.g. ongoing pathology, allergy, or were receiving immune-modifying medication, were excluded from study participation. Demographic data was acquired from all individuals. This included occupational details, i.e. working hours, shift patterns, clinical area, documentation of frequently used anesthetic agents, clinical history, and smoking history. BMI was also calculated, i.e. weight (kg)/height (m2) for each study subject. Each recruit gave informed consent before the study commenced.
This research was approved by research ethics committee of the College of pharmacy, Qassim University and the Ministry of Health of Saudi Arabia (Approval number: 20180526).
Note to readers: The authors are keenly aware that this study does not provide specific levels of WAG exposure, nor reports on numbers of the total numbers of incidental exposures by individuals in each of the study groups. This is the nature of such exposures. Accordingly, the outcomes should be seen as reflective of cumulative incidental exposures. All attempts were made to exclude from study any subject who had been in the operating theater within 24 hr of collection of their blood sample such that acute effects would not be impacting on the overall study group outcomes.
Collection of blood samples
A fasting blood sample (10 ml) was taken from the hand vein into a silicon-coated test-tube between 8 and 9 AM at the end of a work shift. Each sample was split into three aliquots. Plasma extraction was achieved from the first by centrifugation (1000×g, 15 min, 4 °C). The other two aliquots were reappropriated to ethylenediaminetetraacetic acid-coated test-tubes for subsequent serum isolation. All samples were frozen at −20 °C prior to assay.
Plasma fluoride levels
Plasma fluoride concentrations were assayed in duplicate using previously detailed protocols (Levine et al. Citation1996; Şener et al. Citation2007). The subject sample was mixed with an equal volume of total ionic strength adjustment buffer and then analyzed in an Orion 901 Ion Analyzer (Thermo Fisher Scientific, Chelmsford, MA), accessorized with an ion-selective electrode that included fluoride-specific and combination pH electrodes. Parallel standard fluoride curves were generated to aid in calculating plasma fluoride levels by extrapolation. A linear relationship was present over a concentration spectrum of 1–48 µM (correlation coefficient = 0.999). The lower limit of detection was 1 µM.
Plasma HFIP levels
Headspace gas chromatography with flame-ionization detection was used to evaluate the concentration of plasma HFIP (Kharasch et al. Citation1995). Triplicate assays were performed on each sample. Plasma was incubated with β-glucurondiase/sulfatase (2000U Type HI, Sigma, St. Louis, MO) for 15 hr at 37 °C to break down HFIP metabolites into corresponding free alcohols. After cooling to ambient temperature, the pH of the mixture was lowered from 5 to 2 by injection of 200 µl of 3.5 M phosphoric acid. The sample was vortex-mixed and then injected into an HS40 headspace system autosampler (PerkinElmer, Melbourne, FL). A capillary column (30 cm x 0.53 mm x 3.0 µm film thickness) containing RTX 1701 resin (Restek, Bellefonte, PA) was used for material separation. Standard curves, derived from internal standard (2,2,3,3,3-pentafluoro-1-propanol), were generated to aid in extrapolating the plasma HFIP concentrations.
Immunoglobulin assay
IgE titers were evaluated in triplicate using an enzyme-linked immunosorbent assay kit (Immunolab GmbH, Kassel, Germany) and following manufacturer protocols. Elevated serum IgE levels were defined as ≥ 100 IU/ml. Serum IgG, IgM and IgA titers were assayed in triplicate using MININEPHTM nephelometry kits (The Binding Site, Birmingham, UK). In brief, serum samples were thawed and 1:10 dilutions made using 40 μl sample: 400 μl kit diluent. In the assays, 400 μl kit buffer, 40 μl anti-Ig solution, and 10 μl sample for IgG assessment were combined in a cuvette; for IgM and IgA measures, 40 μl sample was added. In each case, the cuvette was then placed in a UV-Vis spectrophotometer (Shimadzu, Columbia, MD) and absorbance values (280 nm) were recorded after 15 min. Standard parallel curves derived for each isotype were used to help in extrapolation of concentration of each Ig type. Normal serum ranges were defined as: IgG, 658–1837 mg/dl; IgM, 40–263 mg/dl; IgA, 71–360 mg/dl.
Lymphocyte subset measurements
In brief, an aliquot of whole blood (100 µl) from each subject was pelleted, and the cells were re-suspended in 0.84% (w/v) ammonium chloride-saline solution on ice for 10 min to lyse erythrocytes. The remaining cells were then pelleted and re-suspended in 1 ml phosphate-buffered saline (PBS, pH 7.4). From this, the total numbers of lymphocytes present were determined. An aliquot containing 106 cells was then removed and combined (in 15 µl total volume) with a specific anti-surface marker fluorochrome-conjugated monoclonal antibody against CD3, CD4, or CD8 (each from Becton Dickenson [BD], San Jose, CA) for 30 min in the dark at room temperature. Thereafter, each sample was examined in a BD FACSCalibur flow cytometry system and data was analyzed with system-associated software. From the data, levels of CD3+, CD4+, and CD8+ cells were determined in each sample. A minimum of 10,000 events per sample was acquired. Each sample was evaluated in triplicate.
Statistical analysis
Analysis was performed using Statistical Package for Social Sciences software, (v.21.0, SPSS Inc., Chicago, IL). Comparison of the exposed sub-groups with the control subjects was performed with one-way analysis of variance (ANOVA). A Dunnett comparison test was used to evaluate any differences between the respective sub-groups. Statistical significance was accepted at p < 0.05. Possible relationships between the concentrations of fluoride, FHIP, and Ig levels were analyzed with Pearson’s correlation coefficient. Software used for the latter was Prism software (v.8.0 for Windows; GraphPad, San Diego, CA). All data are expressed as mean ± SD.
Results
illustrates the matched socio-demographic data for the control (G1) and the WAG-exposed groups (G2–G7). As expected, no significant differences were seen in age, marital status, BMI, or occupational history between the respective cohorts. Of the six sub-groups, only the anesthetists and their assistants (AA) gave a history of headaches, dizziness, syncope and fatigue occurring within work hours.
Table 1. Socio-demographic characteristics and occupational history of the control and waste anesthetic gases exposed groups.
Plasma fluoride and HFIP levels
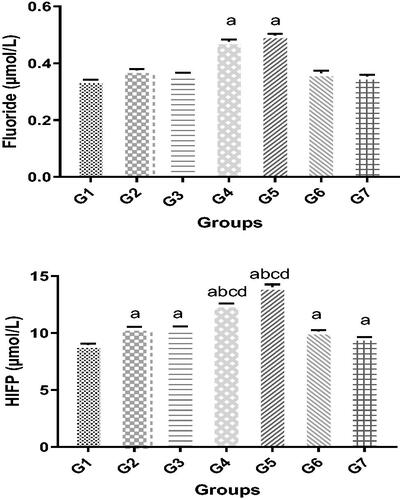
Compared with the control group, elevated plasma fluoride titers were identified in groups G2–G7 (). However, this only achieved significance in the anesthetists and AA groups, i.e. 0.44 [± 0.02] µM and 0.48 [± 0.01] µM, respectively, compared with the controls (0.35 [± 0.01] µM). Plasma HFIP concentrations were also higher in all the exposed groups compared with the controls (). Among the sub-groups, only subjects from the G3 and G4 groups had significantly elevated levels compared with their counterparts; identical levels of HFIP were found is these latter study cohorts.
Figure 1. Changes in blood levels of markers of WAG exposure. Plasma (A) fluoride and (B) HFIP levels. Significance of differences was analyzed by one-way ANOVA and Dunnett test. Values are expressed as mean (in µM) ± SD. Groups (left to right): G1: Control group, G2: Surgeon assistant (SA), G3: Surgeon, G4: Anesthesia specialist, G5: Anesthesia assistant (AA); G6: Nurses; and G7: Workers groups. Value was significantly different from acontrol, bSA, csurgeon, and/or dworker group at p ≤ 0.05.
Serum IgE, IgM, IgG, and IgA levels
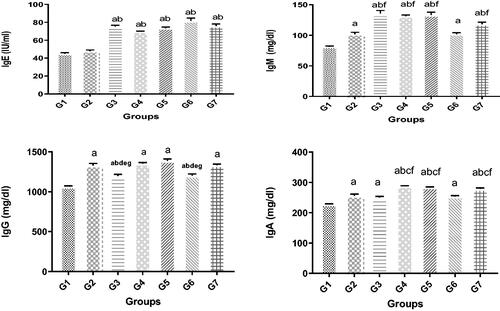
The only WAG-exposed sub-group that failed to show a significant increase in serum IgE compared with the controls was the SA. Levels were even more elevated among some of the sub-groups compared to others, i.e. elevated IgE levels were noted among the surgeons, nurses, and workers compared with not only the SA, but also with the anesthetist and AA groups. IgE titers were equivalent in the latter two groups. IgM concentrations were significantly raised in all WAG-exposed groups compared with the controls. Increased IgM concentrations were more notable in the SA, anesthetists, AA, and the worker groups compared with the surgeons and nurses (). Measurement of IgG showed a similar increase in all the subject groups exposed to WAG compared with the controls. Again, there were disparities in the levels of change relative to the controls, i.e. the observed rise in IgG levels was significantly less in the surgeons and nurses compared with the remaining sub-groups (). As with the other isotypes, serum IgA levels again varied among the different study groups (). Higher quantities of serum IgA were detectable in G2–G7 groups compared with the controls. A significantly greater rise was seen in IgA levels in the anesthetists and AA workers compared with the other professionals evaluated, although the nurses showed a significantly increased level compared with the surgeons, SA, and workers.
Figure 2. Changes in various antibody isotype levels. Significance of differences was analyzed by one-way ANOVA and Dunnett test (compare all vs. control group). Groups (left to right): G1–G7 are characterized in legend. Values are expressed as means ± SD in terms of IU/ml (for IgE), mg/dl (for all other isotypes). Value was significantly different from acontrol, bSA, csurgeon, danesthetist specialist, eAA, fnurse, and/or gworker group at p ≤ 0.05.
Lymphocyte subsets
All WAG-exposed sub-groups showed significant increases in total lymphocyte levels compared with the controls (). Blood levels of CD3+ cells (as percentages) showed no significant change in all subjects exposed to WAG as compared with control. Of the six sub-groups, only the anesthetists and their assistants (AA) displayed a significant increase in CD4+ percentages and CD4/CD8 ratios – and a decrease of CD8+ percentages compared with the controls.
Table 2. Total lymphocytes, CD3+, CD4+, CD8+ cell percentages, and CD4:CD8 ratios in the blood.
Correlations of the studied parameters
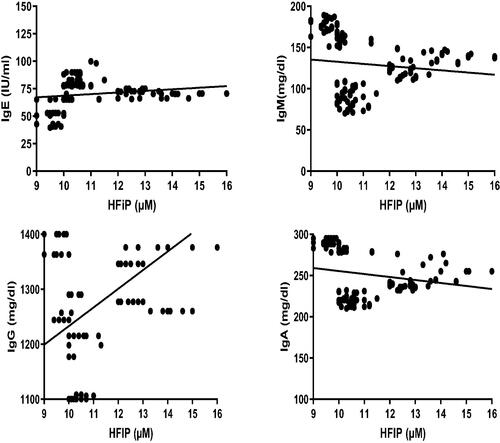
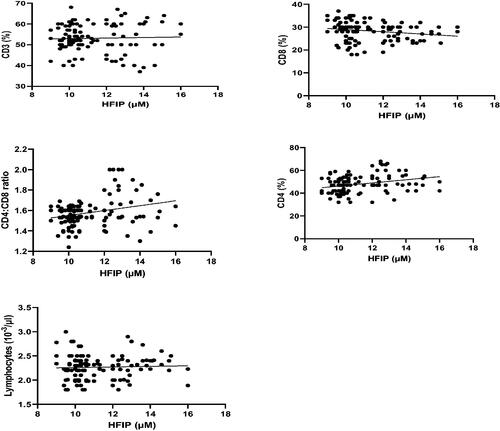
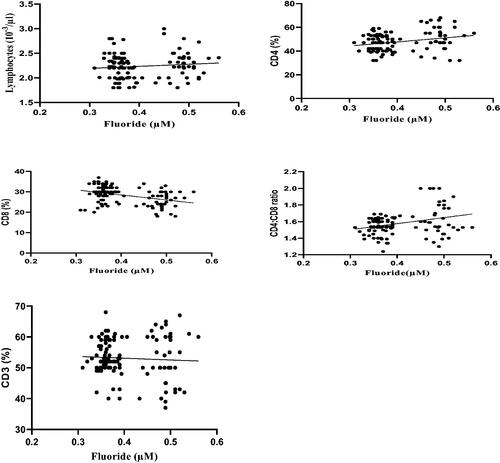
Correlation coefficient analyses of the data revealed that plasma fluoride levels were positively-correlated with serum IgE, IgM, and IgG levels. However, the analyses showed there was no significant correlation between fluoride levels and levels of IgA (). Correlation coefficient analyses also revealed that plasma fluoride levels were positively-correlated with lymphocytic counts, CD4 cell percentages, and CD4/CD8 ratios. In contrast, these analyses revealed that blood fluoride levels were negatively correlated with CD8 cell percentages. Lastly, the analyses showed there was no significant correlation between the fluoride levels and CD3 cell percentages ().
Figure 3. Correlation coefficient between plasma fluoride levels and serum IgE, IgM, IgG, and IgA levels the different WAG-exposed groups (G2–G7). Value significantly differed at p ≤ 0.05.
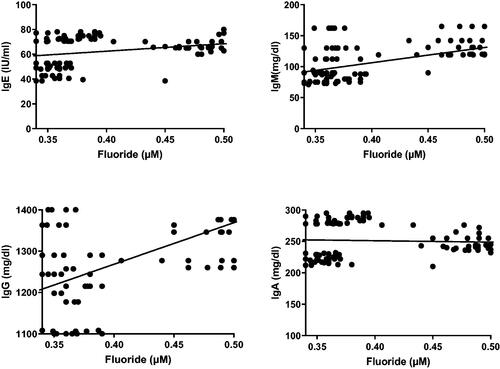
Figure 4. Correlation coefficient between plasma fluoride levels and CD3, CD4, and CD8 levels, and CD4:CD8 ratios, in the different WAG-exposed groups (G2–G7). Value significantly differed at p ≤ 0.05.
Correlation coefficient analyses of the data revealed that blood HFIP levels negatively correlated with serum IgM and IgA levels. In contrast, a positive correlation was noted between blood HFIP levels and serum IgE and IgG levels (). Analyses of the data showed that blood HFIP levels positively-correlated with CD3 and CD4 cell percentages as well as CD4/CD8 ratios. In contrast, correlation coefficient analyses revealed that blood HFIP levels negatively correlated with CD8 cell percentages. The analyses also showed there was no significant correlation between the HFIP levels and lymphocytic counts ().
Discussion
Occupational exposure to toxicants can cause immunomodulation by causing changes in host leukocytic counts, ratios of lymphocyte sub-populations, and lymphocyte activity. This has been shown to be true among workers in oil-industry and, as pertinent to the study here, health-services (Biro et al. Citation2002, Citation2004). Working in operating rooms is known to present risks to the overall health of team workers; clearly, chronic incidental inhalation exposure to a variety of anesthetics, even in low concentrations, could induce adverse health effects. In the context of immune function, occupational inhalation exposures to anesthetics produced declines in cellular anti-oxidant activity, suppressed neutrophil functions, elevated levels of DNA breaks in lymphocytes, etc. (Irwin et al. Citation2009). Aragonés et al. (Citation2016) and Emara et al. (Citation2020) reported occupational exposure of health-team workers to waste anesthetic gases (WAG) resulted in increased hepatic dysfunction, the incidence of spontaneous abortion, and the occurrence of congenital anomalies in their children. Musak et al. (Citation2013) found that incidental exposures to isoflurane or sevoflurane caused chromosomal aberrations in lymphocytes. Schifilliti et al. (Citation2011) demonstrated that repeat exposures to a combination of nitrous oxide and halogenated anesthetics (for periods up to 3 month and longer) elevated the incidence of genotoxicity in the lymphocytes of workers exposed to these agents. Vellore et al. (Citation2006) reported sevoflurane and isoflurane exposures led to increases in the risk for developing asthma or dermatitis, both reflecting changes in host immune status due to the incidental exposures.
There has been much research on patients that evaluated the impact on the immune system from varying anesthetic techniques, agents and dosages in clinical settings of both major and minor surgical procedures. If a volatile anesthetic leaks from the patient respiratory circuit during surgery, it has the potential to cause harm to attending medical personnel. However, there are very few studies that have been published that evaluated effects of such inhalation exposures on immunological function in this population. To address this, the present study was performed using medical personnel working in surgical theaters within the Qassim area of Saudi Arabia. A key study design element employed here was that sociodemographic data, i.e. age, marital status, previous medical history, occupational history, and body mass index for controls (G1) and the exposed professional groups (G2–G7) were matched.
Misra and Koshy (Citation2012) identified an increase in serum fluoride levels with chronic use of sevoflurane and isoflurane; long-term use of these agents is common in critical care management. Sevoflurane causes a greater rise in fluoride than isoflurane. This is attributed to its higher fluoride component and increased breakdown into metabolites by the host (Perbet et al. Citation2014). All subjects exposed to WAG in the current study (i.e. G2–G7) demonstrated elevated plasma fluoride concentrations compared with controls, particularly the anesthetists and their assistants. Fluoride levels in the other professional groups potentially exposed in the operating theater were equivalent. These findings are suggestive that the medical staff in greatest immediate proximity to the sevoflurane circuit receive longer durations and higher degrees of exposure to the drug.
The major role of the immune system is in the identification/disposal of foreign antigens, production of immunologic memory, and bestowing tolerance to self-antigens. The lymphocyte populations of the immune system are comprised of thymus-derived (T-) lymphocytes, bone-marrow-derived (B-) lymphocytes, and natural-killer (NK) cells. CD4+ T-cells along with CD8+ T-cells constitute the majority of T-lymphocytes. CD4+ T-cells have several functions including activation of cells associated with innate immunity, B-cells, cytotoxic T-cells and non-immune cells (Luckheeram et al. Citation2012), in part, via secretion of a variety of cytokines. If the function of the immune system is made suboptimal, recovery from pathological states can be impaired and loss of immune regulation (Waters et al. Citation2018). Thus, effects of anesthesia on a host immune status can have an adverse clinical outcome.
In a healthy host, the above-noted interactions between B- and CD4+ T-cells are essential for optimal immune (humoral) responses (i.e. transplantation, immunization). Any dysregulation of these cells (or their interactions) is already known to be an important factor in the development of some autoimmune diseases, chronic inflammatory pathologies, and changes in host ability to fight cancers. In comparison, as CD4+ and CD8+ T-cells are the effectors of cell-mediated immune (CMI) responses, host maintenance of ratios among CD4 and CD8 cells is important for optimal CMI responses (Hori et al. Citation2003). Any induced shift in this ratio would reflect changes in the relative presence of TH1 and TH2 cells in that host. The implications of an increase in TH2 cell levels in a body include a promotion of B-cell proliferation, induction of immunoglobulin formation, and shifts in key cytokine/chemokine profiles. If unchecked, these changes themselves can lead to damage in a host (i.e. these parameters normally utilize feedback mechanisms to return TH1 and TH2 cells to a “normal” balance once the immunologic challenge to the host is resolved). Accordingly, in the context of a shift in host CD4/CD8 ratios, an induced decreases in TH1 cells would ultimately mean a potential compromise of CMI responses in the host; this would also imply changes in the presence of circulating immunoglobulins and in key cytokines, including TH2 cell-related IL-4, IL-5, and IL-13.
Animal studies have revealed changes in circulating antibody levels and a diminished white blood cell count following sevoflurane administration (Elena et al. Citation2003). These results are consistent with work by Pagel et al. (Citation2000) who described the consequences from exposures to anesthetics on white cell populations and on proportions of lymphocytic components. However, data from human exposures have tended to be less uniform. For example, in surgical patients who received isoflurane, increases in populations of Ig-secreting cells were seen by 1 wk (Salo et al. Citation1997). In patients that underwent major surgical procedures that involved anesthesia, a drop in serum IgG levels was noted (Eskola et al. Citation1984). However this was not the case in patients that underwent a total hip replacement (Salo and Nissila Citation1990). Raised IgG titers, but no change in IgA or IgM, were identified post-operatively in patients receiving sevoflurane or isoflurane (Durlu et al. Citation2002). The findings here of raised IgE, IgM and IgG titers in all the WAG-exposed groups (compared with the controls) are similar to results in a study by Chitkara and Noronha (Citation1977). Even so, the current findings also differ in that those researchers documented a rise in IgA rather than of total IgE after host inhalation of halogenated anesthetics.
The present study also found that total lymphocyte counts were elevated (relative to control values) in all WAG-exposed individuals. These outcomes are similar to those in a study by Morisaki et al. (Citation1998) who reported that sevoflurane induced disorders in leukocyte distribution in anesthetized patients (i.e. manifest as increases in total lymphocyte counts). In addition, the study here found that the WAG-exposed anesthesiologists and their assistants (AA) presented (compared to control values) with significantly increased blood CD4+ levels and CD4/CD8 ratios, and decreased CD8+ cell levels. Such results are consistent with a study by Cocelli et al. (Citation2012) who demonstrated a significant increase in leukocyte and neutrophil counts, CD4+ cell percentages, and CD4/CD8 ratios in patients 24 hr after anesthesia with desflurane. Schneemilch et al. (Citation2005) reported that sevoflurane induced significant increases in lymphocyte levels, CD4+ cell counts and decreases in B-cells in patients 2 hr after anesthesia. Still, the present results do conflict with some data in the literature. Specifically, Liu et al. (Citation2016) reported a significant decrease in CD3+, CD4+, and NK cell levels, and in CD4/CD8 ratios in patients 24 hr after being anesthetized with propofol and sevoflurane. While results of some cited studies are inconsistent with those here, this might be attributable to the fact those data reflected effects of anesthetics for a single acute exposure as opposed to the longer-term incidental exposures to various WAG faced by the health workers in the present study.
In comparison to the above studies evaluating effects of anesthetics in patients, there have a been a handful of studies that examined healthcare workers exposed to such agents. Tompa et al. (Citation2006) reported that nurses exposed incidentally to anesthetics evinced immune alterations, and that these alterations were mitigated by use of protective measures. The study also reported that chronic exposures to these agents resulted in increases in host T-helper (TH) cell levels and in the ratios of activated T- and B-cells. Peric et al. (Citation1994) reported CD4+ cell percentages were increased significantly in surgical personnel (i.e. anesthesiologists) chronically-exposed to halothane and nitrous oxide. On the other hand, chronic exposure to mixtures of nitrous oxide and isoflurane negatively impacted these percentages. Yet another study showed that TH cell levels in these hosts were altered – depending on individual exposure “scores” (Bargellini et al. Citation2001).
The current (as well as previous) studies suggest that any immunomodulating effects of WAG among the evaluated hospital staff were likely dependent on the (A) duration of exposures, (B) type of anesthesia used, and (C) particular duties of an individual in the operating theater.
Conclusions
From the data of this study and the findings of other earlier studies, we can conclude that operating room personnel exposures to WAG are associated with changes in blood levels of IgG and IgM, total lymphocytes, CD4+ and CD8+ subtypes, and accordingly, in CD4:CD8 ratios. It is quite possible these changes would likely be associated with immune dysfunction in these WAG-exposed hosts. As it is not yet clear if this might be reflected as reduced host resistance and/or an increased risk for developing autoimmune diseases, programs need to be in place to manage these WAG to reduce potential risks to the health of hospital staff. Further, continuous efforts to monitor exposures to, and the safety of, all volatilizable anesthetic agents should be required.
Note to readers
The authors are very aware that there are important limitations in this study. Most importantly, this study was limited by a strict inclusion criteria that limited the sample size and excluded females. Such an limitation may have not allowed a fuller range of significant outcomes from being determined. Clearly, a larger more heterogeneous sample size/group would likely provide potentially more significant insights into – and facilitate a deeper understanding of – the effects of incidental WAG exposures on the immune systems of hospital workers.
Acknowledgments
Researchers would like to thank the Deanship of Scientific Research, Qassim University for funding publication of this project. The authors would like to express their thanks to Dr. Ehab Tousson (Tanta University, Egypt) for providing facilities for this study.
Disclosure statement
The authors declare no conflicts of interest.
References
- Aragonés JM, Ayora AA, Ribalta AB, Aparici AG, Lavela JAM, Vidiella JS, López MH. 2016. Occupational exposure to volatile anesthetics: A systematic review. OCCMED. 66:202–207.
- Bargellini A, Rovesti S, Barbieri A, Vivoli R, Roncaglia R, Righi E, Borella P. 2001. Effects of chronic exposure to anaesthetic gases on some immune parameters. Sci Total Environ. 270:149–156.
- Biro A, Pallinger E, Falus A, Tompa A. 2004. [Characterization of chemically-exposed groups by immunotoxicological methods]. Magy Onkol. 48:137–139.
- Biro A, Pallinger E, Major J, Jakab M, Klupp T, Falus A, Tompa A. 2002. Lymphocyte phenotype analysis and chromosome aberration frequency of workers occupationally-exposed to styrene, benzene, polycyclic aromatic hydrocarbons or mixed solvents. Immunol Lett. 81:133–140.
- Braz MG, Carvalho LIM, Chen CYO, Blumberg JB, Souza KM, Arruda NM, Filho DAA, Resende LO, Faria RTBG, Canário CD, et al. 2020. High concentrations of waste anesthetic gases induce genetic damage and inflammation in physicians exposed for three years: A cross-sectional study. Indoor Air. 30:512–520.
- Buckley R. 1986. Humoral immunodeficiency. Clin Immunol Immunopathol. 40:13–24.
- Burton D. 1990. Antibody: The flexible adaptor molecule. Trends Biochem Sci. 15:64–69.
- Canadian Center for Occupational Health and Safety (CCOHS). 2020. Waste anesthetic gases: Hazards. [accessed 2020 Apr 9]. http://www.ccohs.ca/oshanswers/chemicals/waste_anesthetic.html
- Casale T, Caciari T, Rosati M, Gioffrè P, Schifano M, Capozzella A, Pimpinella B, Tomei G, Tomei F. 2014. Anesthetic gases and occupationally-exposed workers. Environ Toxicol Pharmacol. 37:267–274.
- Chaoul M, Braz J, Lucio L, Golim M, Braz L, Braz M. 2015. Does occupational exposure to anesthetic gases lead to increase of pro-inflammatory cytokines? Inflamm Res. 64:939–942.
- Chitkara Y, Noronha A. 1977. Serum immunoglobulins after surgical operations. Intl. Surg. 62:165–168.
- Cocelli L, Ugur M, Karadasli H. 2012. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr Ther Res Clin Exp. 73:41–51.
- Delgado-Herrera L, Ostroff RD, Rogers SA. 2001. Sevoflurance: Approaching the ideal inhalational anesthetic. A pharmacologic, pharmacoeconomic, and clinical review. CNS Drug Rev. 7:48–120.
- Dispenzieri A, Gertz M, Therneau T, Kyle R. 2001. Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc. 76:476–487.
- Durlu N, Batıslam Y, Özatamer O. 2002. The effects of isoflurane and sevoflurane on immune system in minor surgical interventions. J Ankara Med School. 24:105–112.
- Elena G, Amerio N, Ferrero P, Bay M, Valenti J, Colucci D, Puig N. 2003. Effects of repetitive sevoflurane anaesthesia on immune response, select biochemical parameters and organ histology in mice. Lab Anim. 37:193–203.
- Emara A, Alrasheedi K, Aldubayan M, Alhowail A, Elgarabawy R. 2020. Effect of inhaled waste anaesthetic gas on blood and liver parameters among hospital staff. Hum Exp Toxicol. 39:1585–1595.
- Engels N, Wienands J. 2018. Memory control by the B-cell antigen receptor. Immunol Rev. 283:150–160.
- Eskola J, Salo M, Viljanen M, Ruuskanen O. 1984. Impaired B-lymphocyte function during open-heart surgery. Effects of anaesthesia and surgery. Br J Anaesth. 56:333–338.
- Hori Y, Ibuki T, Hosokawa T, Tanaka Y. 2003. The effects of neurosurgical stress on peripheral lymphocyte subpopulations. J Clin Anesth. 15:1–8.
- Irwin M, Trinh T, Yao C. 2009. Occupational exposure to anaesthetic gases: A role for TIVA. Expert Opin Drug Saf. 8:473–483.
- Kharasch E, Karol M, Lanni C, Sawchuk R. 1995. Clinical sevoflurane metabolism and disposi-tion. I. Sevoflurane and metabolite pharmacokinetics. Anesthesiology. 82:1369–1378.
- Kharasch E. 1995. Biotransformation of sevoflurane. Anesth Analg. 81:S27–S38.
- Koutsogiannaki S, Schaefers M, Okuno T. 2016. Prolonged exposure to volatile anesthetic isoflurane worsens the outcome of polymicrobial abdominal sepsis. Toxicol Sci. 156:402–411.
- Levine M, Sarner J, Lerman J, Davis P, Sikich N, Maloney K, Motoyama E, Cook D. 1996. Plasma inorganic fluoride concentrations after sevoflurane anesthesia in children. Anesthesiology. 84:348–353.
- Liu S, Gu X, Zhu L, Wu G, Zhou H, Song Y, Wu C. 2016. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine (Baltimore). 95:e5479.
- Lucchini R, Toffoletto F, Camerino D, Fazioli R, Ghittori S, Gilioli R, Signorini A, Alessio L. 1995. Neurobehavioral functions in operating theatre personnel exposed to anesthetic gases. Med Lav. 86:27–33.
- Luckheeram RV, Zhou R, Verma AD, Xia B. 2012. CD4 + T Cells: Differentiation and functions. Clin Dev Immunol. DOI:https://doi.org/10.1155/2012/925135.
- Miller A, Theodore D, Widrich J, editors. 2020. Inhalational anesthetic. Treasure Island (FL): StatPearls Publishing LLC. [accessed 2020 Mar 6]. https://www.ncbi.nlm.nih.gov/books/NBK554540/
- Misra S, Koshy T. 2012. A review of the practice of sedation with inhalational anaesthetics in the intensive care unit with the AnaConDa(®) device. Indian J Anaesth. 56:518–523.
- Morisaki H, Aoyama Y, Shimada M, Ochiai R, Takeda J. 1998. Leukocyte distribution during sevoflurane anesthesia. Br J Anaesth. 80:502–503.
- Musak L, Smerhovsky Z, Halasova E, Osina O, Letkova L, Vodickova L, Polakova V, Buchancova J, Hemminki K, Vodicka P. 2013. Chromosomal damage among medical staff occupationally-exposed to volatile anesthetics, anti-neoplastic drugs, and formaldehyde. Scand J Work Environ Health. 39:618–630.
- Occupational Safety and Health Administration (OSHA). 2010. Anesthetic gases: Guidelines for workplace exposures. Washington (DC): OSHA.
- Pagel P, Forber N, Waltier D. 2000. Cardiovascular pharmacology. In: Miller R, editor. Anesthesia. 5th ed. Philadelphia (PA): Churchill Livingstone; p. 96–125.
- Perbet S, Bourdeaux D, Sautou V, Pereira B, Chabanne R, Constantin J, Chopineau J, Bazin J. 2014. A pharmacokinetic study of 48-hr sevoflurane inhalation using a disposable delivery system (AnaConDa) in ICU patients. Minerva Anestesiol. 80:655–665.
- Perić M, Petrovečki M, Marušić M. 1994. Age-dependent haematological disturbances in anaesthetic personnel chronically exposed to high occupational concentrations of halothane and nitrous oxide. Anaesthesia. 49:1022–1027.
- Salo M, Nissila M. 1990. Cell-mediated and humoral immune responses to total hip replacement under spinal or general anaesthesia. Acta Anaesthesiol Scand. 34:241–148.
- Salo M, Ma M, Perttila J, Ilonen J. 1997. Enhanced spontaneous antibody response after coronary artery bypass surgery. Can J Anaesth. 44:617–622.
- Schifilliti D, Mondello S, D'Arrigo MG, Chillè G, Fodale V. 2011. Genotoxic effects of anesthetic agents: An update. Expert Opin Drug Saf. 10:891–899.
- Schneemilch CE, Ittenson A, Ansorge S, Hachenberg T, Bank U. 2005. Effect of 2 anesthetic techniques on the post-operative proinflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J Clin Anesth. 17:517–527.
- Şener Y, Tosun G, Kahvecioḡlu F, Gökalp A, Koç H. 2007. Fluoride levels of human plasma and breast milk. Eur J Dent. 1:21–24.
- Tompa A, Jakab M, Magyar ABB, Fodor Z, Klupp T, Major J. 2006. Chemical safety and health conditions among Hungarian hospital nurses. Ann N Y Acad Sci. 1076:635–648.
- Vellore AD, Drought VJ, Sherwood-Jones D, Tunnicliffe B, Moore VC, Robertson AS, Burge PS. 2006. Occupational asthma and allergy to sevoflurane and isoflurane in anaesthetic staff. Allergy. 61(12):1485–1486.
- Vouriot A, Gauchard G, Chau N, Nadif R, Mur J, Perrin P. 2005. Chronic exposure to anesthetic gases affects balance control in operating room personnel. Neurotoxicology. 26:193–198.
- Waters R, Perry S, Han S, Bielekova B, Gedeon T. 2018. The effects of interleukin-2 on immune response regulation. Math Med Biol. 35:79–119.
- Yamamoto K, Terada R, Okamoto R, Hiasa Y, Abe M, Onji M, Tsuji T. 2003. A scoring system for primary biliary cirrhosis and its application for variant forms of autoimmune liver disease. J Gastroenterol. 38:52–59.