Abstract
The immunotoxic potential of drug candidates is assessed through the examination of results from a variety of in vitro and in vivo immunophenotyping and functional study endpoints in pre-clinical studies. CD8+ cytotoxic T-lymphocyte (CTL) activity impairment by immunosuppressive agents is recognized to be a potentiating factor for decreased antiviral defense and increased cancer risk. A bi-specific T-cell engager (BiTE®)-mediated CTL activity assay that applies to ex vivo experimentation in non-human primates in the context of toxicology studies was successfully developed and applied in cynomolgus monkey regulatory studies. While an ex vivo analysis conducted in the context of repeat-dose toxicology studies focuses on the long-term impact on CTL function, an in vitro assay with the same experimental design captures acute effects in the presence of the test article. Here, the in vitro assay was applied to a list of drugs with known clinical immunomodulatory impact to understand the applicability of the assay. The results showed this assay was sensitive to a wide range of immunosuppressants directly targeting cell-intrinsic signaling pathways in activated CTL. However, agents executing immuno-modulation through inhibiting cytokines/cytokine receptors, co-stimulatory molecules, and cell adhesion and migration pathways did not impair the CTL activity in this short-term in vitro culture. In addition, anti-PD-1/PD-L1 immune checkpoint blockers enhanced the CTL activity. Taken together, the results here demonstrate that in concordance with their mechanism of action, the in vitro BiTE®-mediated CTL assay is applicable and sensitive to immunomodulatory agents acting via a variety of mechanisms.
Introduction
In the past decades, there has been an influx of immunomodulatory agents in pre-clinical development and/or approved for clinical use in the therapeutic areas of autoimmunity, inflammation, organ transplant rejection, and cancer. These agents are often designed to interact directly with lymphocytes, antigen-presenting cells, or other immune cell mediators (e.g. cytokines, chemokines, and growth factors) to suppress or stimulate immune responses by a different mechanism of action. Immune-related potential safety liabilities for such therapies and for therapies not intentionally impacting the immune system may be assessed in a variety of pre-clinical studies and endpoints. Along with natural killer (NK) cells, CD8+ cytotoxic T-lymphocytes (CTL) play a critical role in the immunosurveillance of cancerous cells (Dunn et al. Citation2004). CTL-mediated immunity is also important in ameliorating viral infections. For this reason, there has been increasing interest in assessing the impact of investigational drugs on CTL function (ICH Citation2005; Lebrec et al. Citation2016).
Most of the biologics are tested in non-human primates, primarily cynomolgus macaques in consideration of species cross-reactivity. The immunomodulation potential of the drug candidates is often evaluated by measurement of immunophenotyping, T-dependent antibody response (TDAR), and a change of NK or CTL activity in regulatory studies in cynomolgus monkeys. To address this specific need, our laboratory recently developed an assay applicable to an ex vivo experimentation in cynomolgus macaque non-clinical toxicology studies (Frank et al. Citation2018). This assay utilizes a human EGFR Bi-specific T-cell Engager (BiTE®) to induce a robust activation in cynomolgus macaque CD8+ T-cells in purified peripheral blood mononuclear cells (PBMC) (note: hereafter this assay is referred to as the BiTE®-mediated CTL assay). Once PBMC and EGFR target expressing cells are co-cultured, a concentration of BiTE® resulting in 90% of target lysis is added (). Upon ligation of BiTE® on both T-cells and target cells, an immunological synapse is formed and followed by T-cell activation marked by events, such as CD107a degranulation and interferon (IFN)-γ production. The target cell is then killed through redirected lysis (Li et al. Citation2017). While the chronic impact on CD8+ T-cell function can be assessed through ex vivo analysis from PBMC on long-term studies, an in vitro assay utilizing the same experimental design was established simultaneously to capture any acute effects in the presence of the test articles.
Figure 1. Illustration of BiTE®-mediated CTL assay. (1) EGFR expressing HCT-116 target cells and donor PBMC are co-cultured with α-EGFR × CD3 BiTE®. (2,3) Upon binding of BiTE® to both target cell and CD8+ T-lymphocyte, immunological synapse forms, and activation ensues. (4,5) Exocytic granules (marked by CD107a) containing perforin and granzymes along with IFNγ are then secreted, resulting in redirected lysis of target cell (5) (Created with BioRender.com).
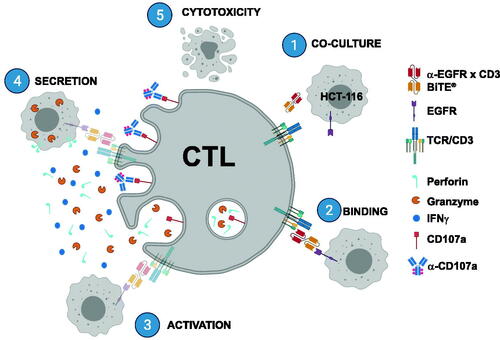
The objective of this study was to describe the applicability of the BiTE®-mediated CTL assay to a variety of drugs with known immunomodulation in the clinic. The agents included were small molecule inhibitors of lymphocyte signaling, proliferation, and activation (e.g. dexamethasone, prednisolone, tofacitinib, ruxolitinib, mycophenolate mofetil, rapamycin, teriflunomide, tacrolimus/FK506, and apremilast), biologics or small molecules inhibiting either cytokine signaling (e.g. adalumimab, infliximab, tocilizumab, and ustekinumab), co-stimulatory receptor activation (abatacept), lymphocyte trafficking (natalizumab and fingolimod/FTY720), or immune checkpoints (anti-PD-1 and anti-PD-L1 monoclonal antibodies). This report describes the sensitivity of this assay to a wide range of immunomodulating agents, in a mechanism-specific manner.
Materials and methods
Immunomodulatory agents
Dexamethasone was purchased from Tocris Bioscience (Minneapolis, MN, USA). Prednisone, prednisolone, tofacitinib, ruxolitinib, mycophenolic acid (MPA), rapamycin, teriflunomide, Tacrolimus/FK506, apremilast, and tocilizumab were all purchased from Selleckchem (Houston, TX, USA). Adalimumab (Humira®, AbbVie, North Chicago, IL, USA), Infliximab (Remicade®, Janssen Biotech, Horsham, PA, USA), ustekinumab (Stelara®, Janssen Biotech, Horsham, PA, USA), abatacept (Orencia®, Bristol-Myers Squibb, New York, NY, USA), natalizumab (Tysabri®, Biogen Idec, Cambridge, MA, USA) were reconstituted with diluent and stored according to manufacturer instructions. Fingolimod/FTY720 (S)-Phosphate was purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti-PD-1 (PL39505) and anti-PD-L1 (PDL1.243) monoclonal antibodies were generated in-house with equivalent bioactivity to Nivolumab and Atezolizumab.
Antibodies and additional reagents
CD3 (clone SP34-2, BUV395), CD4 (clone L200, PerCP-Cy5.5), CD8 (clone RPA-T8, AF700), CD107a (clone H4A3, APC), and IFNγ (B27, FITC) and Cytofix/Cytoperm™ were purchased from BD Biosciences (San Diego, CA, USA). Live/Dead Fixable NIR was supplied separately (Thermo Fisher Scientific, Waltham, MA, USA). PharmLyse (BD Biosciences) was employed to lyse red blood cells. Cytofix/Cytoperm (BD) served as a fixative and permeabilization reagent for intracellular cytokine staining. Staining buffer was purchased from BD Biosciences as 2% bovine serum albumin and 0.1% sodium azide in phosphate-buffered saline. EGFR/CD3 BiTE® was generated internally (Frank et al. Citation2018).
Isolation of PBMC
Peripheral blood was drawn directly into sodium heparin-coated Vacutainer tubes (BD Biosciences). All blood was obtained from adult (4–11-year-old, male or female) Cynomolgus macaques (Macaca fascicularis) of Mauritius origin housed at Worldwide Primates Inc. (Miami, FL, USA). All blood was delivered overnight at ambient temperature to the Amgen facilities located in South San Francisco, CA, USA. All in vivo work/blood sampling was conducted under an IACUC approved protocol in an AAALAC-accredited facility.
PBMC were subsequently isolated using Ficoll (GE Healthcare, Chicago, IL, USA) density gradient sedimentation. In brief, blood samples were diluted with an equal volume of RPMI 1640 media (Gibco, Grand Island, NY, USA), layered onto either 90% Ficoll (male donors) or 95% Ficoll (female donors), and then centrifuged at 1830 × g for 30 min at room temperature. These differences in need for Ficoll strengths were based on empirical testing. The resulting mononuclear cell fraction in each case was washed with media, and then any remaining red blood cells in the pellets were lysed using PharmLyse for 10 min at room temperature. Resulting PBMC were cryopreserved in Recovery™ Cell Culture Freezing Medium (Invitrogen, Waltham, MA, USA), placed in a Biocision Freezing Container at −80 °C for >24 h before transfer to a −150 °C freezer for long-term storage. Each PBMC sample was used to evaluate several test articles to optimize sample usage based on assay requirements.
In vitro BiTE®-mediated CTL assay
Human colon cancer cells (HCT-116) sourced from the American Type Culture Collection (ATCC) (Manassas, VA, USA) were lifted from the culture vessel with Cell Dissociation Buffer (Gibco) before washing with PBS, counting (and checked for viability), and subsequently being added to co-cultures. In brief, 105 viable PBMC were co-cultured with HCT-116 cells at an effector to target ratio (E:T) equal to 5:1 in the presence or absence of 200 pM (EC90) recombinant anti-EGFR × anti-CD3 bi-specific T-cell engager (BiTE®, Amgen Inc.) that cross-binds Cynomolgus CD3 antigen on T-cells in 96-well flat-bottom tissue culture plates. The EC90 value was predetermined in a cytotoxicity assay (Lutterbuese et al. Citation2010).
Cells were then incubated at 37 °C for a total of 48 h. At 5 h before harvest, APC-conjugated anti-human CD107a (H4A3), GolgiStop, and GolgiPlug (BD Biosciences) were added to the cells. At the end of incubation, the cells were subjected to Live/Dead Fixable NIR staining for 30 min at 4 °C. After washing, cells were fixed and permeabilized with Cytofix/Cytoperm according to manufacturer instructions. After permeabilization, cells were stained with BUV395-anti-CD3 (SP34-2), PE-anti-CD4 (L200), BV421-anti-CD8 (RP8-T8), and FITC-anti-IFNγ (B27) for 30 min on ice protected from light. After thorough washing with Stain Buffer, the cells were re-suspended in Stain Buffer and promptly analyzed in a FACSymphony flow cytometer (BD Biosciences). Data were initially acquired using DiVa acquisition software (BD Biosciences) before final analysis using FlowJo v10.4 software (BD Biosciences). A minimum of 10 000 events/samples was acquired. The assay principle and associated steps are described in .
Testing of immunomodulatory agents
A range of concentrations of each agent was added into the co-culture of PBMC/HCT-116 during the entire incubation period of the assay. Stock solutions were prepared in either DMSO, water, or PBS according to manufacturer recommendations. Subsequent dilutions of each agent were made in McCoy’s 5 A medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) and concomitantly added to the PBMC/HCT-116 co-culture for 48 h in the BiTE®-mediated CTL assay. The quantity of diluent contained in the highest concentration of drug was always used as a control (in the absence of drug).
Data analysis
All data analysis was done using Prism v.8.4.3 software (GraphPad, San Diego, CA, USA). Each IC50 was calculated with non-linear regression analysis and the value represents the concentration of a test compound where 50% of its maximal inhibition effect is observed.
Results
Evaluation of immunosuppressive therapeutic agents in the BiTE®-mediated CTL assay
Small molecules inhibiting lymphocyte signaling pathways, proliferation, and activation
A panel of nine immunosuppressant agents directly influencing lymphocyte-intrinsic signaling pathways, cell proliferation, and activation () was tested in the BiTE®-mediated CTL assay to determine the change of CD8+ T-cell responses. Flow cytometry gating strategy was presented with representative plots (Supplementary Figure 1). Dexamethasone and prednisone are both synthetic corticosteroids used to suppress immune responses and treat many inflammatory conditions including arthritis, colitis, asthma, bronchitis, and allergies (Wust et al. Citation2008; Lutterbuese et al. Citation2010). Consistent with previous findings (Frank et al. Citation2018), dexamethasone effectively inhibited CD107a surface staining and IFNγ production from CD8+ T-cells upon activation (). While prednisone did not directly affect CD8+ T-cell responses (data not shown), prednisolone, an active metabolite of prednisone induced a dose-dependent suppression of CD107a and IFNγ (). A group of marketed small molecular inhibitors (e.g. tofacitinib, ruxolitinib, mycophenolic acid, teriflunomide, tacrolimus/FK506, rapamycin, and apremilast) targeting different pathways of T-cell responses were then tested with multiple donor PBMC (, ). In each case, dose-dependent suppression of CD107a and IFNγ from CD8+ T-cells was evident.
Figure 2. Immunosuppressive agents targeting T cell-intrinsic signals, proliferation, and activation reduced CTL activity. Percentage CD8+ T-cells with CD107a surface staining (left panel) and IFNγ production (right panel) were measured at different drug concentrations. Each data point is mean (±SD) from a single donor run in duplicate. Data are representative individual donors of multiple independent experiments with several donors (n). (A) Dexamethasone (n = 4). (B) Prednisolone (n = 6). (C) Tofacitinib (n = 4). (D) Ruxolitinib (n = 6). (E) Mycophenolic acid (n = 6). (F) Teriflunomide (n = 8). (G) Tacrolimus/FK506 (n = 5). (H) Rapamycin (n = 6). (I) Apremilast (n = 8). Drugs were tested in separate experiments.
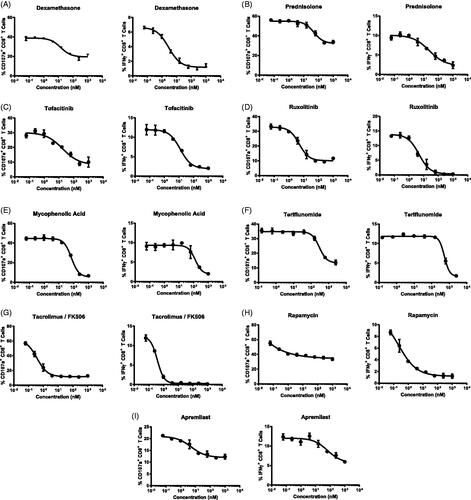
Table 1. Immunomodulatory agents and mechanisms of action.
At the concentrations evaluated with each of the agents, there was no effect on T-cell viability—except a slight reduction for teriflunomide at concentrations > 2500 nM (Supplementary Figure 2). Averaging the data from all the donors tested, the IC50 of CD107a surface expression and the IC50 of IFNγ production for all nine drugs were listed and compared (). A good correlation of IC50 between both analytes CD107a and IFNγ was observed for each drug. Dexamethasone is more potent than prednisolone in the donors tested. Two Janus kinase (JAK) inhibitors tofacitinib and ruxolitinib had similar IC50 values regarding CD8+ T-cell responses. Tacrolimus/FK506 (calcineurin inhibitor) and rapamycin (mTOR inhibitor) displayed the most potent inhibition among all tested drugs. Though potent, rapamycin only partially reduced CD107a surface staining (by 50%). In contrast, the suppression by rapamycin of IFNγ production reached 80% across all donors tested. Nucleotide synthesis inhibitors mycophenolic acid and teriflunomide influenced CTL activity in the assay with much lower potency than other compounds. The PDE4 inhibitor apremilast also partially inhibited CTL activity, with an IC50 similar to that of mycophenolic acid. Of note, three of the donors tested with teriflunomide and two of the donors tested with apremilast demonstrated no apparent suppression of CD8+ T-cell activity under all concentrations tested (). Moreover, teriflunomide demonstrated the most variability in immunosuppression between donors. Overall, all tested compounds affecting intrinsic T-cell activation pathways successfully suppressed CD107a and IFNγ responses in the BiTE® mediated CTL assay.
Table 2. IC50 values of immunosuppressant agents.
Therapeutic agents targeting specific cytokine signaling, lymphoid trafficking, and co-stimulatory pathways
To evaluate the application of this BiTE®-mediated CTL assay for immunosuppressant biologics, a panel of four monoclonal antibodies (e.g. adalimumab, infliximab, tociluzimab, and ustekinumab) targeting cytokine or cytokine receptors () was tested in the assay. A wide concentration range was selected to cover the concentrations achieved in vitro in potency assays and in vivo at efficacious dose levels in patients (Moller et al. Citation1990; Cornillie et al. Citation2001; Weisman et al. Citation2003; Mihara et al. Citation2005; Clarke et al. Citation2010; Ogata et al. Citation2012; Buurman et al. Citation2018; Tsakok et al. Citation2019). No apparent effects on CD8+ T-cell CD107a surface staining or IFNγ production were observed at the concentrations evaluated for each of the antibodies (). Upon BiTE® induced T-cell activation, tumor necrosis factor (TNF)-α was produced (Brehm et al. Citation2005; Ross et al. Citation2017). However, anti-TNFα antibodies adalimumab and infliximab did not affect CTL activity () or change the levels of TNFα-producing CD8+ T-cells (Supplementary Figure 3).
Figure 3. Biologics and compounds targeting cytokine or cytokine receptors, co-stimulation, or lymphocyte migration did not impact CTL activity in vitro. Percentage CD8+ T-cells with CD107a surface staining (left panel) and IFNγ production (right panel) were measured at different drug concentrations. Each data point is mean (±SD) from a single donor run in duplicate. Data are representative of individual donors of multiple independent experiments with several donors (n). (A) Adalumumab (n = 3). (B) Infliximab (n = 3). (C) Tociluzumab (n = 3). (D) Ustekinumab (n = 3). (E) Abatacept (n = 6). (F) Nataluziumab (n = 3). (G) Fingolimod/FTY720 (n = 6). Drugs were tested in separate experiments.
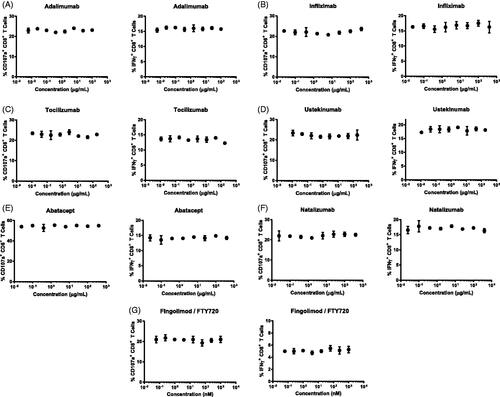
The study here next tested abatacept, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) Ig fusion protein known to suppress T-cell activation by interrupting CD80/86-CD28 co-stimulatory pathways (Douthwaite et al. Citation2017; Li et al. Citation2019b). Abatacept did not impact the CD107a and IFNγ response at any of the concentrations tested, suggesting BiTE®-mediated CTL activation may bypass the requirement of interaction between CD80/CD86 and CD28 ().
Two therapeutic agents targeting lymphocyte adhesion, and trafficking (natalizumab, and fingolimod/FTY720) were tested in the BiTE®-mediated CTL activity assay (, ). At all concentrations evaluated, there were no apparent effects on CD8+ T-cell CD107a surface staining or IFNγ production.
Evaluation of immunostimulatory agents in BiTE®-mediated CTL assay
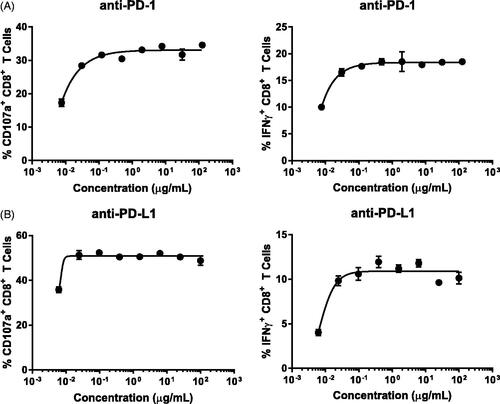
To determine if the BiTE®-mediated CTL assay was able to detect an enhancement on CD8+ T-cell activity, immune checkpoint modifying antibodies were tested (). The immune checkpoint blockers nivolumab and atezolizumab target the programmed cell death-1 (PD-1) on lymphocytes and programmed cell death ligand-1 (PD-L1) on tumors, respectively. Both molecules are designed to enhance the anti-tumor activity of lymphocytes by removing the inhibitory effect from PD-1/PD-L1 interaction. Two monoclonal antibodies similar to nivolumab and atezoluzimab were each tested with multiple donors (). In all donors tested with each therapeutic agent, increased CD107a surface staining and IFNγ production from CD8+ T-cells were evident. The concentrations achieving a maximal response in the assay are similar to the other potency published data (Wang et al. Citation2014; de Sousa Linhares et al. Citation2019).
Figure 4. Anti-PD-1/PD-L1 immune checkpoint blockades enhanced CTL activity in vitro. Percentage CD8+ T-cells with CD107a surface staining (left panel) and IFNγ production (right panel) were measured at different drug concentrations. Each data point is mean (±SD) from a single donor run in duplicate. Data are representative of individual donors of multiple independent experiments with several donors (n). (A) Anti-PD-1 (n = 3). (B) Anti-PD-L1 (n = 3). Drugs were tested in separate experiments.
Discussion
Our laboratory previously developed a Cynomolgus macaque CD8+ CTL assay to inform primarily on immunosuppressive potentials of immunomodulatory therapeutics in non-human primate preclinical studies. The assay used a BiTE® molecule as a tool to activate Cynomolgus T-cells with a target expressing cell line () (Frank et al. Citation2018). The BiTE®-mediated CTL assay consistently elicited robust CTL responses across multiple donors while in the presence of target cells and was shown to be sensitive to dexamethasone, commonly used as an immunosuppressant in the clinic (Barnes Citation2006). Here, the assay was further applied to a list of therapeutic agents with the known immunomodulatory ability to understand the applicability of this assay. Prednisolone (an active metabolite of prednisone) suppressed the Cynomolgus macaque CTL activity with slightly less potency than dexamethasone; this is aligned with an observation of a lower potency in the clinic (Shefrin and Goldman Citation2009). Both dexamethasone and prednisone are glucocorticoids that inhibit cytokine production and T-cell proliferation notably via binding glucocorticoid receptor and subsequent transactivation or trans-repression of gene expression.
All agents that directly influence lymphocyte intrinsic pathways involved in proliferation and activation effectively reduced cynomolgus monkey CTL activity in the assay (, ). This activity, measured by CD107a surface staining and IFNγ production, was suppressed under in vitro exposure to tofacitinib, ruxolitinib, mycophenolic acid, rapamycin, teriflunomide, tacrolimus/FK506, and apremilast. Tofacitinib and ruxolitinib are JAK inhibitors that inhibit the JAK-signal transducer and activator of transcription (STAT) pathways that play a major role in cytokine receptor signaling (Hodge et al. Citation2016; Elli et al. Citation2019). Mycophenolic acid (active moiety of mycophenolate mofetil) is an inhibitor of inosine-5′-monophosphate dehydrogenase (IMPDH) critical for de novo purine synthesis necessary for rapidly dividing T-cells (Allison and Eugui Citation2000). Teriflunomide (an active metabolite of leflunomide) is a de novo pyrimidine synthesis inhibitor that binds mitochondrial dihydroorotate dehydrogenase (DHODH) during activated lymphocyte replication (Fox et al. Citation1999; Bar-Or Citation2014). The well-characterized calcineurin inhibitor tacrolimus/FK506 impaired cynomolgus monkey CTL activity with the highest potency in this assay. Tacrolimus/FK506 acts directly on T-cells by disrupting the obligatory calcium cascade required for T-cell activation and transcription of interleukin (IL)-2 (Thomson et al. Citation1995). Rapamycin impedes progression through the cell cycle G1/S transition in IL-2 stimulated T-cells by inhibiting the mammalian target of rapamycin (mTOR) (Dumont and Su Citation1996). Apremilast inhibits phosphodiesterase 4 (PDE4), the dominant enzyme responsible for the breakdown of cyclic adenosine monophosphate (cAMP), resulting in the down-regulation of several pro-inflammatory factors (Schafer Citation2012).
In contrast, the therapeutic drugs that affect immunity by impacting cytokine pathways and lymphocyte adhesion/trafficking did not alter CTL function in the assay. This is consistent with an expectation that some drugs modify immunity in vivo through chronic treatments or via specific mechanisms not captured in this short-term in vitro assay that only measures an acute direct immunomodulatory effect of the drugs. Adalimumab, a TNF antagonist, was recently shown to have no impact on CD3 bi-specific-mediated cytotoxic T-cell activity despite the prevention of TNFα activity (Li et al. Citation2019a). Another TNF antagonist, infliximab, is a chimeric (mouse/human) monoclonal antibody that only binds chimpanzee and human TNF; hence, it was not suited for testing here (Cornillie et al. Citation2001). Tocilizumab binds and inhibits both soluble and membrane-bound IL-6 receptors. IL-6 signaling is dispensable for activated CD8+ T-cells where the density of IL-6R is significantly decreased as the membranous form of the receptor is shed (Bottcher et al. Citation2014). The IL-12/IL-23 inhibitor ustekinumab binds the shared p40 subunit on both cytokines (Benson et al. Citation2011). Together, IL-12 and IL-23 induce T-helper (TH)-1 and TH17 cell differentiation in CD4 T-cells. Although IL-12 acts as a third signal in CD8+ T-cell activation (Schurich et al. Citation2013), it has been shown in the clinic that ustekinumab treatment did not affect CD8+ T-cell proliferation or cytokine production (Tsuda et al Citation2012; Narita et al. Citation2014).
Agents that inhibit lymphocyte adhesion/transmigration have demonstrated therapeutic benefits for patients with rheumatoid arthritis or multiple sclerosis. Natalizumab and fingolimod/FTY720 did not suppress CTL activity in this in vitro assay. Natalizumab blocks the interaction of α4-integrin with adhesion molecules on the vascular endothelium (Leger et al. Citation1997). The impact of a selective adhesion inhibitor is not predicted to manifest in this in vitro co-culture assay. The sphingosine-1-phosphate (S1P) receptor agonist Fingolimod (FTY720) induces sequestration of lymphocytes in secondary lymphoid organs and prevents lymphocyte egress into circulation (Matloubian et al. Citation2004). Here, the larger systemic effects of lymphocyte sequestration observed in vivo are not expected to be recapitulated in this in vitro assay.
Interestingly, abatacept, a CTLA-4-Ig fusion protein that inhibits T-cell activation by binding to CD80 and CD86 on antigen-presenting cells, did not dampen the CTL response (); however, the anti-PD-1/PD-L1 checkpoint inhibitors were capable of enhancing CTL responses induced by BiTE® (). BiTE® molecules stimulate a robust T-cell activation through directly engaging CD3 and bypassing the antigen-presenting cells (APC), therefore, interruption of CD80/CD86 signals on APC may not impact the response. However, given that tumor cells express abundant PD-L1, disruption of PD-1/PD-L1 interactions between target and T-cells can further augment T-cell activation in this assay. While both checkpoint inhibitors enhanced CTL activity at all concentrations tested, only modest effects were observed. More substantial effects of checkpoint inhibition on CTL activity are predicted using suboptimal concentrations of BiTE®-mediated stimulation (Sam et al. Citation2020).
The primary goal of the current work was to further understand the utility of a recently developed BiTE®-mediated cynomolgus macaque CTL activity assay. To that end, several marketed therapeutics with known immunosuppressive and immune-enhancing profiles with various mechanisms of action were tested in vitro. The results of these studies demonstrated there was a varying impact on CD8+ CTL activity. The immunosuppressive effects observed on CD107a surface expression and IFNγ production from these agents were highly correlative when looking at potency (). Despite pharmacodynamic responses in preclinical cynomolgus macaque studies, investigational molecules that disrupt cognate antigen presentation, inhibit specific cytokines dispensable for cytolytic function, alter the adhesion properties or trafficking of lymphocytes were found to be insensitive in this BiTE®-mediated activity assay.
While larger systemic biological processes cannot be recapitulated in this in vitro assay, any immunomodulatory agents that alter the intrinsic properties (e.g. activation, cell cycle progression, signaling cascades, transcription, checkpoint inhibition, etc.) of the CD8+ CTL demonstrated sensitivity (immunosuppression or immune enhancement) in this assay. Taken together, the current in vitro results demonstrate that the BiTE®-mediated CTL assay to be applicable to a wide range of immunomodulatory agents directly targeting T-cell activation associated intrinsic signaling pathways and immune checkpoints, providing a promising addition to the toolbox of assays used to assess immunotoxicity.
Supplemental Material
Download PDF (697.1 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Allison A, Eugui E. 2000. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 47(2–3):85–118.
- Bar-Or A. 2014. Teriflunomide (Aubagio(R)) for the treatment of multiple sclerosis. Exp Neurol. 262:57–65.
- Barnes P. 2006. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 148(3):245–254.
- Benson J, Sachs C, Treacy G, Zhou H, Pendley C, Brodmerkel C, Shankar G, Mascelli M. 2011. Therapeutic targeting of the IL-12/23 pathways: Generation and characterization of ustekinumab. Nat Biotechnol. 29(7):615–624.
- Bottcher J, Schanz O, Garbers C, Zaremba A, Hegenbarth S, Kurts C, Beyer M, Schultze J, Kastenmuller W, Rose-John S, et al. 2014. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. 8(5):1318–1327.
- Brehm M, Daniels K, Welsh R. 2005. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. J Immunol. 175(8):5043–5049.
- Buurman D, Blokzijl T, Festen E, Pham B, Faber K, Brouwer E, Dijkstra G. 2018. Quantitative comparison of the neutralizing capacity, immunogenicity and cross-reactivity of anti-TNFα biologicals and an Infliximab-biosimilar. PLOS One. 13(12):e0208922.
- Clarke A, Poulton L, Wai H, Walker S, Victor S, Domagala T, Mraovic D, Butt D, Shewmaker N, Jennings P, et al. 2010. A novel class of anti-IL-12p40 antibodies: Potent neutralization via inhibition of IL-12-IL-12Rβ2 and IL-23-IL-23R. MAbs. 2(5):539–549.
- Cornillie F, Shealy D, D'Haens G, Geboes K, van Assche G, Ceuppens J, Wagner C, Schaible T, Plevy S, Targan S, et al. 2001. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn's disease. Aliment Pharmacol Ther. 15(4):463–473.
- de Sousa Linhares A, Battin C, Jutz S, Leitner J, Hafner C, Tobias J, Wiedermann U, Kundi M, Zlabinger G, Grabmeier-Pfistershammer K, et al. 2019. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep. 9(1):11472.
- Douthwaite J, Moisan J, Privezentzev C, Soskic B, Sabbah S, Cohen S, Collinson A, England E, Huntington C, Kemp B, et al. 2017. A CD80-biased CTLA4-Ig fusion protein with superior in vivo efficacy by simultaneous engineering of affinity, selectivity, stability, and FcRn Binding. J Immunol. 198(1):528–537.
- Dumont F, Su Q. 1996. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 58(5):373–395.
- Dunn G, Old L, Schreiber R. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 21(2):137–148.
- Elli E, Barate C, Mendicino F, Palandri F, Palumbo G. 2019. Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front Oncol. 9:1186.
- Fox R, Herrmann M, Frangou C, Wahl G, Morris R, Strand V, Kirschbaum B. 1999. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 93(3):198–208.
- Frank B, Wei Y, Kim K, Guerrero A, Lebrec H, Balazs M, Wang X. 2018. Development of a BiTE®-mediated CD8+ cytotoxic T-lymphocyte activity assay to assess immunomodulatory potential of drug candidates in Cynomolgus macaque. J Immunotoxicol. 15(1):119–125.
- Herrero-Beaumont G, Martinez Calatrava M, Castaneda S. 2012. Abatacept mechanism of action: Concordance with its clinical profile. Reumatol Clin. 8(2):78–83.
- Hodge J, Kawabata T, Krishnaswami S, Clark J, Telliez J, Dowty M, Menon S, Lamba M, Zwillich S. 2016. The mechanism of action of tofacitinib – an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 34(2):318–328.
- ICH. 2005. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Immunotoxicity Studies for Human Pharmaceuticals S8.
- Jacobson P, Uberti J, Davis W, Ratanatharathorn V. 1998. Tacrolimus: A new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant. 22(3):217–225.
- Lebrec H, Brennan F, Haggerty H, Herzyk D, Kamperschroer C, Maier C, Ponce R, Preston B, Weinstock D, Mellon R. 2016. HESI/FDA workshop on immunomodulators and cancer risk assessment: Building blocks for a weight-of-evidence approach. Regul Toxicol Pharmacol. 75:72–80.
- Leger O, Yednock T, Tanner L, Horner H, Hines D, Keen S, Saldanha J, Jones S, Fritz L, Bendig M. 1997. Humanization of mouse antibody against human α4 integrin: Potential therapeutic for the treatment of multiple sclerosis. Hum Antibodies. 8(1):3–16.
- Li J, Piskol R, Ybarra R, Chen Y, Li J, Slaga D, Hristopoulos M, Clark R, Modrusan Z, Totpal K, et al. 2019a. CD3 bi-specific antibody-induced cytokine release is dispensable for cytotoxic T-cell activity. Sci Transl Med. 11:eaax8861.
- Li J, Stagg N, Johnston J, Harris M, Menzies S, DiCara D, Clark V, Hristopoulos M, Cook R, Slaga D, et al. 2017. Membrane-proximal epitope facilitates efficient T-cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell. 31(3):383–395.
- Li X, Roy A, Murthy B. 2019b. Population pharmacokinetics and exposure-response relationship of intravenous and subcutaneous abatacept in patients with rheumatoid arthritis. J Clin Pharmacol. 59(2):245–257.
- Lutterbuese R, Raum T, Kischel R, Hoffmann P, Mangold S, Rattel B, Friedrich M, Thomas O, Lorenczewski G, Rau D, et al. 2010. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci USA. 107(28):12605–12610.
- Matloubian M, Lo C, Cinamon G, Lesneski M, Xu Y, Brinkmann V, Allende M, Proia R, Cyster J. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427(6972):355–360.
- Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, Matsumoto Y, Ohsugi Y. 2005. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 5(12):1731–1740.
- Moller A, Emling F, Blohm D, Schlick E, Schollmeier K. 1990. Monoclonal antibodies to human TNFα: In vitro and in vivo application. Cytokine. 2(3):162–169.
- Narita M, Nishizawa Y, Iwaya S, Oiwa E, Iwabuchi M, Uchiyama T, Matsuyama A, Masuko M, Takahashi M. 2014. Ustekinumab improves psoriasis without suppressing tumor antigen-specific cytotoxic T-lymphocytes. Int Arch Allergy Immunol. 165(1):52–60.
- Ogata A, Hirano T, Hishitani Y, Tanaka T. 2012. Safety and efficacy of tocilizumab for the treatment of rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord. 5:27–42.
- Ross S, Sherman M, McElroy P, Lofgren J, Moody G, Baeuerle P, Coxon A, Arvedson T. 2017. Bi-specific T-cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing. PLOS One. 12(8):e0183390.
- Sam J, Colombetti S, Fauti T, Roller A, Biehl M, Fahrni L, Nicolini V, Perro M, Nayak T, Bommer E, et al. 2020. Combination of T-cell bi-specific antibodies with PD-L1 checkpoint inhibition elicits superior anti-tumor activity. Front Oncol. 10:575737.
- Schafer P. 2012. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 83(12):1583–1590.
- Schurich A, Pallett L, Lubowiecki M, Singh H, Gill U, Kennedy P, Nastouli E, Tanwar S, Rosenberg W, Maini M. 2013. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLOS Pathog. 9(3):e1003208.
- Sehr T, Proschmann U, Thomas K, Marggraf M, Straube E, Reichmann H, Chan A, Ziemssen T. 2016. New insights into pharmacokinetics and pharmacodynamics of natalizumab treatment for patients with multiple sclerosis, obtained from clinical and in vitro studies. J Neuroinflammation. 13:164.
- Shefrin A, Goldman R. 2009. Use of dexamethasone and prednisone in acute asthma exacerbations in pediatric patients. Can Fam Physician. 55(7):704–706.
- Stuve O, Bennett J. 2007. Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri) in inflammatory diseases. CNS Drug Rev. 13(1):79–95.
- Thomson A, Bonham C, Zeevi A. 1995. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther Drug Monit. 17(6):584–591.
- Tsakok T, Wilson N, Dand N, Loeff F, Bloem K, Baudry D, Duckworth M, Pan S, Pushpa-Rajah A, Standing J, et al. 2019. Association of serum ustekinumab levels with clinical response in psoriasis. JAMA Dermatol. 155(11):1235–1243.
- Tsuda K, Yamanaka K, Kondo M, Matsubara K, Sasaki R, Tomimoto H, Gabazza E, Mizutani H. 2012. Ustekinumab improves psoriasis without altering T-cell cytokine production, differentiation, and T-cell receptor repertoire diversity. PLOS One. 7(12):e51819.
- Volpi C, Orabona C, Macchiarulo A, Bianchi R, Puccetti P, Grohmann U. 2019. Preclinical discovery and development of fingolimod for the treatment of multiple sclerosis. Expert Opin Drug Discov. 14(11):1199–1212.
- Wang C, Thudium K, Han M, Wang X, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al. 2014. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2(9):846–856.
- Weisman M, Moreland L, Furst D, Weinblatt M, Keystone E, Paulus H, Teoh L, Velagapudi R, Noertersheuser P, Granneman G, et al. 2003. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-TNFα monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: Pilot study. Clin Ther. 25(6):1700–1721.
- Wust S, van den Brandt J, Tischner D, Kleiman A, Tuckermann J, Gold R, Luhder F, Reichardt H. 2008. Peripheral T-cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 180(12):8434–8443.
