Abstract
The aging immune system is characterized by a low-grade chronic systemic inflammatory state (“inflammaging”) marked by elevated serum levels of inflammatory molecules such as interleukin (IL)-6 and C-reactive protein (CRP). These inflammatory markers were also reported to be strong predictors for the development/severity of Type 2 diabetes, obesity, and COVID-19. The levels of these markers have been positively associated with those of advanced glycation end-products (AGEs) generated via non-enzymatic glycation and oxidation of proteins and lipids during normal aging and metabolism. Based on the above observations, it is clinically important to elucidate how dietary AGEs modulate inflammation and might thus increase the risk for aging-exacerbated diseases. The present narrative review discusses the potential pro-inflammatory properties of dietary AGEs with a focus on the inflammatory mediators CRP, IL-6 and ferritin, and their relations to aging in general and Type 2 diabetes in particular. In addition, underlying mechanisms – including those related to gut microbiota and the receptors for AGEs, and the roles AGEs might play in affecting physiologies of the healthy elderly, obese individuals, and diabetics are discussed in regard to any greater susceptibility to COVID-19.
Introduction and methods
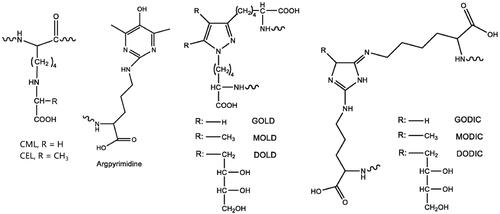
Advanced glycation end-products (AGEs) are a class of heterogenous irreversible products primarily generated during the late stage of Maillard reactions, non-enzymatic glycation reactions between reducing sugars and amino acids/lipids/nucleic acids (Hodge Citation1953; Namiki and Hayashi Citation1983). AGE precursors can also be produced from the oxidation of sugars (Dyer et al. Citation1991), lipids (Fu et al. Citation1996), and via polyol pathways, mainly following the formation of intermediary highly reactive dicarbonyls. Among frequently-reported AGEs () are Nε-carboxymethyl lysine (CML) (Ahmed et al. Citation1986), Nε-carboxymethyl lysine (CEL) (Ahmed et al. Citation1997), pentosidine (Miyata et al. Citation1996), argpyrimidine (Gomes et al. Citation2005), GOLD, MOLD or DOLD (lysine dimers crosslinked by two dicarbonyl molecules [glyoxal {GO}, methylglyoxal {MG} or 3DG, respectively]) (Nagaraj et al. Citation1996; Wells-Knecht et al. Citation1996), as well as GODIC, MODIC, and DODIC (arginine and lysine crosslinks) (Lederer and Klaiber Citation1999; Biemel et al. Citation2001). All these AGEs are referenced by their core structures. As free amino acids, peptides, and proteins are involved in the crosslinking, the actual molecular weights (MWs) of AGEs are highly diverse, and there is no clear separation between high and low MW AGEs (Poulsen et al. Citation2013).
AGEs are known for imparting detrimental effects on human health, in part because they accumulate in the extracellular matrix of various tissues; ultimately, such effects contribute to aging and chronic diseases (Kellow and Coughlan Citation2015). The modes of action by which AGEs act in situ include: (1) crosslinking of proteins, lipids, and nucleic acids, leading to alterations in cell structures and functions; (2) activation of receptors for AGEs, resulting in cell proliferation, autophagy, inflammation, and/or apoptosis; (3) generation of reactive oxygen species (ROS) that contribute to oxidative stress; and, (4) impairing mitochondrial function. Furthermore, some AGEs can be recognized as antigens to induce immune responses. Dietary AGEs are also known to possess allergenicity and immunogenicity properties that may play a role in food allergy (Gupta et al. Citation2018).
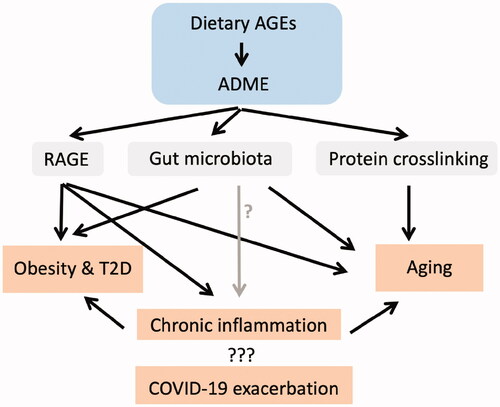
In this review, the immunotoxic characteristics of dietary AGEs are reviewed in terms of pro-inflammatory potentials, with a focus on relationships with biomarkers of aging and Type 2 diabetes, that is, C-reactive protein (CRP), interleukin (IL)-6, ferritin, and overall lymphopenia. Cross-disciplinary approaches, including those in food science, toxicology, physiology, and immunology, have been used to critically assess the contributions of dietary AGEs to disease progression through immune disruption. Potential underlying mechanisms of action for these AGEs in a host, including changes induced in gut microbiota and their receptors for AGEs that lead to aging-exacerbated diseases, are discussed here as well ().
Figure 2. Dietary advanced glycation end-products (AGEs) induce toxicological effects by activating RAGE, modulating gut microbiota and inducing collagen crosslinking – potential roles in severity of COVID-19, aging, obesity and Type 2 diabetes. ADME: Absorption, distribution, metabolism, and excretion.
For this review, various databases including Google Scholar and PubMed were searched using terms such as aging, Type 2 diabetes, COVID-19, inflammation, advanced glycation end-products, and microbiome.
AGEs in food
AGEs can be generated both endogenously and exogenously. Food is a major exogenous source of AGEs, especially those prepared under high-temperature conditions and stored for long periods or with food additives (Luevano-Contreras and Chapman-Novakofski Citation2010). AGEs are naturally present in animal-derived foods, and cooking processes result in additional AGE formation (Uribarri et al. Citation2010). The absorption rate for dietary AGEs is ≈10% in the human gastrointestinal (GI) tract; this correlates with AGE levels in circulation and tissues (Koschinsky et al. Citation1997). Due to heterogeneity in composition, various markers are used to quantify AGEs in various specimens. The most commonly used marker for AGEs is the non-fluorescent CML, because of their high abundance/wide distribution in biological (Reddy et al. Citation1995) and non-biological (Uribarri et al. Citation2010) samples. Fluorescence of AGEs is another marker; however, not all AGEs fluoresce and any fluorescence characteristics are specific to each individual AGE. Thus, any analytical readouts are highly dependent on the composition of the AGEs present and a combination of excitation/emission wavelengths applied (Schmitt et al. Citation2005).
Several studies have been conducted in attempts to establish a database for AGEs. Most of the large-scale studies quantified CML levels using ELISA (Goldberg et al. Citation2004; Uribarri et al. Citation2010; Takeuchi et al. Citation2015) or LC-MS/MS (Hull et al. Citation2012; Scheijen et al. Citation2016). One study used ELISA to compare CML to glyceraldehyde, glucose, or fructose-derived AGEs in a total of 1650 beverages/foods commonly consumed in Japan (Takeuchi et al. Citation2015). Another study compared CML to two other markers of AGEs, CEL and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1), in 190 foods using LC-MS/MS (Scheijen et al. Citation2016). In general, foods with high AGE content include nuts, biscuits, and cooked meat (Uribarri et al. Citation2010; Hull et al. Citation2012; Scheijen et al. Citation2016). Heating steps, such as occur in cooking and industrial processing, dramatically increase AGE levels in food. For example, the AGE level in beef was >10-times higher after 4 min of grilling (Uribarri et al. Citation2010) and 5- to 10-times higher in evaporated semi-skimmed milk than in semi-skimmed milk (Scheijen et al. Citation2016). Kinetically, the Maillard reaction rate can be increased by 4- to 8-fold/10 °C (Kaanane and Labuza Citation1989). In comparison, fruits and vegetables are low in AGEs (Uribarri et al. Citation2010). Lastly, most beverages are low in CML, CEL, or MG-H1 (Uribarri et al. Citation2010; Takeuchi et al. Citation2015; Scheijen et al. Citation2016), but high in fructose- and glucose-derived AGEs (especially the latter).
Large discrepancies have been found regarding AGE content in high-fat foods and cereals when using different quantifying methodologies. For example, higher AGE levels were detected in fatty foods by ELISA, and in cereals by LC-MS/MS (Uribarri et al. Citation2010; Hull et al. Citation2012; Scheijen et al. Citation2016). One study compared GC-MS and ELISA approach in the quantitation of CML levels in three types of milk samples: (1) powdered infant formula, (2) milk consisting of whey protein isolate (WPI), lactose and ascorbate, and (3) hydrolyzed liquid infant formula. The two methods correlated well for powdered infant formula (r2 = 0.966) and milk consisting of WPI, lactose, and ascorbate (r2 = 0.996), although a higher CML level was detected in the powdered formula with the ELISA. In contrast, no satisfactory correlation was obtained for hydrolyzed liquid infant formula, with a much higher CML detection when ELISA was used (Charissou et al. Citation2007). This might be explained by an unspecific interference of ELISA by the lipid matrix, which could account for the overestimation of AGEs in fatty foods by ELISA in general. Another study quantitated CML levels in gruel samples by ELISA and ESI-LC-MS/MS: average CML levels measured by ELISA was 54% of that measured by ESI-LC-MS/MS (Tareke et al. Citation2013), suggesting under-estimation of AGE levels in cereal by quantitating CML using ELISA. Using UPLC-MS, CML levels in cereals were identified as low; however, the intake of cold breakfast cereals could lead to elevated serum and urinary CML levels in adults (Semba et al. Citation2012). A “fructositis” hypothesis has been proposed to explain this phenomenon: high fructose-to-glucose ratios promote the intestinal in situ formation of fructose-associated AGEs (de Christopher Citation2017).
Absorption, distribution, metabolism, and excretion (ADME) of dietary AGEs
Exposure to dietary AGEs is dependent on eating habits and age since AGE content varies in different foods. A Western diet (WD) high in processed and red meats, high-fat dairy, refined grains, sweets, and desserts, contains much higher AGE levels than a prudent diet high in fruits, vegetables, fish, legumes, whole grains) (Lopez-Garcia et al. Citation2004). A 70-kg adult fed a WD is estimated to take in 1 mg CML/kg body weight (BW) daily, while a 6-kg infant takes in > 2.5 mg CML/kg BW/day through consumption of 1 L infant formula (Delgado-Andrade et al. Citation2007; Hull et al. Citation2012; van Rooijen et al. Citation2014). Breastfeeding significantly reduces levels of AGEs in infants compared to those who are formula-fed (Federico et al. Citation2016).
It was estimated ≈ 10% of dietary AGEs can be absorbed after oral ingestion and then transported into circulation, with two-thirds of these AGEs remaining in the body (Koschinsky et al. Citation1997). Due to this low absorption rate, pathological effects of dietary AGEs have been mostly neglected, even though both human and animal studies have shown dietary AGEs can contribute to the pool of AGEs in a body. In a cohort of 450 participants, uptake of high-dietary AGEs resulted in an elevation of free CML, CEL, and MG-H1 levels in plasma and urine, but not in the protein-bound forms (Scheijen et al. Citation2018), suggesting protein-bound AGEs likely arise endogenously. Another study of 90 healthy people showed a reduction of dietary intake of AGEs was associated with an average 30–40% decrease in serum AGE levels (Uribarri et al. Citation2005). Exposure to heat-treated (200 °C, 10 min) high-fat diet by male ApoE−/− mice for 8 weeks produced increases in plasma CML and CEL levels and in spleen weight when compared to values in mice fed a control high-fat diet (Marungruang et al. Citation2016).
Interestingly, there seemed to be a threshold for dietary AGEs to have an effect on AGE levels in the body. It was seen that dietary consumption of AGEs at levels < 0.5 × 106 U would not result in increases in serum AGE levels. Once the threshold was reached, a significant correlation (r2 = 0.8, p < 0.05) was found between the amounts of AGEs ingested and resultant elevations in serum AGE kinetics (Koschinsky et al. Citation1997). This plateau phenomenon for serum AGEs has also been observed in mice. C57BL/6 male mice fed chow containing 323 ng CML/g had no detectable CML in their sera. However, further oral administration of WPI-glucose-derived AGEs at a dose equivalent to the amount of CML the mouse received in the diet produced an average serum CML level of 150 ng/ml (Chen and Guo Citation2019). In another mouse study, serum CML levels almost doubled when dietary CML intake increased from 16.0 × 104 to 24.4 × 104 U/day, and remained at that level even when dietary CML intake was 30 × 104 U/day (Cai et al. Citation2012).
It was reported in animal studies that oral AGE exposure was associated with increased AGE levels in the kidney, liver, lung, heart, tendons (Roncero-Ramos et al. Citation2013, Citation2014; Li et al. Citation2015), and GI tract (Yuan et al. 2018). A limitation with those studies was that they were unable to differentiate if increases in tissue AGE levels were directly a result of deposition of exogenous AGEs or indirectly from the boosted accumulation of endogenous AGEs. One study used dietary protein-bound [13C]-labeled CML that directly traced the distribution of dietary AGEs to discriminate it from endogenous AGEs. After chronic oral exposure, the [13C]-CML was directly deposited in organs, with high levels found in the kidney, ileum, colon, and lung; the material was found at > 10-times lower levels in the brain, testis, heart, skeletal muscle, liver, and fat. Moreover, an intake of CML that was ≈ 10 times higher than the dietary level increased endogenous CML levels in the colon (almost doubled) and muscles, but not in other organs (Tessier et al. Citation2016).
The fate of ingested AGEs is under extensive investigation; there are many reports available on AGE deposition and distribution in organs and tissues (). A human study showed that 1/3 of absorbed AGEs was secreted into the urine within 48 h (Koschinsky et al. Citation1997). Another study found urinary CML secretion was related to the forms and complexity of CML, that is, high MW and insoluble fractions from bread crust extractions decreased urinary secretion rates compared to whole bread crust extraction (Roncero-Ramos et al. Citation2013); this was due to the anti-digestive properties of insoluble protein-bound CML. Fecal excretion is another major route for AGE disposition, that is, ≈1/3 of dietary AGEs eliminated based on CML quantitation (Roncero-Ramos et al. Citation2013). This quantity might be under-estimated because part of the dietary AGEs was likely degraded to low MW compounds by gut microbiota (Tuohy et al. Citation2006). Also, a small portion of serum CML can be passed into breast milk (Dittrich et al. Citation2006).
In terms of metabolism, AGEs are not typical substrates for detoxifying Phase 1 and 2 enzymes (Poulsen et al. Citation2013). Small endogenously-formed glycated and misfolded proteins are targets for intracellular degradation by the ubiquitin-proteasome-system 20S proteasome (Jung et al. Citation2009). Large bulky glycated proteins can also form after oxidation and cross-linking. If not eliminated by the lysosomal system, they can accumulate in cells and tissues (Teodorowicz et al. Citation2018).
Mechanisms of immunotoxicity following dietary exposure to AGEs
AGEs are considered immunotoxicants as part of their overall toxicologic profile (Kellow and Coughlan Citation2015). The main effects of AGEs on immunity are to induce pro-inflammatory responses. In the current review, two mechanisms, including regulation of receptor for AGEs (RAGE) and gut microbiota, are discussed to illustrate how dietary AGE induces immunotoxicity (). Other mechanisms have also been reported. For example, the AGE receptor 1 (AGER1, responsible for endocytic uptake and degradation of AGEs) can suppress RAGE expression and negatively regulate any oxidative stress and inflammation induced by AGEs (Lu et al. Citation2004; Ott et al. Citation2014). Consumption of dietary AGEs can deplete AGER1 in adipocytes, resulting in increases in inflammation, oxidative stress, and insulin resistance (Cai et al. Citation2012).
Receptor for AGEs
RAGE (a 35 kD transmembrane receptor of immunoglobulin superfamily; Neeper et al. Citation1992) is expressed on a range of cell types, including immune cells (Ott et al. Citation2014). RAGE plays an important role in inflammatory processes and endothelial activation. In vitro application of AGEs induces inflammatory responses in macrophages (van der Lugt et al. Citation1975; Jin et al. Citation2015) and promotes differentiation of native CD4+ T-cells toward a pro-inflammatory status by its binding to RAGE (Han et al. Citation2014). Up-regulation of RAGE expression in different organs and tissues has been observed in rodents on diets/drinking water containing AGE/MG (Cai et al. Citation2012; Sena et al. Citation2012). Activation of RAGE results in intracellular ROS production (Coughlan et al., Citation2009) and activation of p21(ras)-dependent mitogen-activated protein kinase (MAPK) pathways (Lander et al. Citation1997), which eventually lead to up-regulation of NF-κB and inflammation (). The consequent elevations in circulating levels of cytokines such as IL-1, IL-6, and tumor necrosis factor (TNF)-α ultimately will support a persistent state of inflammation. In a study that investigated the effects of various AGEs (BSA + D-glyceraldehyde, BSA + D-glycolaldehyde, BSA + MG, BSA + GO) on monocyte expression of adhesion molecules, interferon (IFN)-γ and TNF-α production, and T-cell proliferation, it was found that the effect of AGEs on immune cells depended on the AGE subtype present (Ohashi et al. Citation2010).
Figure 3. Schematic representation of paradoxical control of pro- and anti-inflammation by RAGE activation.
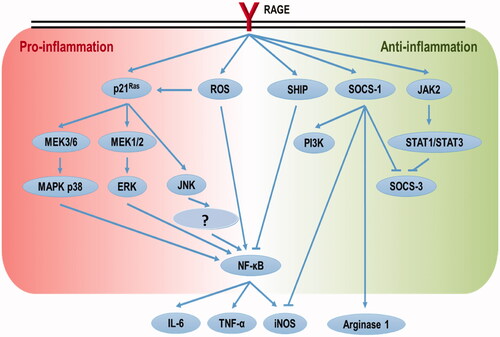
In contrast, it was reported that mixed and purified Maillard reaction products (MRPs) containing AGEs imparted anti-oxidative and anti-inflammatory effects when applied to human Caco-2 epithelial colorectal adenocarcinoma cells (Chen and Kitts Citation2011; Kitts et al. Citation2012). It was also shown that ribose-tryptophan MRPs had anti-inflammatory effects in the lipopolysaccharide (LPS)-treated murine macrophage RAW 264.7 cell line (Qin et al. Citation2018). Those investigators identified one anti-inflammatory ribose-tryptophan MRP as 532.24 Da 3-((1H-indol-3-yl)-methyl)-8-(5-((1H-indol-3-yl)methyl)-6-oxomorpholin-2-yl)-9-hydroxy-1,7,4-dioxazecan-2-one (Qin et al. Citation2018). In another study, Huang et al. (Citation2015) found that AGEs attenuated nitric oxide effects on human renal tubular cells via RAGE-JAK2-STAT1/STAT3 activation and consequent SOCS-3 suppression. These apparently contradictory findings to the main literature reflect the fact that binding of RAGE ligands may not only lead to pro- but to anti-inflammation as well (). Treatment of THP-1 macrophage cells with 1 μg/ml high mobility group box (HMGB) 1 (RAGE ligand) polarized the cells to an anti-inflammatory M2 state; this too was via impact on RAGE-SHIP/SOCS1 (Rojas et al. Citation2016). The dual roles of RAGE in a pro-\anti-inflammatory balance seem dose-related; however, this needs further study. Interestingly, anti-inflammatory “functions” of AGEs can be RAGE-independent. For example, BSA-glucose-derived AGEs suppressed LPS-induced M1 polarization of bone marrow-derived macrophages, and the effect was due to a dampening of NLRP3 inflammasome assembly (Son et al. Citation2017).
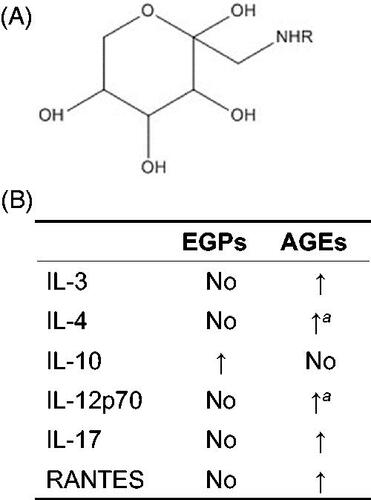
Another possibility could be some MRPs (other than AGEs) in the above-tested mixtures imparted anti-inflammatory effects. Studies have shown WPI-glucose-derived early glycation products (MRPs ; ) and AGEs differentially-modulated host macrophage cytokine/chemokine profiles (Chen et al. Citation2018; Chen and Guo Citation2019). AGEs induced inflammation (consistent with a majority of reports) and EGPs were anti-inflammatory (Chen et al. Citation2018). The latter effect was further evidenced by a dramatic elevation in serum IL-10 levels () and enhanced M2 polarization in C57BL/6 male mice with prostate tumors orally administered EGPs (Chen and Guo Citation2019). Though the inflammatory responses induced by WPI-glucose-derived AGEs were conceivable via RAGE, anti-inflammatory responses induced by EGPs could be using a different mechanism. One study found that EGPs at levels up to 10 mg/ml were unable to induce RAGE expression in human macrophages (unpublished observation). In addition to RAGE, AGEs can also interact with scavenger receptors predominantly involved in the capture, removal, and degradation of AGEs. This group includes Type I and Type II macrophage scavenger receptors, CD36, FEEL-1 and FEEL-2, SR-BI and SR-BII, and Lox-1 (Byun et al. Citation2017). Thus, EGPs might bind one of these receptors to initiate effects.
Figure 4. Differential immunomodulating capabilities of EGPs and AGEs. (A) Structure of glucose-derived Amadori compounds (early glycation products). R = protein/peptide/amino acid. (B) Comparison of EGPs and AGEs in modulating cytokine/chemokine production. Modified from Chen and Guo (Citation2019). No: no significant change when compared to non-reacted control. aValue significantly different from EGPs but not from non-reacted control.
Gut microbiota
The microbiota plays a major role in inflammation because of its tight relationship with immune system development and maturation. Much dietary AGEs has high MW and are not absorbed in the intestine; instead, they pass through the GI to the colon and are potentially metabolized by gut microbiota (GMB). Thus, it is not surprising to see an increasing body of literature that shows that dietary AGEs can induce gut dysbiosis, for example, a GMB imbalance ().
Table 1. Summary of human and lab animal microbiome studies on Maillard reaction products (AGE and EGP).
In male BALB/c mice orally exposed to CML, Bacteroidaceae levels were increased while those of Lachnospraceae decreased (Al Jahdali et al. Citation2017). In male ApoE−/− mice, there were increases in plasma CML and CEL levels after host exposures to heat-treated high-fat diets; this was accompanied by a decreased α diversity (increases in Allobaculum and unclassified genus of Clostridiales and decreases in Bacteroides, unclassified genera of Lachnospiraceae, Rikenellaceae and Ruminococcaceae at genus level) (Marungruang et al. Citation2016). Decreased α diversity was also seen in male C57BL/6 mice exposed to an AIN-93G diet enriched with AGEs; these hosts manifest increased gut levels of Alloprevotella, Helicobacter, Parabacteroides, Ruminococcaceae_UCG-014, and unclassified genus of Rhodospirillaceae, and decreased Alistipes, Desulfovibrio, Lachnospiraceae_NK4A136_group, and Rikenellaceae_RC9_ gut group levels. The diet with high AGE levels also altered fecal short-chain fatty acid (SCFA) levels in the hosts, for example, increasing isobutyrate and isovalerate while decreasing acetate and butyrate levels (Qu et al. Citation2018). The same high-AGE diet also decreased α diversity (i.e. decreased Alloprevotella and Ruminococcaceae, while increased Allobaculum and Bacteroides) in male Sprague-Dawley rats (Qu et al. Citation2017). The ammonia concentration in the rat cecal contents was increased, while that of acetate concentration was decreased. In addition, increased epithelial damage and lymphocyte infiltration, and decreased tight junction in the colon were noted.
One conclusion reached based on all the above data is that AGE consumption decreases GMB species richness – based on consistent patterns of decreased α diversity. As for individual bacteria, no broad conclusions can yet be drawn due to inconsistencies among the animal studies. However, Alistipes (decreased in mice by a diet containing high levels of AGEs [Qu et al. Citation2018]), was found at a lower abundance in the gut of peritoneal dialysis patients (Yacoub et al. Citation2017) who had higher serum AGE levels () and in patients with nonalcoholic fatty liver disease (Jiang et al. Citation2015). In addition, gut levels of some strains of Helicobacter were augmented by increases in AGEs (Qu et al. Citation2018).
In contrast, some GMB changes induced by AGEs seem to be beneficial. For example, colonization/prevalence of several gut inflammation-inducing strains from the Desulfovibrio genus was decreased by consumption of diets containing high AGE levels (Loubinoux et al. Citation2002; CitationFigliuolo et al., 2017; Qu et al. Citation2018). Increasing levels of Allobaculum due to consumption of an AGE-rich diet by ApoE−/− mice (Marungruang et al. Citation2016) and Sprague-Dawley rats (Qu et al. Citation2017) were suggested to be beneficial to maintaining a healthy colon mucus layer and a reduced overall inflammatory status (Jakobsson et al. Citation2015). Interestingly, there is one report to show that oral CML exposure alleviated gut dysbiosis induced by dextran sulfate sodium salt, but not by trinitro-benzenesulfonic acid, in colitic mice (Al Jahdali et al. Citation2017).
As discussed above, the disagreement between AGE-induced beneficial and detrimental changes in GMB could partially be due to the composition and abundance of MRP in the test diet or samples (Snelson and Coughlan Citation2019). Two experiments were conducted in which adolescent male humans consumed diets that were either high or low in hydroxymethylfurfural (HMF, an MRP generated in intermediate stage) and CML, and male weanling Wister rats fed diets with or without a glucose-lysine mixture high in Amadori compounds, HMF and CML (Seiquer et al. Citation2014). No significant differences were detected for plasma biochemical\anthropometric parameters in either experiment; however, discrepancies between the two studies occurred in trying to correlate GMB and MRP markers as further discussed below. In the human study, negative correlations were found between Lactobacilli numbers and dietary advanced MRPs (e.g. AGEs), whereas Bifidobacteria counts were negatively correlated with Amadori compound intake (e.g. EGPs). In the rats, total bacteria and Lactobacilli levels negatively correlated with MRP intake, and no correlations were found for Bifidobacteria (). The authors concluded specific effects of dietary MRPs were likely due to dietary amounts of the different browning compounds with distinct chemical structures. This notion was verified in a study using different fractions isolated from bread crust to feed weanling rats (Delgado-Andrade et al. Citation2017). Low and high MW fractions rich in Amadori compounds were found to up-regulate total levels of gut SCFAs, formic, and propionic acid, while the same agents down-regulated gut Lactobacillus spp. levels. It was also seen that the insoluble fraction abundant in HMF and CML up-regulated gut formic and acetic acid levels, while down-regulating gut Eubacterium rectale/Colletotrichum coccoides and Clostridium leptum levels.
The effects of Amadori compounds on GMB have been explored. When exposed to a diet high in furosine (Amadori compound-derived marker for the initial stage of Maillard reaction), CML and CEL, Sprague–Dawley rats (when compared to counterparts fed a heated-control diet) had decreased colonic levels of inflammatory TNFα and IL-6, and altered GMB (increased Akkermansia, Allobaculum, and Lachnospiraceae_UCG-006, and decreased Erysipelatoclostridium at genus level). In addition, these rats displayed normal colons with only some decreases in crypt depth (Han et al. Citation2018). These likely beneficial effects on gut health suggested a regulatory effect for Amadori compounds on GMB and anti-inflammatory responses.
In recent studies, aged male non-obese diabetic (NOD) mice were treated by gavage with EGPs that contained only Amadori compounds generated from a WPI-glucose system (Chen et al. Citation2019). These EGP-treated mice had an increased survival rate and decreased inflammation and immune infiltration into their prostatic lobes (Chen, Guo, et al. Citation2020). When the microbial taxa at the genus level were compared, EGP treatment led to increases in gut levels of Anaerostipes, Parabacteroides, Prevotella, Allobaculum, and Bacteroides, but decreases in Adlercreutzia and Roseburia (in terms of relative abundance; ). The up-regulated Bacteroides acidifaciens was correlated with most of the immune parameters measured in the rats. Anaerostipes spp. express enzymes required for the production of butyrate that protects NOD mice against diabetes (Mariño et al. Citation2017), and it is associated with a reduction of plasma glucose, insulin resistance, and body weight in diabetic mice fed with a high-fat diet (Xu et al. Citation2018). Bacteroides acidifaciens is important for promoting IgA production in the large intestine, and it is a potential treatment for metabolic diseases like obesity (Yanagibashi et al. Citation2013). Overall, EGP-treated mice exhibited a healthier GMB than that of the controls.
Toxicological effects of dietary AGEs on diseases through immune disruption
Formation of AGEs takes place as a part of normal aging and metabolism and occurs at an accelerated rate in hyperglycemic, inflammatory, and oxidative stress conditions. In this section, the toxicological effects of dietary AGEs on aging and Type 2 diabetes in relation to RAGE and gut dysbiosis are discussed ().
Aging
A substantial body of evidence shows that AGEs and their functionally-compromised adducts are linked to, and perhaps responsible for, changes seen in the function of cells and tissues during aging, and then in the development of many age-related morbidities, for example, atherosclerosis, nephropathies, retinopathy, osteoarthritis, neurodegenerative diseases, diabetes mellitus (Ott et al. Citation2014; Spauwen et al. Citation2015; Drenth et al. Citation2018). High levels of circulating AGEs can be used to predict cardiovascular disease mortality among older community-dwelling women (Semba et al. Citation2009). Administration of aminoguanidine (inhibitor of AGE formation) for 24–30 weeks in normotensive WAG/Rij rats prevented age-related cardiac hypertrophy and arterial stiffness (Corman et al. Citation1998). Similarly, a presence of AGEs was also associated with motor function decline in aging, and it was speculated that high levels of AGEs may be a biomarker for low physical activity (Drenth et al. Citation2018). In a study of 559 moderate-to-severely disabled women (age 65 and older), women with higher CML concentrations had less grip strength than those with lower CML; from this, it was concluded that women with higher AGEs have more muscle weakness (Dalal et al. Citation2009). Interestingly, brain tissues of Alzheimer’s disease patients were found to contain higher AGE levels than brains of age-matched controls (Cruz-Sánchez et al. Citation2010).
It is likely that increases in endogenous production and exogenous intake, and lower clearance and detoxification, lead to the accumulation of AGEs in older populations. However, higher AGE levels occur in both healthy older adults and those with chronic diseases. Studies have/are being tried to identify mechanisms to explain why some human tissues are damaged while others are not in those states. One mechanism involves increased crosslinking within collagen and the extracellular matrix with age-related increases in AGE levels (Sims et al. Citation1996). Glycated low-density lipoproteins can crosslink with collagen to prevent uptake by cell receptors. These modified low-density lipoproteins are instead more likely phagocytosed by macrophages to form foam cells and, ultimately, the development of atheroma (Bucala et al. Citation1994). Tissue accumulation of AGEs can be further enhanced by some cardiovascular changes associated with aging, such as vascular stiffening, diastolic dysfunction, and endothelial dysfunction (Fishman et al. Citation2018).
The aging immune system is characterized by a low-grade chronic systemic inflammatory state (“inflammaging”) marked by elevated inflammatory molecules, such as IL-6, CRP, ferritin, and lymphopenia (Dennis et al. Citation1998; Li et al. Citation2011; Cankurtaran et al. Citation2012). In hemodialysis patients, tissue levels of AGEs are an independent determinant of CRP levels (Nagano et al. Citation2011). Though CRP is an acute-phase protein of hepatic origin, AGEs cannot directly stimulate hepatocytes to produce CRP, but they enhance its expression by stimulating monocytes/macrophages to produce cytokines like IL-6 (Li et al. Citation2007). Circulating AGE levels correlate with those for IL-6 and other inflammatory markers in rheumatoid arthritis (Hein et al. Citation2005). In gingival fibroblasts, AGEs also increase IL-6 expression (Nonaka et al. Citation2018). In a group of elderly patients with mild cognitive impairment, serum RAGE levels were positively correlated with both AGE and CRP levels (Gorska-Ciebiada et al. Citation2015). Further, CRP can up-regulate RAGE expression in endothelial cells (Zhong et al. Citation2006) and in THP-1 cells (Mahajan et al. Citation2010). Thus, AGEs may contribute to the aging processes through exacerbating “inflammaging”.
Ferritin is a major tissue iron-storage protein that exhibits a variety of activities relevant to the immune system, including binding to T-cells, suppressing delayed-type hypersensitivity reactions (to induce anergy), suppressing B-cell antibody production, reducing phagocytosis by granulocytes, and regulating granulo-monocytopoietic processes (Zandman-Goddard and Shoenfeld Citation2008). Ferritin level increases with aging as a part of “inflammaging” (Cankurtaran et al. Citation2012). Macrophages accumulate ferritin during inflammation and polarization to pro-inflammatory M1. In β-thalassemia patients, circulating ferritin levels were seen to positively correlate with levels of pentosidine, a fluorescent protein crosslink used as a biomarker for AGEs (Mirlohi et al. Citation2018).
Studies in animals have suggested GMB alterations might cause aging. The GMB from old mice contributes to “inflammaging” after fecal microbiota transplantation to young germ-free mice (Fransen et al. Citation2017). Work with African turquoise killifish has shown that acute transfer of GMB from young donors to antibiotic-treated middle-age recipients extends lifespan and delays behavioral aging (Smith et al. Citation2017). The elderly have a different GMB profile when compared to healthy adults. Generally, the diversity of GMB and abundance of commensals that maintain immune tolerance in the gut are reduced, while that of opportunistic pathogens that stimulate gut inflammation is increased (Nagpal et al. Citation2018) – this is somewhat consistent with gut dysbiosis induced by AGEs [discussed earlier]. Aging generally leads to chronic systemic inflammatory states with hyperactive innate immune responses, particularly in the form of elevated neutrophil (PMN) accumulation following respiratory infection (Chen, Kelley, et al. Citation2020). Depletion of GMB using antibiotics significantly reduces levels of circulating aged neutrophils (Zhang et al. Citation2015).
It is of note that older people have greater susceptibility to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2; COVID-19), and that COVID-19 patients have increased circulating PMN-to-lymphocyte ratios (Liu, Liu, et al. Citation2020). Adverse effects of AGEs on PMN are well-known, including inhibited bacterial killing (Collison et al. Citation2002), suppressed migration (Touré et al. 2008), and induction of both oxidative stress and respiratory burst (Wong et al. Citation2003; Bansal et al. Citation2012). AGEs also impact promotion of CD4+ T-cell differentiation to pro-inflammatory states (Lu et al. Citation2019). Thus, it is likely modulation of innate immunity induced by AGEs contributes to the observed “age-enhanced” mortality to SARS-CoV-2 infection. It is possible these outcomes are mediated in part through AGE-induced alterations in the host GMB.
Type 2 diabetes (T2D)
T2D, which accounts for ≈ 90% of all diabetes cases, is characterized by insufficient secretion of insulin from pancreatic β-islet cells, coupled with impaired insulin actions in target tissues such as muscle, liver, and fat (termed insulin resistance). Approximately one-third of U.S. adults > 65 year-of-age have T2D, and an additional one-third of older adults have pre-diabetes (Cowie et al. 2009). Serum or plasma AGE levels are generally elevated in T2D patients due to hyperglycemia (Vlassara et al. Citation2002). RAGE was also dramatically up-regulated in T2D patients (Yan et al. Citation2009). Hyperglycemia increases glycation processes in insulin-independent tissues and cells (like red blood cells, peripheral nerves, endothelial cells, eye lenses, and kidneys) (Tessier Citation2010). In vitro, pancreatic β-cells exposed to AGEs displayed insulin secretory defects; in vivo, islets were damaged in Sprague-Dawley rats after chronic intraperitoneal injections of AGEs in the form of modified rat serum albumin (Coughlan et al. Citation2011).
The immune system plays an important role in controlling whole-body metabolism and contributes significantly to the pathogenesis of T2D (Tsalamandris et al. Citation2019). Intake of AGEs in the diet increases levels of inflammatory mediators (i.e. CRP, TNF-α, VCAM-1) found in the sera of T2D patients (Vlassara et al. Citation2002), and the increased serum AGE level is related to the rapid development of diabetic complications (Zheng et al. Citation2002; Rhee and Kim, Citation2018). For example, AGEs have also been implicated in a delayed wound healing in T2D patients (Peppa et al. Citation2009). In elderly T2D patients with mild cognitive impairment, serum AGE, RAGE, and CRP levels were increased (Gorska-Ciebiada et al. Citation2015). On the other hand, Vlassara et al. (Citation2002) noted that reduced intake of AGEs in T2D patients contributed to decreased levels of circulating AGEs and inflammatory markers like TNFα and CRP. Another study suggested blood IL-6 and AGE levels were significant independent determinants of CRP in diabetics (Tan et al. Citation2004).
T2D is frequently associated with elevated levels of serum ferritin (Lecube et al. Citation2004). T2D is also associated with intestinal dysbiosis. Among the commonly reported findings, the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia were negatively associated with T2D, while the genera of Ruminococcus, Fusobacterium, and Blautia were positively associated with T2D (Gurung et al. Citation2020).
COVID-19
While this review was in preparation, various investigators have hypothesized a role for the RAGE axis in COVID-19 pathogenesis (Kerkeni and Gharbi Citation2020; Stilhano et al. Citation2020), as well as in diabetes (de Francesco et al. Citation2020) and lung inflammation (Andersson et al. Citation2020; Rojas et al. Citation2020). Unfortunately, at present, there do not appear to be any studies specifically tackling the topic of dietary AGEs and any potential contribution to COVID-19 morbidity. It is worth noting a recent spike in studies surrounding soluble RAGE measures in COVID patients (see Dozio et al. Citation1975; Lim et al. Citation2021). AGEs may contribute to organ damage by promoting host cell death (Mao et al. Citation2018). Importantly, the levels of soluble RAGE in bronchoalveolar lavage fluid – which reflect tissue RAGE expression (Nakamura et al. Citation2007) – were found to correlate with the severity of various inflammatory lung diseases (Uchida et al. Citation2006; Kamo et al. 2015; Stockley et al. Citation2019). Thus, it seems it would be clinically important to elucidate if AGEs help to exacerbate inflammation, and by doing so increase the risk for COVID-19 development and severity in susceptible populations.
Conclusions
In this review, dietary sources, ADME, immunotoxic effects, and underlying mechanisms of action by AGEs were discussed. Dietary AGEs are an important exogenous source of AGEs and may contribute to an AGE pool in a body. Some studies indicated effects of AGEs are subtype-dependent. Most studied AGEs were mixtures generated in reactions between BSA and glucoses. Even with the same reactants, the composition/abundance of each component of AGEs can vary; these are often primarily determined by the incubation conditions (e.g. time, pH, temperature, reactant ratio). A complete reaction leads to the production of melanoidins, while an incomplete reaction results in the generation of EGPs in the initial or intermediate stages. Mixture impurity could also affect assay outcomes. Therefore, further identification and purification of functional AGE(s) would be a strategy to permit stronger conclusions to be reached. Nonetheless, elevated serum and organ levels of AGEs can induce chronic inflammation and contribute to the progression of various diseases, including aging, Type 2 diabetes, and possibly COVID-19.
Acknowledgments
The authors greatly appreciate Dr. Steven D. Holladay (Department Head, Professor, Department of Veterinary Biomedical Sciences at the University of Georgia) for his critical comments.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Funding
References
- Ahmed M, Brinkmann Frye E, Degenhardt T, Thorpe S, Baynes J. 1997. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 324(2):565–570.
- Ahmed M, Thorpe S, Baynes J. 1986. Identification of Nε-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 261(11):4889–4894.
- Akıllıoğlu H, Gökmen V. 2019. Advanced glycation end-products (AGE). In: Wang S, editor. Chemical hazards in thermally-processed foods. Singapore: Springer, p. 121–151.
- Al Jahdali N, Gadonna-Widehem P, Delayre-Orthez C, Marier D, Garnier B, Carbonero F, Anton P. 2017. Repeated oral exposure to Nε-carboxymethyllysine, a Maillard reaction product, alleviates gut microbiota dysbiosis in colitic mice. Dig Dis Sci. 62(12):3370–3384.
- Andersson U, Ottestad W, Tracey K. 2020. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 26(1):42.
- Bansal S, Siddarth M, Chawla D, Banerjee B, Madhu S, Tripathi A. 2012. Advanced glycation end-products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol Cell Biochem. 361(1–2):289–296.
- Biemel K, Reihl O, Conrad J, Lederer M. 2001. Formation pathways for lysine-arginine crosslinks derived from hexoses and pentoses by Maillard processes: Unraveling the structure of a pentosidine precursor. J Biol Chem. 276(26):23405–23412.
- Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. 1994. Modification of low density lipoprotein by advanced glycation end-products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 91(20):9441–9445.
- Byun K, Yoo Y, Son M, Lee J, Jeong G-B, Park YM, Salekdeh GH, Lee B. 2017. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol Ther. 177:44–55.
- Cai W, Ramdas M, Zhu L, Chen X, Striker G, Vlassara H. 2012. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the anti-oxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA. 109(39):15888–15893.
- Cankurtaran M, Yavuz B, Halil M, Ulger Z, Haznedaroğlu I, Arıoğul S. 2012. Increased ferritin levels could reflect ongoing aging-associated inflammation and may obscure underlying iron deficiency in the geriatric population. Eur Geriat Med. 3(5):277–280.
- Charissou A, Ait-Ameur L, Birlouez-Aragon I. 2007. Evaluation of a gas chromatography/mass spectrometry method for the quantification of carboxymethyllysine in food samples. J Chromatogr A. 1140(1–2):189–194.
- Chen J, Kelley W, Goldstein D. 2020. Role of aging and the immune response to respiratory viral infections: Potential implications for COVID-19. J Immunol. 205(2):313–320.
- Chen X, Kitts D. 2011. Anti-oxidant and anti-inflammatory activities of Maillard reaction product isolated from sugar-amino acid model systems. J Agric Food Chem. 59(20):11294–11303.
- Chen Y, Filipov N, Guo T. 2018. Dietary glycation products regulate immune homeostasis: Early glycation products promote prostate cancer cell proliferation through modulating macrophages. Mol Nutr Food Res. 62(3):201700641.
- Chen Y, Guo K, Nagy T, Guo T. 2020. Chronic oral exposure to glycated whey proteins increases survival of aged male NOD mice with autoimmune prostatitis by regulating the gut microbiome and anti-inflammatory responses. Food Funct. 11(1):153–162.
- Chen Y, Guo T. 2019. Dietary early glycation products promote the growth of prostate tumors more than advanced glycation end-products through modulation of macrophage polarization. Mol Nutr Food Res. 63(4):e1800885.
- Chen Y, Nagy T, Guo T. 2019. Glycated whey proteins protect NOD mice against Type 1 diabetes by increasing anti-inflammatory responses and decreasing autoreactivity to self-antigens. J Funct Foods. 56:171–181.
- Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, Stern DM, Al-Mohanna FA. 2002. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end-products (AGEs). J Leukoc Biol. 71(3):433–444.
- Corman B, Duriez M, Poitevin P, Heudes D, Bruneval P, Tedgui A, Levy B. 1998. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci USA. 95(3):1301–1306.
- Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan ALY, Fukami K, Thallas-Bonke V, Nawroth PP, et al. 2009. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 20(4):742–752.
- Coughlan MT, Yap FYT, Tong DCK, Andrikopoulos S, Gasser A, Thallas-Bonke V, Webster DE, Miyazaki J-I, Kay TW, Slattery RM, et al. 2011. Advanced glycation end-products are direct modulators of β-cell function. Diabetes. 60(10):2523–2532.
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, et al. 2009. Full accounting of diabetes and pre-diabetes in U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 32(2):287–294.
- Cruz-Sánchez F, Gironès X, Ortega A, Alameda F, Lafuente J. 2010. Oxidative stress in Alzheimer’s disease hippocampus: A topographical study. J Neurol Sci. 299(1–2):163–167.
- Dalal M, Ferrucci L, Sun K, Beck J, Fried L, Semba R. 2009. Elevated serum advanced glycation end-products and poor grip strength in older community-dwelling women. J Gerontol A Biol Sci Med Sci. 64(1):132–137.
- de Francesco E, Vella V, Belfiore A. 2020. COVID-19 and diabetes: The importance of controlling RAGE. Front Endocrinol. 11:526.
- de Christopher L. 2017. The paradox in dietary advanced glycation end-products research the source of the serum and urinary advanced glycation end-products is the intestines, not the food. Adv Nutr. 8:679–683.
- Delgado-Andrade C, de la Cueva S, Peinado M, Rufian-Henares J, Navarro M, Rubio L. 2017. Modifications in bacterial groups and short chain fatty acid production in the gut of healthy adult rats after long-term consumption of dietary Maillard reaction products. Food Res Intl. 100:134–142.
- Delgado-Andrade C, Seiquer I, Navarro M, Morales F. 2007. Maillard reaction indicators in diets usually consumed by adolescent population. Mol Nutr Food Res. 51(3):341–351.
- Dennis M, Nicolson A, Lehmann A, Junaid O, Byrne E, Hopkinson N. 1998. Clinical associations of lymphopenia in elderly persons admitted to acute medical and psychiatric wards. Gerontology. 44(3):168–171.
- Dittrich R, Hoffmann I, Stahl P, Muller A, Beckmann M, Pischetsrieder M. 2006. Concentrations of Nε-carboxymethyllysine in human breast milk, infant formulas, and urine of infants. J Agric Food Chem. 54(18):6924–6928.
- Dozio E, Sitzia C, Pistelli L, Cardani R, Rigolini R, Ranucci M, Corsi Romanelli M. 1975. Soluble receptor for advanced glycation end-products and its forms in COVID-19 patients with and without diabetes mellitus: A pilot study on their role as disease biomarkers. J Clin Med. 9(11):3785.
- Drenth H, Zuidema S, Krijnen W, Bautmans I, Smit A, van der Schans C, Hobbelen H. 2018. Advanced glycation end-products are associated with physical activity and physical functioning in the older population. J Gerontol A Biol Sci Med Sci. 73(11):1545–1551.
- Dyer D, Blackledge J, Thorpe S, Baynes J. 1991. Formation of pentosdine during non-enzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 266(18):11654–11660.
- Federico G, Gori M, Randazzo E, Vierucci F. 2016. Skin advanced glycation end-products evaluation in infants according to the type of feeding and mother’s smoking habits. SAGE Open Med. 4:2050312116682126.
- Figliuolo VR, Dos Santos LM, Abalo A, Nanini H, Santos A, Brittes NM, Bernardazzi C, de Souza HSP, Vieira LQ, Coutinho-Silva R, et al. 2017. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 189:29–38.
- Fishman S, Sonmez H, Basman C, Singh V, Poretsky L. 2018. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol Med. 24(1):59.
- Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, et al. 2017. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 8:1385.
- Fu M, Requena J, Jenkins A, Lyons T, Baynes J, Thorpe S. 1996. The advanced glycation end-product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 271(17):9982–9986.
- Goldberg T, Cai W, Peppa M, Dardaine V, Baliga B, Uribarri J, Vlassara H. 2004. Advanced glycoxidation end-products in commonly consumed foods. J Am Diet Assoc. 104(8):1287–1291.
- Gomes R, Sousa Silva M, Quintas A, Cordeiro C, Freire A, Pereira P, Martins A, Monteiro E, Barroso E, Ponces Freire A. 2005. Argpyrimidine, a methylglyoxal-derived advanced glycation end-product in familial amyloidotic polyneuropathy. Biochem J. 385(2):339–345.
- Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J. 2015. C-reactive protein, advanced glycation end-products, and their receptor in Type 2 diabetic, elderly patients with mild cognitive impairment. Front Aging Neurosci. 7:209.
- Gupta R, Gupta K, Sharma A, Das M, Ansari I, Dwivedi P. 2018. Maillard reaction in food allergy: Pros and cons. Crit Rev Food Sci Nutr. 58(2):208–226.
- Gurung M, Li Z, You H, Rodrigues R, Jump D, Morgun A, Shulzhenko N. 2020. Role of gut microbiota in Type 2 diabetes pathophysiology. EBioMedicine. 51:102590.
- Han K, Jin W, Mao Z, Dong S, Zhang Q, Yang Y, Zeng M. 2018. Microbiome and butyrate production are altered in gut of rats fed glycated fish protein diet. J Funct Foods. 47:423–433.
- Han X-Q, Gong Z-J, Xu S-Q, Li X, Wang L-K, Wu S-M, Wu J-H, Yang H-F. 2014. Advanced glycation end-products promote differentiation of CD4(+) T-helper cells toward pro-inflammatory response. J Huazhong Univ Sci Technolog Med Sci. 34(1):10–17.
- Hein G, Köhler M, Oelzner P, Stein G, Franke S. 2005. The advanced glycation end-product pentosidine correlates to IL-6 and other relevant inflammatory markers in rheumatoid arthritis. Rheumatol Int. 26(2):137–141.
- Hodge J. 1953. Dehydrated foods - Chemistry of Browning reactions in model systems. J Agric Food Chem. 1(15):928–943.
- Huang J, Lee Y, Chuang L, Guh J, Hwang J. 2015. Cinnamaldehyde and nitric oxide attenuate advanced glycation end-products-induced the Jak/STAT signaling in human renal tubular cells. J Cell Biochem. 116(6):1028–1038.
- Hull G, Woodside J, Ames J, Cuskelly G. 2012. Nε-(Carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 131(1):170–174.
- Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC, Johansson MEV. 2015. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 16(2):164–177.
- Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. 2015. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 5:8096.
- Jin X, Yao T, Zhou Z, Zhu J, Zhang S, Hu W, Shen C. 2015. Advanced glycation end-products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κB pathway. Biomed Res Int. 2015:732450.
- Jung T, Catalgol B, Grune T. 2009. The proteasomal system. Mol Aspects Med. 30(4):191–296.
- Kaanane A, Labuza T. 1989. The Maillard reaction in foods. Prog Clin Biol Res. 304:301–327.
- Kamo T, Tasaka S, Tokuda Y, Suzuki S, Asakura T, Yagi K, Namkoong H, Ishii M, Hasegawa N, Betsuyaku T. 2015. Levels of soluble receptor for advanced glycation end-products in bronchoalveolar lavage fluid in patients with various inflammatory lung diseases. Clin Med Insights Circ Respir Pulm Med. 9(1):147–154.
- Kellow N, Coughlan M. 2015. Effect of diet-derived advanced glycation end-products on inflammation. Nutr Rev. 73(11):737–759.
- Kerkeni M, Gharbi J. 2020. RAGE receptor: May be a potential inflammatory mediator for SARS-COV-2 infection? Med Hypotheses. 144:109950.
- Kitts D, Chen X, Jing H. 2012. Demonstration of anti-oxidant and anti-inflammatory bioactivities from sugar-amino acid Maillard reaction products. J Agric Food Chem. 60(27):6718–6727.
- Koschinsky T, He C-J, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. 1997. Orally-absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 94(12):6474–6479.
- Lander H, Tauras J, Ogiste J, Hori O, Moss R, Schmidt A. 1997. Activation of the receptor for advanced glycation end-products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 272(28):17810–17814.
- Lecube A, Hernández C, Genescà J, Esteban J, Jardí R, García L, Simó R. 2004. Diabetes is the main factor accounting for the high ferritin levels detected in chronic Hepatitis C virus infection. Diabetes Care. 27(11):2669–2675.
- Lederer M, Klaiber R. 1999. Cross-linking of proteins by Maillard processes: Characterization and detection of lysine-arginine crosslinks derived from glyoxal and methylglyoxal. Bioorg Med Chem. 7(11):2499–2507.
- Li H, Manwani B, Leng S. 2011. Frailty, inflammation, and immunity. Aging Dis. 2(6):466–473.
- Li J, Hou F, Guo Z, Shan Y, Zhang X, Liu Z. 2007. Advanced glycation end-products up-regulate C-reactive protein synthesis by human hepatocytes through stimulation of monocyte IL-6 and IL-1 beta production. Scand J Immunol. 66(5):555–562.
- Li M, Zeng M, He Z, Zheng Z, Qin F, Tao G, Zhang S, Chen J. 2015. Increased accumulation of protein-bound Nε-(carboxymethyl)lysine in tissues of healthy rats after chronic oral Nε-(carboxymethyl)lysine. J Agric Food Chem. 63(5):1658–1663.
- Lim A, Radujkovic A, Weigand M, Merle U. 2021. Soluble receptor for advanced glycation end-products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann Intensive Care. 11(1):50.
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R 2020. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 18(1):206. DOI:https://doi.org/10.1186/s12967-020-02374-0.
- Lopez-Garcia E, Schulze M, Fung T, Meigs J, Rifai N, Manson J, Hu F. 2004. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 80(4):1029–1035.
- Loubinoux J, Bronowicki J, Pereira I, Mougenel J, Faou A. 2002. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 40(2):107–112.
- Lu C, He J, Cai W, Liu H, Zhu L, Vlassara H. 2004. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci USA. 101(32):11767–11772.
- Lu H, Xu S, Liang X, Dai Y, Huang Z, Ren Y, Lin J, Liu X. 2019. Advanced glycated end-products alter neutrophil effect on regulation of CD4+ T-cell differentiation through induction of myeloperoxidase and neutrophil elastase activities. Inflammation. 42(2):559–571.
- Luevano-Contreras C, Chapman-Novakofski K. 2010. Dietary advanced glycation end-products and aging. Nutrients. 2(12):1247–1265.
- Mahajan N, Bahl A, Dhawan V. 2010. C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end-products (RAGE) and its inflammatory ligand EN-RAGE in THP-1 cells: Inhibitory effects of atorvastatin. Intl J Cardiol. 142(3):273–278.
- Mao YX, Cai WJ, Sun XY, Dai PP, Li XM, Wang Q, Huang XL, He B, Wang PP, Wu G, et al. 2018. RAGE-dependent mitochondria pathway: A novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end-products. Cell Death Dis. 9(6):674.
- Mariño E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, et al. 2017. Gut microbial metabolites limit the frequency of autoimmune T-cells and protect against Type 1 diabetes. Nat Immunol. 18(5):552–562.
- Marungruang N, Fak F, Tareke E. 2016. Heat-treated high-fat diet modifies gut microbiota and metabolic markers in apoe-/- mice. Nutr Metab. 13:22.
- Mirlohi M, Yaghooti H, Shirali S, Aminasnafi A, Olapour S. 2018. Increased levels of advanced glycation end-products positively correlate with iron overload and oxidative stress markers in patients with β-thalassemia major. Ann Hematol. 97(4):679–684.
- Miyata T, Taneda S, Kawai R, Ueda Y, Horiuchi S, Hara M, Maeda K, Monnier VM. 1996. Identification of pentosidine as a native structure for advanced glycation end-products in β-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci USA. 93(6):2353–2358.
- Nagano M, Fukami K, Yamagishi S-I, Sakai K, Kaida Y, Matsumoto T, Hazama T, Tanaka M, Ueda S, Okuda S. 2011. Tissue level of advanced glycation end-products is an independent determinant of high-sensitivity C-reactive protein levels in haemodialysis patients. Nephrology. 16(3):299–303.
- Nagaraj R, Shipanova I, Faust F. 1996. Protein crosslinking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine crosslink derived from methylglyoxal. J Biol Chem. 271(32):19338–19345.
- Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. 2018. Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging. 4(4):267–285.
- Nakamura K, Yamagishi S, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, Imaizumi T. 2007. Serum levels of sRAGE, the soluble form of receptor for advanced glycation end-products, are associated with inflammatory markers in patients with Type 2 diabetes. Mol Med. 13(3–4):185–189.
- Namiki M, Hayashi T. 1983. A new mechanism of the Maillard reaction involving sugar fragmentation and free-radical formation. ACS Symp Series. 215:21–46.
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. 1992. Cloning and expression of a cell surface receptor for advanced glycosylation end-products of proteins. J Biol Chem. 267(21):14998–15004.
- Nonaka K, Kajiura Y, Bando M, Sakamoto E, Inagaki Y, Lew JH, Naruishi K, Ikuta T, Yoshida K, Kobayashi T, et al. 2018. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J Periodontal Res. 53(3):334–344.
- Ohashi K, Takahashi HK, Mori S, Liu K, Wake H, Sadamori H, Matsuda H, Yagi T, Yoshino T, Nishibori M, et al. 2010. Advanced glycation end-products enhance monocyte activation during human mixed lymphocyte reaction. Clin Immunol. 134(3):345–353.
- Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. 2014. Role of advanced glycation end-products in cellular signaling. Redox Biol. 2:411–429.
- Peppa M, Stavroulakis P, Raptis S. 2009. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 17(4):461–472.
- Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO. 2013. Advanced glycation end-products in food and their effects on health. Food Chem Toxicol. 60:10–37.
- Qin D, Li L, Li J, Li J, Zhao D, Li Y, Li B, Zhang X. 2018. A new compound isolated from the reduced ribose-tryptophan Maillard reaction products exhibits distinct anti-inflammatory activity. J Agric Food Chem. 66(26):6752–6761.
- Qu W, Nie C, Zhao J, Ou X, Zhang Y, Yang S, Bai X, Wang Y, Wang J, Li J. 2018. Microbiome-metabolomics analysis of the impacts of long-term dietary advanced glycation end-product consumption on C57BL/6 mouse fecal microbiota and metabolites. J Agric Food Chem. 66(33):8864–8875.
- Qu W, Yuan X, Zhao J, Zhan Y, Hu J, Wang J, Li J. 2017. Dietary advanced glycation end-products modify gut microbial composition and partially increase colon permeability in rats. Mol Nutr Food Res. 61(10):201700118.
- Reddy S, Bichler J, Wells-Knecht K, Thorpe S, Baynes J. 1995. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end-product (AGE) antigen in tissue proteins. Biochemistry. 34(34):10872–10878.
- Rhee S, Kim Y. 2018. The role of advanced glycation end-products in diabetic vascular complications. Diabetes Metab J. 42(3):188–195.
- Rojas A, Delgado-López F, Perez-Castro R, Gonzalez I, Romero J, Rojas I, Araya P, Añazco C, Morales E, Llanos J. 2016. HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumour Biol. 37(3):3321–3329.
- Rojas A, Gonzalez I, Morales M. 2020. SARS-CoV-2-mediated inflammatory response in lungs: Should we look at RAGE? Inflamm Res. 69(7):641–643.
- Roncero-Ramos I, Delgado-Andrade C, Tessier F, Niquet-Leridon C, Strauch C, Monnier V, Navarro M. 2013. Metabolic transit of N(ε)-carboxymethyl-lysine after consumption of AGEs from bread crust. Food Funct. 4(7):1032–1039.
- Roncero-Ramos I, Niquet-Leridon C, Strauch C, Monnier V, Tessier F, Navarro M, Delgado-Andrade C. 2014. An advanced glycation end-product (AGE)-rich diet promotes Nε-carboxymethyl-lysine accumulation in the cardiac tissue and tendons of rats. J Agric Food Chem. 62(25):6001–6006.
- Scheijen R, Clevers E, Engelen L, Dagnelie P, Brouns F, Stehouwer C, Schalkwijk C. 2016. Analysis of advanced glycation end-products in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 190:1145–1150.
- Scheijen J, Hanssen N, van Greevenbroek M, van der Kallen C, Feskens E, Stehouwer CDA, Schalkwijk C. 2018. Dietary intake of advanced glycation end-products is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin Nutr. 37(3):919–925.
- Schmitt A, Schmitt J, Munch G, Gasic-Milencovic J. 2005. Characterization of advanced glycation end-products for biochemical studies: Side-chain modifications and fluorescence characteristics. Anal Biochem. 338(2):201–215.
- Seiquer I, Rubio L, Peinado M, Delgado-Andrade C, Navarro M. 2014. Maillard reaction products modulate gut microbiota composition in adolescents. Mol Nutr Food Res. 58(7):1552–1560.
- Semba RD, Ang A, Talegawkar S, Crasto C, Dalal M, Jardack P, Traber MG, Ferrucci L, Arab L. 2012. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end-products, in adults: The energetics study. Eur J Clin Nutr. 66(1):3–9.
- Semba RD, Ferrucci L, Sun K, Beck J, Dalal M, Varadhan R, Walston J, Guralnik JM, Fried LP. 2009. Advanced glycation end-products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res. 21(2):182–190.
- Sena C, Matafome P, Crisostomo J, Rodrigues L, Fernandes R, Pereira P, Seica R. 2012. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res. 65(5):497–506.
- Sims T, Rasmussen L, Oxlund H, Bailey A. 1996. The role of glycation crosslinks in diabetic vascular stiffening. Diabetologia. 39(8):946–951.
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano D. 2017. Regulation of lifespan by gut microbiota in short-lived African turquoise killifish. E-life. 6:e27014.
- Snelson M, Coughlan M. 2019. Dietary advanced glycation end-products: Digestion, metabolism and modulation of gut microbial ecology. Nutrients. 11(2):215.
- Son S, Hwang I, Han S, Shin J, Shin O, Yu J. 2017. Advanced glycation endproducts impair NLRP3 inflammasome-mediated innate immune responses in macrophages. J Biol Chem. 292(50):20437–20448.
- Spauwen PJJ, van Eupen MGA, Köhler S, Stehouwer CDA, Verhey FRJ, van der Kallen CJH, Sep SJS, Koster A, Schaper NC, Dagnelie PC, et al. 2015. Associations of advanced glycation end-products with cognitive functions in individuals with and without Type 2 diabetes: The Maasttricht study. J Clin Endocrinol Metab. 100(3):951–960.
- Stilhano RS, Costa AJ, Nishino MS, Shams S, Bartolomeo CS, Breithaupt-Faloppa AC, Silva EA, Ramirez AL, Prado CM, Ureshino RP. 2020. SARS-CoV-2 and the possible connection to ERs, ACE2, and RAGE: Focus on susceptibility factors. FASEB J. 34(11):14103–14119.
- Stockley R, Halpin D, Celli B, Singh D. 2019. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med. 199(10):1195–1204.
- Takeuchi M, Takino J-i, Furuno S, Shirai H, Kawakami M, Muramatsu M, Kobayashi Y, Yamagishi S-i. 2015. Assessment of concentrations of various advanced glycation end-products in beverage and foods commonly consumed in Japan. PLoS One. 10(3):e0118652.
- Tan K, Chow W, Tam S, Bucala R, Betteridge J. 2004. Association between acute-phase reactants and advanced glycation end-products in Type 2 diabetes. Diabetes Care. 27(1):223–228.
- Tareke E, Forslund A, Lindh C, Fahlgren C, Ostman E. 2013. Isotope dilution ESI-LC-MS/MS for quantification of free and total Nε-(1-carboxymethyl)-L-lysine and free Nε-(1-carboxy-ethyl)-L-Lysine: Comparison of total Nε-(1-carboxymethyl)-L-lysine levels measured with new method to ELISA assay in gruel samples. Food Chem. 141(4):4253–4259.
- Teodorowicz M, Hendriks W, Wichers H, Savelkoul H. 2018. Immunomodulation by processed animal feed: Role of Maillard reaction products and advanced glycation end-products (AGEs). Front Immunol. 9:2088.
- Tessier F. 2010. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol Biol. 58(3):214–219.
- Tessier FJ, Niquet-Léridon C, Jacolot P, Jouquand C, Genin M, Schmidt A-M, Grossin N, Boulanger E. 2016. Quantitative assessment of organ distribution of dietary protein-bound [13C]-labeled Nε-carboxymethyllysine after chronic oral exposure in mice. Mol Nutr Food Res. 60(11):2446–2456.
- Touré F, Zahm J-M, Garnotel R, Lambert E, Bonnet N, Schmidt AM, Vitry F, Chanard J, Gillery P, Rieu P. 2008. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem J. 416(2):255–261.
- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis G-A, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. 2019. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol. 14(1):50–59.
- Tuohy K, Hinton D, Davies S, Crabbe M, Gibson G, Ames J. 2006. Metabolism of Maillard reaction products by the human gut microbiota-implications for health. Mol Nutr Food Res. 50(9):847–857.
- Uchida T, Shirasawa M, Ware L, Kojima K, Hata Y, Makita K, Mednick G, Matthay Z, Matthay M. 2006. Receptor for advanced glycation end-products is a marker of Type I cell injury in acute lung injury. Am J Respir Crit Care Med. 173(9):1008–1015.
- Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. 2005. Diet-derived advanced glycation end-products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci. 1043:461–466.
- Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. 2010. Advanced glycation end-products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 110(6):911–916.
- van der Lugt T, Weseler A, Gebbink W, Vrolijk M, Opperhuizen A, Bast A. 1975. Dietary advanced glycation end-products induce an inflammatory response in human macrophages in vitro. Biochem Pharmacol. 24(17):1639–1641.
- van Rooijen C, Bosch G, van der Poel A, Wierenga P, Alexander L, Hendriks W. 2014. Quantitation of Maillard reaction products in commercially-available pet foods. J Agric Food Chem. 62(35):8883–8891.
- Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. 2002. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 99(24):15596–15601.
- Wells-Knecht KJ, Brinkmann E, Wells-Knecht MC, Litchfield JE, Ahmed MU, Reddy S, Zyzak DV, Thorpe SR, Baynes JW. 1996. New biomarkers of Maillard reaction damage to proteins. Nephrol Dial Transplant. 11(S5):41–47.
- Wong R, Pettit A, Quinn P, Jennings S, Davies J, Ng L. 2003. Advanced glycation end-products stimulate an enhanced neutrophil respiratory burst mediated through activation of cytosolic phospholipase A2 and generation of arachidonic acid. Circulation. 108(15):1858–1864.
- Xu Y-H, Gao C-L, Guo H-L, Zhang W-Q, Huang W, Tang S-S, Gan W-J, Xu Y, Zhou H, Zhu Q. 2018. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol. 238(3):231–244.
- Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, Abyad S, Honan AM, Thomas SA, Zheng W, Valiyaparambil SA, et al. 2017. Advanced glycation end-products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One. 12(9):e0184789.
- Yan S, Yan S, Ramasamy R, Schmidt A. 2009. Tempering the wrath of RAGE: An emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med. 41(6):408–422.
- Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, Takahashi Y, Momose Y, Itoh K, Hirayama K, et al. 2013. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA + B-cells. Immunobiology. 218(4):645–651.
- Yuan X, Zhao J, Qu W, Zhang Y, Jia B, Fan Z, He Q, Li J. 2018. Accumulation and effects of dietary advanced glycation end-products on the gastrointestinal tract in rats. Int J Food Sci Technol. 53(10):2273–2281.
- Zandman-Goddard G, Shoenfeld Y. 2008. Hyper-ferritinemia in autoimmunity. Isr Med Assoc J. 10:83–84.
- Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang J-E, Scheiermann C, et al. 2015. Neutrophil aging is regulated by the microbiome. Nature. 525(7570):528–532.
- Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H. 2002. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 18(3):224–237.
- Zhong Y, Li S, Liu S, Szmitko P, He X, Fedak P, Verma S. 2006. C-Reactive protein up-regulates receptor for advanced glycation end-products expression in human endothelial cells. Hypertension. 48(3):504–511.