Abstract
Epicutaneous exposure to protein allergens, such as papain, house dust mite (HDM), and ovalbumin (OVA), represents an important mode of sensitization for skin diseases including protein contact dermatitis, immunologic contact urticaria, and atopic dermatitis. These diseases are inducible by re-exposure to an allergen at both original skin sensitization and distant skin sites. In this study, we examined the serum IgE/IgG1 response, differentiation of T-helper (TH) cells, and epicutaneous TH recall response in mice pre-sensitized with protein allergens through the back skin and subsequently challenged on the ear skin. Repeated epicutaneous sensitization with allergenic proteins including papain, HDM, OVA, and protease inhibitor-treated papain, but not bovine serum albumin, induced serum allergen-specific antibody production, passive cutaneous anaphylaxis responses, and TH2 differentiation in the skin draining lymph node (DLN) cells. Sensitization with papain or HDM, which have protease activity, resulted in the differentiation of TH17 as well as TH2. In papain- or HDM-sensitized mice, a subsequent single challenge on the ear skin induced the expression of TH2 and TH17/TH22 cytokines. These results suggest that allergenic proteins induce the differentiation of TH2 in skin DLN cells and an antibody response. These findings may be useful for identifying proteins of high and low allergenic potential. Moreover, allergenic proteins containing protease activity may also differentiate TH17 and induce TH2 and TH17/TH22 recall responses at epicutaneous challenge sites. This suggests that allergen protease activity accelerates the onset of skin diseases caused by protein allergens.
Introduction
Protein allergens, such as papain and house dust mite (HDM), contain proteases and are known to cause respiratory allergic reactions and, more rarely, allergic skin diseases including protein contact dermatitis and immunologic contact urticaria (Amaro and Goossens Citation2008; Basketter and Lahti Citation2011; Takai and Ikeda Citation2011; Barbaud Citation2020). With respect to protein allergies, inhalation and dietary exposure are associated with sensitization, which results in respiratory and food allergy (Ramirez and Bahna Citation2009; Paller et al. Citation2019). Exposure of the skin to enzymes used in laundry and cleaning products does not appear to pose a significant risk of allergic disease, although it is recognized that epicutaneous exposure to other proteins may result in sensitization in some circumstances (Kimber et al. Citation2014; Coenraads Citation2016; Basketter and Kimber Citation2022). In murine models, several studies have demonstrated that epicutaneous sensitization with such allergens can induce airway and skin inflammation as well as serum IgE/IgG1 responses (Kikuchi et al. Citation2006; Iida et al. Citation2014; Stremnitzer et al. Citation2014; Shimura et al. Citation2016; Suzuki et al. Citation2016; Deckers et al. Citation2017; Ochi et al. Citation2017; Kamijo et al. Citation2021; Yokozeki et al. Citation2021).
Some studies indicated the importance of allergen protease activity for skin sensitization following repeated exposure (Iida et al. Citation2014; Shimura et al. Citation2016). Moreover, allergic skin diseases are inducible by re-exposure to an allergen at the distant skin sites as well as initial sensitization sites. In a previous study, it was demonstrated that the protease activity of papain contributed to the induction of T-helper (TH) 2 and TH17/TH22 recall responses at distant epicutaneous challenge sites in mice that were pre-sensitized at the sensitization sites (Ogasawara et al. Citation2021). However, additional experiments are necessary using other protein allergens that are known to cause allergic skin diseases (e.g. HDM) (Kikuchi et al. Citation2006; Stremnitzer et al. Citation2014; Suzuki et al. Citation2016; Deckers et al. Citation2017) as well as non-protease allergens, such as ovalbumin (OVA; He et al. Citation2007; Wang et al. 2007, 2009). With respect to toxicological evaluation, it is also important to test a protein with low allergenic potential as a negative control and confirm that smaller antibody production and TH2 and TH17/TH22 recall responses occur.
Based on these findings, we conducted a study using papain and HDM as proteases, OVA as a non-protease, and bovine serum albumin (BSA), which is considered a low allergenic protein (Hilton et al. Citation1994, Citation1997; Dearman et al. Citation2000), as a “negative” control. We evaluated the differentiation of TH cells, serum IgE/IgG1 responses, and epicutaneous TH recall responses at both sensitization and challenge sites in mice that were pre-sensitized through the back skin and subsequently challenged on the ear skin. The role of protease activity to the epicutaneous sensitization phase was also examined using a protease inhibitor E64-treated papain (E64-papain), which lacks protease activity, but retains its T/B epitope structures (Kikuchi et al. Citation2006; Nishioka et al. Citation2018).
Material and methods
Materials
Papain (Calbiochem, San Diego, CA), HDM (Stallergenes Greer, Lenoir, NC), OVA (Fujifilm Wako Pure Chemical, Osaka, Japan), and BSA (Fujifilm) were purchased. E64-papain and E64-HDM were prepared according to a previously described protocol using E-64 (Sigma, St Louis, MO, USA; Kamijo et al. Citation2013). According to manufacturer instructions, biotinylated protein allergens were prepared using the EZ-Link™ Sulfo-NHS-LC-Biotinylation Kit (ThermoFisher Scientific, Tokyo, Japan). Protein concentrations were determined by the Lowry method using the DC™ Protein Assay Kit II (BioRad, Hercules, CA, USA).
Mice
HOS:HR-1 hairless mice (7-wk-old, female) were obtained from Japan SLC (Shizuoka, Japan). The animals were housed in a pathogen-free facility maintained at 23 °C with a 55% relative humidity and a 12 h light:dark cycle. All mice were given access to standard feed pellets and filtered tap water ad libitum. The Kao Corporation Animal Care Committee approved all of the animal experiments. The studies were conducted according to committee guidelines.
Epicutaneous sensitization
According to an earlier described method, epicutaneous sensitization was done through a 24 h occlusive patch (Ogasawara et al. Citation2021). In brief, the protein allergens [100 μg/100 μl phosphate-buffered saline (PBS)/2.25 cm2/site] or PBS (100 μl/2.25 cm2/site) were perfused onto a square piece of gauze (PIP, Osaka, Japan) under an acrylic adhesive waterproof film (3 M Japan, Tokyo, Japan), placed onto the back skin of the mice, and immobilized using a polyurethane film (Alcare, Tokyo, Japan). The application was performed twice weekly for 5 weeks (total number of applications = 10). Three days after the final application, the mice were anesthetized using isoflurane (Fujifilm) and blood samples were collected from the abdominal aorta. The mice were then euthanized by cervical dislocation. The sensitization sites (back skin) were collected at necropsy and stabilized in RNA-later (QIAGEN, Manchester, UK).
Draining lymph node (DLN) cells were collected aseptically from the axillary lymph and inguinal lymph nodes. The cells were harvested, washed, and resuspended in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 1% (v/v) penicillin-streptomycin solution, and 0.05 mM 2-mercaptoethanol (all Invitrogen). The cells were counted for use in the assays described below.
Epicutaneous challenge
The epicutaneous challenge was conducted according to a previously described protocol (Ogasawara et al. Citation2021). In brief, 1.0% protein allergen (25 µl/ear) in PBS containing 0.5% Tween-20 (Kanto Chemical, Tokyo, Japan) was applied to the back of the mouse ear. Before and 24 h after challenge, the mice were anesthetized and euthanized. The ear skin challenge sites were collected into RNA-later for preservation.
Passive cutaneous anaphylaxis (PCA) test
The PCA test was done according to the method described in Hilton et al. (Citation1994) and Dearman et al. (Citation2000), with minor modification. Serum samples were diluted in saline and injected into the dermis of the ears of naïve, recipient mice (10 µl/ear). After 48 h, 5.0 µg of protein and 1.0 mg of Evans Blue dye (Fujifilm) in 200 µl of PBS were injected intravenously. The leaked dye was measured 30 min after injection.
Quantitative RT-PCR (qRT-PCR)
Back and ear skin samples were placed in Buffer RLT (QIAGEN) containing 1% 2-mercaptoethanol (Serva, Heidelberg, Germany) and homogenized with a micro-homogenizer (Microtec, Chiba, Japan). Total RNA was extracted and purified using the RNeasy Fibrous Tissue Mini Kit and the RNeasy MinElute Cleanup Kit (QIAGEN). The purity and concentration of the isolated RNA were determined with a NanoDrop system (ThermoFisher Scientific). First-strand cDNA was synthesized from total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA). qRT-PCR was then performed using Taqman probes on a QuantStudio 5 Real-Time PCR system (Applied Biosystems). mRNA levels of target genes were normalized to expression levels of GAPDH (glyceraldehyde 3-phosphate dehydrogenase) housekeeping gene. All data are presented as relative expression levels to the control group ( and —back skin of vehicle-treated mice; —ear skin of vehicle-treated mice before challenge).
Serum total IgE ELISA
Total serum IgE antibody levels were measured as described in Ogasawara et al. (Citation2021). In brief, total serum IgE was detected using plates coated with 2 µg/ml anti-mouse IgE antibody (BD Biosciences, San Jose, CA, USA) in PBS. Plates were pre-blocked with 20% Immunoblock (DS Pharma Biomedical, Osaka, Japan) in distilled water. Sera and detection antibodies were diluted with PBS containing 0.05% Tween-20 and 5% Immunoblock (PBS-T/IB, serum dilution 1:50). Plates were treated with TMB Microwell Peroxidase Substrate (Seracare, Milford, MA, USA) according to the manufacturer’s instructions and the absorbance at 450 nm was recorded for each well using a CLARIOstar plate reader (BMG Labtech, Ortenberg, Germany).
Serum antigen-specific IgE/IgG1 ELISA
Serum antigen-specific IgE/IgG1 levels were measured using a previously described protocol (Strid et al. Citation2005) with minor modification. In brief, serum antigen-specific IgE or IgG1 levels were detected using plates coated with 5 µg/ml anti-mouse IgE or IgG1 antibody (both BD Biosciences) in PBS. The plates were pre-blocked as described above and sera were diluted using PBS-T/IB (serum dilutions of 1:50 for antigen-specific IgE and 1:5000 for antigen-specific IgG1). Biotinylated protein allergens were added at a concentration of 0.24 µg/ml in PBS-T/IB and incubated for 1 h at room temperature. For detection, streptavidin-horseradish peroxidase conjugate (GE Healthcare, Amersham, UK; 1:1000 in PBS-T/IB) was added and the plates incubated for 1 h at room temperature. Plates were then treated with TMB Microwell Peroxidase Substrate and the absorbance at 450 nm in each well was recorded.
Re-stimulation of DLN cells
Re-stimulation of DLN cells was done according to the protocol in Ogasawara et al. (Citation2021). Here, DLN cells were plated at a density of 5 × 105 cells/well in flat-bottom 96-well plates (Nippon Genetics, Tokyo, Japan) containing 100 µg/ml protein allergen or medium alone. DLN cells from papain- or HDM-sensitized mice were re-stimulated in vitro for cytokine production with E64-papain or -HDM to avoid possible stimulatory effects by protease activity, respectively (Kamijo et al. Citation2013). After 72 h, the culture supernatants were collected and analyzed for specific cytokines (e.g. interleukin [IL]-4, -17, and interferon [IFN]-γ) using mouse Quantikine ELISA Kits (R&D Systems, Minneapolis, MN, USA), following manufacturer instructions.
Statistical analysis
A Mann-Whitney U test (two-tailed) was used to determine any significant differences between mean values. A p-value < 0.05 was considered statistically significant. All data were analyzed using EZR v.1.40 software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
Epicutaneous sensitization with papain, HDM, and OVA, but not with BSA, on the back skin of mice induces serum allergen-specific IgE and IgG1, and differentiation of TH2 cells
HOS:HR-1 hairless mice were epicutaneously sensitized by applying protein allergens on the back skin through an occlusive patch (). Papain and HDM up-regulated total serum IgE levels (). Papain, HDM, and OVA, but not BSA, induced the production of allergen-specific IgE and IgG1 (). Undiluted or 10-fold diluted serum from BSA-sensitized mice showed a positive PCA reaction rate of 20% or 0%, respectively (). In contrast, serum from papain-, HDM-, and OVA-sensitized mice exhibited positive PCA reactions rate of 100%, 20%, and 100%, respectively, even at dilutions of 1:10 (). These results indicate that sensitization with papain, HDM, and OVA induced stronger antibody responses compared with BSA.
Figure 1. Epicutaneous sensitization with papain, HDM, and OVA, but not with BSA, on the back skin of mice induces serum allergen-specific IgE and IgG1, and differentiation of TH2 cells. (A) Timeline. (B) Serum total IgE. (C) Serum allergen-specific IgE and IgG1. (D) Positive PCA reaction rates. (E) Cytokine production in DLN cells re-stimulated with protein allergens. (F) Gene expression in back skin. Data shown are means ± SD of 5 mice/group representing two independent experiments with similar results. *p < 0.05 vs. vehicle (B, C, F) or no re-stimulation (E) by Mann-Whitney U test. N.D.: not detected; N.T.: not tested.
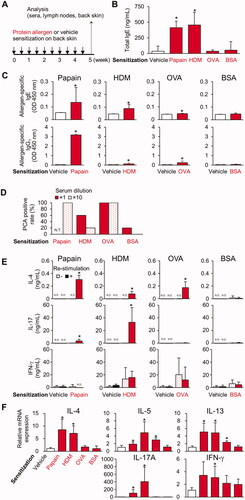
Production of IL-4 in antigen-re-stimulated skin DLN cells was observed in the papain-, HDM-, and OVA-, but not BSA-, sensitized mice (). IL-17 production was also observed in papain- and HDM-sensitized mice (). The OVA-treated skin demonstrated higher expression levels of TH2 cytokine (IL-5 and -13) mRNA, whereas papain- and HDM-treated skins had elevated expression of both TH2 (IL-4, -5, and -13) and TH17 cytokine (IL-17A) mRNA, although the BSA-treated skin did not up-regulate these cytokines (). These results indicate that differentiation of allergen-specific TH2 was commonly induced in papain-, HDM-, and OVA-sensitized mice. In contrast, allergen-specific TH17 was induced only in papain- and HDM-sensitized mice.
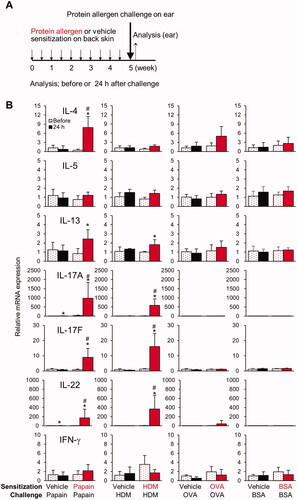
Epicutaneous challenge with papain and HDM, but not with OVA and BSA, on ear skin stimulates TH2 and TH17/TH22 cytokine gene expression in pre-sensitized mice
After sensitization of the back skin, the mice were subsequently challenged with protein allergens on the surface of the ear (). Epicutaneous challenge with papain and HDM, but not with OVA or BSA, in pre-sensitized mice resulted in the increased expression of TH2 (IL-4 or -13) and TH17/TH22 cytokine (IL-17A, -17 F, and -22) mRNA (). None of these responses was detected in vehicle-treated mice, indicating that epicutaneous papain or HDM pre-sensitization through the back skin was indispensable for the responses. Moreover, adaptive skin immune responses induced by pre-sensitization (such as increases in allergen-specific TH cells and antibodies) may contribute to the responses during the elicitation phase.
Figure 2. Epicutaneous challenge with papain and HDM, but not with OVA and BSA, on the ear skin stimulates TH2 and TH17/TH22 cytokine gene expression dependent upon epicutaneous pre-sensitization. (A) Timeline. (B) Gene expression in ear skin before and 24 h after challenge. Data shown are means ± SD of 4 mice/group and represent two independent experiments with similar results. *p < 0.05 vs. before challenge, #p < 0.05 vs. vehicle (Mann-Whitney U test).
Epicutaneous papain sensitization accelerates serum antibody production and differentiation of TH17 cells dependent on protease activity
Mice were epicutaneously sensitized with E64-papain using the same protocol as in . E64-papain induced the production of E64-papain-specific IgE and IgG1, whereas the total serum IgE and E64-papain-specific IgG1 were significantly smaller compared with serum from papain-sensitized mice (). Serum from papain-sensitized mice at a dilution of 1:10 and 1:40 both showed a positive PCA reaction rate of 100%, whereas serum from E64-papain-sensitized mice exhibited positive PCA reaction rates of 80% or 20%, respectively (). These results indicate that sensitization with E64-papain induced a weaker antibody response compared with papain.
Figure 3. Epicutaneous papain sensitization accelerates serum antibody production and differentiation of TH17 cells dependent on protease activity. (A) Serum antibodies. (B) Positive PCA reaction rates. (C) Cytokine production in DLN cells re-stimulated with E64-papain. (D) Gene expression in back skin. Data shown are means ± SD of 5 mice/group representing two independent experiments with similar results. *p < 0.05 by Mann-Whitney U test. N.D.: not detected; N.T.: not tested.
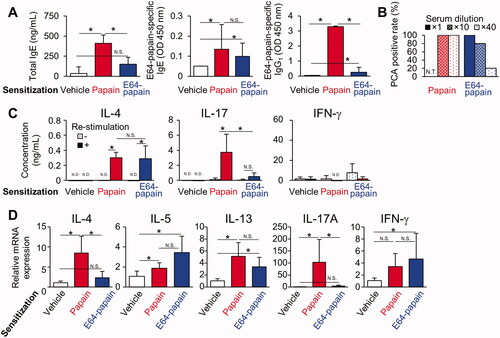
The production of IL-4 in antigen-re-stimulated skin DLN cells was observed in E64-papain-sensitized mice as well as papain-sensitized mice, whereas that of IL-17 was not observed in E64-papain-sensitized mice and was significantly smaller compared with papain-sensitized mice (). The E64-papain-treated skin exhibited higher expression of IL-5 and IL-13 mRNA compared with vehicle-treated skin (). However, up-regulation of IL-17A was not observed and IL-17 levels were significantly lower compared with papain-treated skin (). These results indicated that differentiation of allergen-specific TH2 was induced in E64-papain-sensitized mice, whereas induction of allergen-specific TH17 was down-regulated compared with papain-sensitized mice.
Discussion
It was previously demonstrated that epicutaneous papain sensitization induced serum papain-specific IgE/IgG1 as well as TH2 and TH17 differentiation and epicutaneous papain challenge induced local TH2 and TH17/TH22 recall responses that were dependent upon papain protease activity (Ogasawara et al. Citation2021). Using epicutaneous sensitization and challenge with other protein allergens in mice, the universality of the serum IgE/IgG1 responses, differentiation of TH cells, and epicutaneous TH recall responses were examined. The novelties of this study are those universal phenomena among protein allergens and the importance of protease activity both in the sensitization and elicitation phases.
During the sensitization phase, allergen-specific antibody production and TH2 differentiation were commonly observed in papain-, HDM-, and OVA-, but not BSA-, sensitized mice. Although only the undiluted serum from one BSA-sensitized mouse showed a positive result in the PCA test, papain-, HDM-, and OVA-sensitized mice induced a greater response compared with BSA. Therefore, it is suggested that the antibody and TH2 immune responses observed in the previous study using papain were commonly induced by other allergenic proteins, such as HDM and OVA. These agents may have greater potential to cause immediate-type hypersensitivity reactions compared with low allergenic proteins, such as BSA.
Here in the elicitation phase, on the other hand, TH2 and TH17/TH22 recall responses at the challenge sites were observed in papain- and HDM-, but not in OVA-, sensitized mice. Der f 1 and Der p 1, the major protein allergens in HDM, belong to the cysteine protease family along with papain (Chua et al. Citation1988; Thomas Citation2015). It was demonstrated previously that the TH recall response depends on the protease activity of papain and the response may be suppressed by inhibition of its protease activity (Ogasawara et al. Citation2021). Thus, it was considered that one of the reasons why the epicutaneous OVA challenge did not induce a TH recall response might be the lack of protease activity, and that allergenic proteins with protease activity could induce such TH2 and TH17/TH22 recall responses.
As protease activity dependency during the elicitation phase was addressed in a previous study (Ogasawara et al. Citation2021), the contribution of protease activity to the epicutaneous sensitization phase in a murine model was explored here. The results showed that antibody production and PCA reactions were suppressed in E64-papain-sensitized mice compared with in papain-sensitized mice, suggesting the protease activity of papain contributes not only to epicutaneous challenge, but also to sensitization. Moreover, other studies using papain models with epicutaneous sensitization on intact or tape-stripped ear skin indicated a dependency on protease activity (Iida et al. Citation2014; Shimura et al. Citation2016). Papain protease activity causes the breakdown of tight junction proteins, which are essential for the epithelial barrier (Stremnitzer et al. Citation2015). Studies in our laboratory previously reported that papain protease activity enhanced transdermal permeability in a reconstructed human epidermal model (Ogasawara et al. Citation2021). Thus, one can consider that allergen protease activity increases the likelihood of an encounter between allergen and dermal dendritic cells, thereby contributing to epicutaneous sensitization. However, antibody production and PCA reactions were observed even in E64-papain-sensitized mice, although they were weaker compared with those in papain-sensitized mice. This suggested to us that epicutaneous sensitization may be achieved without protease activity. Allergen-specific antibody production and PCA reactions were also observed in OVA-sensitized mice. Other studies have demonstrated that OVA-specific antibody production was induced through shaved skin with an occlusive patch dressing (He et al. Citation2007; Wang et al. Citation2007). Therefore, it can be concluded that the protease activity of protein allergens is not essential, but can enhance the process of epicutaneous sensitization.
In addition, in the present study, production of IL-17 in response to allergen re-stimulation was observed in the DLN cells from papain- and HDM-sensitized mice, suggesting both induced TH17 differentiation. However, no significant increase was observed in OVA- and E64-papain-sensitized mice. Moreover, E64-papain-sensitized mice showed significantly lower production compared with papain-sensitized mice under the same conditions. These results suggest that OVA and E64-papain exhibit lower potential to induce TH17 differentiation compared with papain and HDM. In a previous study (Kunimine et al. Citation2021), the induction of TH17 was compared between papain and E64-papain using a murine model with epicutaneous sensitization on ear skin and was suppressed in E64-papain-sensitized mice compared with papain-sensitized mice. Protein allergens, such as HDM, contribute to the induction of danger-associated molecular patterns (DAMPs), such as ATP and uric acid, in a protease activity-dependent manner (Ramu et al. Citation2018). DAMPs induce IL-6 and IL-23, cytokines that contribute to TH17 differentiation through the TLR pathway (Dennehy et al. Citation2009; McFadden et al. Citation2011; Glowczyk et al. Citation2017). Thus, the protease activity of protein allergens may contribute to TH17 differentiation by acting as an adjuvant during epicutaneous sensitization. Some studies have demonstrated TH17 differentiation by epicutaneous OVA sensitization with shaving and a 1 week occlusive patch (He et al. Citation2007), suggesting that TH17 differentiation may be induced even with non-protease allergens through epicutaneous exposure through barrier-disturbed skin or for more extended time periods. Cytokines, such as IL-17A, produced by TH17 decrease the synthesis of tight junction proteins (Yuki et al. Citation2016). Taken together, the results allow for a conclusion that the protease activity of protein allergens can facilitate a vicious circle between TH17 immune responses and skin barrier dysfunction, contributing to the aggravation of allergic skin diseases.
In conclusion, the present study demonstrated that epicutaneous sensitization with papain, HDM, and OVA, but not BSA, commonly induces allergen-specific antibody production, PCA responses, and TH2 differentiation in a murine model. Papain and HDM, which exhibit protease activity, differentiate TH17 and TH2 as well as elicit TH2 and TH17/TH22 recall skin responses. This study provides important insights into the role of protein allergens and their protease activities in eliciting skin responses during protein contact dermatitis, immunologic contact urticaria, and atopic dermatitis. Furthermore, although formally validated or widely recognized methods to evaluate skin allergic hazard of proteins have not been developed (Basketter and Kimber Citation2018), universal phenomena among allergenic proteins observed in the current study (e.g. allergen-specific antibody production, PCA response, TH2 differentiation) would contribute to establishing such a method.
Author contribution
AO, TY, AK, YL, and YT performed the experiments. AO and TY designed the experiments and analyzed the data. AO and TY wrote the manuscript. DB and HS provided scientific suggestions and review.
Acknowledgements
The authors thank Dr. Frank Gerberick (GF3 Consultancy LLC) for expertise and advice. The authors would also like to thank all the members of their laboratory for their informative help and advice.
Declaration of interest
Author DB received a consultation fee for this project from Kao Corporation. All other authors declare no conflicts of interest.
Data availability statement
The data that support the findings of this study are available from AO upon reasonable request.
References
- Amaro C, Goossens A. 2008. Immunological occupational contact urticaria and contact dermatitis from proteins: A review. Contact Dermatitis. 58(2):67–75.
- Barbaud A. 2020. Mechanism and diagnosis of protein contact dermatitis. Curr Opin Allergy Clin Immunol. 20(2):117–121.
- Basketter D, Kimber I. 2018. Are skin sensitisation test methods relevant for proteins? Regul Toxicol Pharmacol. 99:244–248.
- Basketter D, Kimber I. 2022. Enzymes and sensitization via skin exposure: A critical analysis. Regul Toxicol Pharmacol. 129:105112.
- Basketter D, Lahti A. 2011. Immediate contact reactions. In: Johansen J, Frosch P, and Lepoittevin J, editors. Contact dermatitis. Berlin, Heidelberg: Springer Berlin Heidelberg; p. 137–153.
- Chua KY, Stewart GA, Thomas WR, Simpson RJ, Dilworth RJ, Plozza TM, Turner KJ. 1988. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 167(1):175–182.
- Coenraads P. 2016. Sensitization potential of hydrolysed wheat proteins. Contact Dermatitis. 74(6):321–322.
- Dearman R, Caddick H, Basketter D, Kimber I. 2000. Divergent antibody isotype responses induced in mice by systemic exposure to proteins: A comparison of ovalbumin with bovine serum albumin. Food Chem Toxicol. 38(4):351–360.
- Deckers J, Sichien D, Plantinga M, van Moorleghem J, Vanheerswynghels M, Hoste E, Malissen B, Dombrowicz D, Guilliams M, de Bosscher K, et al. 2017. Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells. J Allergy Clin Immunol. 140(5):1364–1377.
- Dennehy K, Willment J, Williams D, Brown G. 2009. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol. 39(5):1379–1386.
- Glowczyk I, Wong A, Potempa B, Babyak O, Lech M, Lamont R, Potempa J, Koziel J. 2017. Inactive gingipains from P. gingivalis selectively skews T-cells toward a TH17 phenotype in an IL-6-dependent manner. Front Cell Infect Microbiol. 7:140.
- He R, Oyoshi M, Jin H, Geha R. 2007. Epicutaneous antigen exposure induces a TH17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA. 104(40):15817–15822.
- Hilton J, Dearman R, Basketter D, Kimber I. 1994. Serological responses induced in mice by immunogenic proteins and by protein respiratory allergens. Toxicol Lett. 73(1):43–53.
- Hilton J, Dearman R, Sattar N, Basketter D, Kimber I. 1997. Characteristics of antibody responses induced in mice by protein allergens. Food Chem Toxicol. 35(12):1209–1218.
- Iida H, Takai T, Hirasawa Y, Kamijo S, Shimura S, Ochi H, Nishioka I, Maruyama N, Ogawa H, Okumura K, et al. 2014. Epicutaneous administration of papain induces IgE and IgG responses in a cysteine protease activity-dependent manner. Allergol Int. 63(2):219–226.
- Kamijo S, Hara M, Suzuki M, Nakae S, Ogawa H, Okumura K, Takai T. 2021. Innate IL-17A enhances IL-33-independent skin eosinophilia and IgE response on subcutaneous papain sensitization. J Invest Dermatol. 141(1):105–113.
- Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, Matsuda H, Matsuda A, Oboki K, Ohno T, et al. 2013. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 190(9):4489–4499.
- Kikuchi Y, Takai T, Kuhara T, Ota M, Kato T, Hatanaka H, Ichikawa S, Tokura T, Akiba H, Mitsuishi K, et al. 2006. Crucial commitment of proteolytic activity of a purified recombinant major house dust mite allergen Der p1 to sensitization toward IgE and IgG responses. J Immunol. 177(3):1609–1617.
- Kimber I, Griffiths C, Basketter D, McFadden J, Dearman R. 2014. Epicutaneous exposure to proteins and skin immune function. Eur J Dermatol. 24(1):10–14.
- Kunimine S, Takai T, Kamijo S, Maruyama N, Kimitsu T, Masutani Y, Yoshimura T, Suchiva P, Shimizu S, Ogawa H, et al. 2021. Epicutaneous vaccination with protease inhibitor-treated papain prevents papain-induced TH2-mediated airway inflammation without inducing TH17 in mice. Biochem Biophys Res Commun. 546:192–199.
- McFadden J, Dearman R, White J, Basketter D, Kimber I. 2011. The Hapten-Atopy Hypothesis II: The 'cutaneous hapten paradox'. Clin Exp Allergy. 41(3):327–337.
- Nishioka I, Takai T, Maruyama N, Kamijo S, Suchiva P, Suzuki M, Kunimine S, Ochi H, Shimura S, Sudo K, et al. 2018. Airway inflammation after epicutaneous sensitization of mice requires protease activity of low-dose allergen inhalation. J Allergy Clin Immunol. 141(6):2271–2273.
- Ochi H, Takai T, Shimura S, Maruyama N, Nishioka I, Kamijo S, Iida H, Nakae S, Ogawa H, Okumura K, et al. 2017. Skin treatment with detergent promotes protease allergen-dependent epicutaneous sensitization in a manner different from tape stripping in mice. J Invest Dermatol. 137(7):1578–1582.
- Ogasawara A, Yuki T, Takai T, Yokozeki K, Katagiri A, Takahashi T, Yokozeki H, Basketter D, Sakaguchi H. 2021. Epicutaneous challenge with protease allergen requires its protease activity to recall TH2 and TH17/TH22 responses in mice pre-sensitized via distant skin. J Immunotoxicol. 18(1):118–126.
- Paller A, Spergel J, Mina-Osorio P, Irvine A. 2019. The atopic march and atopic multi-morbidity: Many trajectories, many pathways. J Allergy Clin Immunol. 143(1):46–55.
- Ramirez D, Bahna S. 2009. Food hypersensitivity by inhalation. Clin Mol Allergy. 7:4.
- Ramu S, Menzel M, Bjermer L, Andersson C, Akbarshahi H, Uller L. 2018. Allergens produce serine proteases-dependent distinct release of metabolite DAMPs in human bronchial epithelial cells. Clin Exp Allergy. 48(2):156–166.
- Shimura S, Takai T, Iida H, Maruyama N, Ochi H, Kamijo S, Nishioka I, Hara M, Matsuda A, Saito H, et al. 2016. Epicutaneous allergic sensitization by cooperation between allergen protease activity and mechanical skin barrier damage in mice. J Invest Dermatol. 136(7):1408–1417.
- Stremnitzer C, Manzano-Szalai K, Starkl P, Willensdorfer A, Schrom S, Singer J, Reichart U, Akira S, Jensen-Jarolim E. 2014. Epicutaneously applied Der p 2 induces a strong TH2 2-biased antibody response in C57BL/6 mice, independent of functional TLR4. Allergy. 69(6):741–751.
- Stremnitzer C, Manzano-Szalai K, Willensdorfer A, Starkl P, Pieper M, Konig P, Mildner M, Tschachler E, Reichart U, Jensen-Jarolim E. 2015. Papain degrades tight junction proteins of human keratinocytes in vitro and sensitizes C57BL/6 mice via the skin independent of its enzymatic activity or TLR4 activation. J Invest Dermatol. 135(7):1790–1800.
- Strid J, Hourihane J, Kimber I, Callard R, Strobel S. 2005. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 35(6):757–766.
- Suzuki M, Hara M, Ichikawa S, Kamijo S, Nakazawa T, Hatanaka H, Akiyama K, Ogawa H, Okumura K, Takai T. 2016. Presensitization to Ascaris antigens promotes induction of mite-specific IgE upon mite antigen inhalation in mice. Allergol Int. 65(1):44–51.
- Takai T, Ikeda S. 2011. Barrier dysfunction caused by environmental proteases in the pathogenesis of allergic diseases. Allergol Int. 60(1):25–35.
- Thomas W. 2015. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 64(4):304–311.
- Wang G, Savinko T, Wolff H, Dieu-Nosjean M, Kemeny L, Homey B, Lauerma A, Alenius H. 2007. Repeated epicutaneous exposures to ovalbumin progressively induce atopic dermatitis-like skin lesions in mice. Clin Exp Allergy. 37(1):151–161.
- Wang L, Chiu H, Hsu C, Liu C, Hsueh Y, Miaw S. 2009. Epicutaneous sensitization with a protein antigen induces TH17 cells. J Dermatol Sci. 54(3):192–197.
- Yokozeki K, Yuki T, Ogasawara A, Katagiri A, Takahashi Y, Basketter D, Sakaguchi H. 2021. Total dose defines the incidence of percutaneous IgE-/IgG1-mediated immediate-type hypersensitivity caused by papain. J Appl Toxicol. 41(6):898–906.
- Yuki T, Tobiishi M, Kusaka-Kikushima A, Ota Y, Tokura Y. 2016. Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17. PLoS One. 11(9):e0161759.
