Abstract
The potential for effector functions of therapeutic antibodies, including antibody-dependent cell-mediated cytotoxicity (ADCC), is a biological activity of interest for characterization, regardless of if ADCC is an intended primary pharmacological effect. The composition of the conserved antibody Fc glycan can vary as a function of post-translational processing which may affect the binding affinity to Fc receptors, leading to a change of effector activity. Ordesekimab (AMG 714 or PRV-015), a fully human immunoglobulin G1-kappa anti-interleukin (IL)-15 monoclonal antibody, is in clinical development for celiac disease. The binding of ordesekimab to IL-15 inhibits the interaction of IL-15 with the IL-2Rβ and common γ chain of the IL-15 receptor complex, but not with the IL-15Rα chain. Therefore, the simultaneous binding of ordesekimab to the Fcγ receptor (R) IIIα expressed on natural killer (NK) cells and to the IL-15/IL-15Rα complex on cells such as monocytes may theoretically enable ADCC toward the IL-15Rα-expressing cells. The high mannose (HM) levels on the Fc glycan were found to vary in different lots of ordesekimab resulting from refinements to the manufacturing process, and the impact on ordesekimab-mediated ADCC activity was evaluated in in vivo and in vitro studies. A review of nonclinical and clinical data found no evidence of ordesekimab-induced depletion of monocytes, or cytotoxicity in organs with wide IL-15Rα expression, suggesting a lack of in vivo ADCC activity. In addition, in vitro peripheral blood mononuclear cells-based ADCC assay did not reveal any cytolytic effect of ordesekimab with various levels of HM content when cocultured with recombinant human IL-15. Taken together, these data demonstrate that ADCC is not a potential liability for ordesekimab and does not contribute to the reduction of IL-15-mediated inflammation, the intended pharmacological effect.
Introduction
Ordesekimab, also known as AMG 714 or PRV-015, is a fully human immunoglobulin G1-kappa (IgG1κ) anti-interleukin (IL)-15 monoclonal antibody (mAb), that is in clinical development by Amgen Inc. and Provention Bio Inc. for gluten-free diet nonresponsive celiac disease (NRCD). Celiac disease is a chronic autoimmune condition characterized by inflammation and tissue damage in the gastrointestinal system stemming from an abnormal reaction to ingested gluten (Green and Cellier Citation2007; Penny et al. Citation2020); the underlying pathogenesis of disease involves up-regulation of IL-15, which leads to activation of intraepithelial lymphocytes and development of mucosal injury (Abadie and Jabri Citation2014). The binding of ordesekimab to IL-15 inhibits its interaction with the IL-2Rβ and common γ chain of the IL-15 receptor complex, but not the interaction of IL-15 with the IL-15Rα chain (). Ordesekimab is capable of binding to IL-15 both free in solution as well as when bound to IL-15Rα. IL-15Rα is widely expressed on immune cells, including antigen-presenting cells (APC) such as dendritic cells (DC) and monocytes, and patients with celiac disease have increased serum levels of IL-15 (Dubois et al. Citation2002; Stonier et al. Citation2008; Vorobjova et al. Citation2019).
Figure 1. Illustrations of the mechanism of action of ordesekimab and potential ADCC triggered by ordesekimab. (A) IL-15 is produced and presented on IL-15Rα by APC. Ordesekimab blocks binding of IL-15 to the IL-2Rβ and common γ chain of IL-15 receptor complex, but not to IL-15Rα. (B) Potential effector function (ADCC) mediated by ordesekimab in the presence of IL-15. Created with BioRender.com.
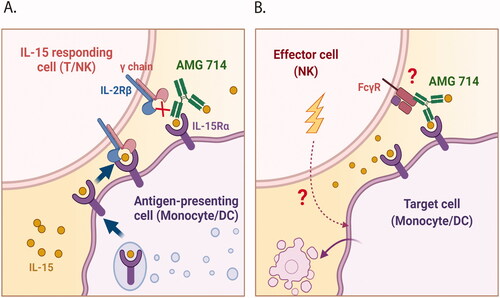
Antibody-dependent cell-mediated cytotoxicity (ADCC) is an Fc-mediated effector function of antibodies that requires the engagement of cytolytic effector cells and target cells, resulting in killing of target cells. The Fcγ receptors (FcγR), in particular FcγRIIIα, contribute to ADCC as mediated through natural killer (NK) effector cells (Wang et al. Citation2018; Gogesch et al. Citation2021). The Fc region of human IgG1 is able to interact with FcγR on NK cells; thus, ordesekimab could potentially engage NK cells and APC through FcγR-Fc-IL-15-IL-15Rα interactions (), potentially leading to ADCC-mediated death of APC. Elimination of IL-15Rα-expressing cells may help alleviate inflammatory conditions induced by over-expressed IL-15. However, the potential clinical benefits of ADCC far outweigh the risks and are of significant interest to therapeutic antibody development programs.
The Fc region of IgG1 antibodies carries an N-linked glycan at asparagine 297 (N297), which affects FcγR binding and ADCC activity of antibodies (Lee and Im Citation2017). Post-translational modification of glycans attached to the N297 residue can affect the Fc binding affinity to FcR, possibly influencing the effector function. During process development, different glycan profiles were associated with refinements in the manufacturing processes of ordesekimab, with a range of high mannose (HM) content being associated with different manufacturing lots. Although high levels of N-linked mannose glycan on antibodies have previously been linked to enhanced ADCC (Yu et al. Citation2012), direct influence has been difficult to demonstrate because HM is often linked with afucosylation, which strongly impacts ADCC (Shinkawa et al. Citation2003).
While ADCC was not an intended primary pharmacological effect of ordesekimab, the potential for effector function is a biological activity of interest of the molecule. Thus, to investigate the potential for ordesekimab-mediated ADCC activity, a retrospective evaluation of circulating monocytes, which represent a potential target cell expressing cell surface IL-15Rα, in nonclinical cynomolgus macaque toxicology studies and in human clinical trials, was undertaken. In addition, the cytotoxicity of monocytes in human peripheral blood mononuclear cells (PBMC) when co-cultured with ordesekimab in an in vitro ADCC assay in the presence of exogenous recombinant human (rh)IL-15 was investigated. Taken together, the in vivo and in vitro investigations here evaluated the potential effector function and the significance of critical product quality attributes of ordesekimab mAb expressing glycan variants and provided confirmation for the lack of ordesekimab-mediated ADCC.
Materials and methods
Antibodies and reagents
All mAb were obtained from BD Biosciences (San Diego, CA, USA), unless otherwise stated. The following antibodies were used: CD3 (clone SP34-2), CD4 (clone L200), CD45 (clone HI30), CD11c (clone 3.9), CD14 (clone M5E2), CD16 (clone 3G8), CD19 (clone HIB19), CD20 (clone 2H7), CD56 (clone NCAM16.2), CD8 (clone RPA-T8), HLA-DR (clone G46-6), CD69 (clone FN50), IL-15Rα (clone JM7A4, BioLegend, San Diego, CA, USA), mouse IgG2b κ isotype control (clone MPC-11, BioLegend), and Live/Dead Fixable Near-IR (Thermo Fisher Scientific, Waltham, MA, USA). Fetal bovine serum (FBS) and ≤0.09% sodium azide in phosphate-buffered saline (PBS; BD Biosciences) was used as the staining buffer.
The medium used for the ADCC assay was RPMI-1640 containing GlutaMAX (1X) medium, supplemented with 10% FBS, 100 U penicillin/ml, and 100 µg streptomycin/ml (all from Gibco, Waltham, MA, USA), was used as the medium for the ADCC assay. Anti-CD20 antibody ABP 798 reference standard (ABP 798 RS, Amgen Lot 0010177775) was used as a positive control for targeting B-cells in the ADCC assay (Golay et al. Citation2003).
Ordesekimab glycan variants
Test article samples were provided by Amgen Process Development (Thousand Oaks, CA) and ordesekimab first-in-human (FIH) RS (Amgen Lot A0410270004-A) was used as a reference for the improved manufacturing processes of the ordesekimab lots. Total HM levels were calculated by adding Man5, Man6, Man7, and Man8, as measured by hydrophilic interaction liquid chromatography. The percent of HM for ordesekimab FIH RS and lots #1, #2, #3, and #4 were 4.7, 5.0, 7.0, 8.1, and 10.6%, respectively.
Isolation of PBMC
Human peripheral blood was drawn directly into sodium heparin vacutainer tubes (BD Biosciences) from an internal Amgen human blood donor program on the day of PBMC isolation. Peripheral blood from Mauritius-origin adult cynomolgus macaque (Macaca fascicularis) was collected in sodium heparin vacutainer tubes from Alpha Genesis (Yemassee, SC, USA) and delivered to Amgen overnight at ambient temperature, followed by PBMC isolation the next day. Peripheral blood from Chinese-origin cynomolgus macaque was collected in sodium heparin vacutainer tubes and shipped from Valley Biosystems (West Sacramento, CA, USA) on the same day of PBMC isolation. All in vivo work/blood sampling was conducted under an Institutional Animal Care and Use Committee (IACUC)-approved protocol in an American Association for Accreditation of Laboratory Animal Care-accredited facility.
Different isolation techniques of PBMC were applied to compensate for the compartmental differences between blood samples from different species. In brief, PBMC were isolated by density gradient centrifugation without acceleration or brake. Blood samples were diluted with an equal volume of RPMI-1640 media and layered onto either 100% Ficoll (GE Healthcare, Chicago, IL, USA) and centrifuged at 400 × g for 20 min for human, or onto 92% Ficoll diluted in Dulbecco’s phosphate-buffered saline and centrifuged at 1830 × g for 25 min for Mauritius-origin cynomolgus macaques, or onto Lympholyte-Mammal (Cedarlane, Burlington, NC, USA) gradient and centrifuged at 800 × g for 20 min for Chinese-origin cynomolgus macaques. Each resulting mononuclear cell fraction was washed with corresponding media, followed by flow cytometry analysis or use in the in vitro ADCC assay.
Analysis of IL-15Rα expression by flow cytometry
For IL-15Rα expression in the human and macaque PBMC samples, an antibody master mix containing Brilliant Stain Buffer (1:20 dilution; BD Biosciences) and fluorophore-conjugated antibodies against cell markers, with 5 µl of either anti-human IL-15Rα or mouse IgG2b κ isotype control, was then added to each sample, mixed, and incubated at 4°C for 30 min. After a thorough washing step, stained cells were resuspended in stain buffer and promptly acquired with a FACSymphony™ A3 Flow Cytometer (BD Biosciences). Data were acquired using DiVa acquisition software and final analysis was done with FlowJo™ v10.5 software (both BD Life Sciences). A minimum of 250,000 events/sample was analyzed each time.
Samples were analyzed for the following cell types (after exclusion of doublets and dead cells) and subsets were identified with the following clusters of differentiation (CD) or markers: monocytes (CD14+HLA-DR+), DC (CD11c+HLA-DR+), NK cells (CD56+ for human and CD16+ for cynomolgus macaque), B-cells (CD20+), CD4+ (CD3+CD4+), and CD8+ T-cells (CD3+CD8+) (Supplementary Figure S1). Cells expressing IL-15Rα were determined relative to the mouse IgG2b κ isotype control. Graphical analysis was generated using Prism software (v.7.04, GraphPad, San Diego, CA, USA).
ADCC assay
A two-fold serial dilution of test articles or the positive control anti-CD20 IgG1 antibody was prepared in assay media. Human PBMC in 100 µl assay media (at 2 × 106 cells/ml) were pre-mixed with rhIL-15 and added to a 96-well U-bottom plate. Then, 100 µl of diluted test and control articles were transferred to corresponding wells (final top concentrations of 150 µg/ml for ordesekimab and 1.5 µg/ml for the CD20 antibody) with 500 pg/ml of rhIL-15 per well. The plate was incubated for 48 h at 37°C in a CO2 incubator, and then the cells were stained with fluorophore-conjugated antibodies. The antibody staining panels for human cells were CD3-BUV395, CD45-BV421, HLA-DR-BV605, CD16-BV711, CD14-AF488, CD19-PE, and CD56-PE-Cy7. A master mix containing Brilliant Stain Buffer (1:20 dilution) and panel antibodies was then added to each sample and the plate was left for 30 min protected from light. After thorough washing, cells were resuspended in 100 µl of stain buffer with 50 nM of TO-PRO3 (Invitrogen, Waltham, MA). This was followed by the addition of 50 µl CountBright™ Absolute Counting Beads solution (Life Technologies, Carlsbad, CA) to each sample and analysis of the samples in the FACSymphony flow cytometer. Absolute numbers of live immune cells were calculated using CountBright™ Absolute Counting Beads, according to manufacturer instructions. The viable CD45+ populations were defined as monocytes (CD14+, CD16+, HLA-DR+), NK cells (CD56+), total T-cells (CD3+), and B-cells (CD19+).
The percentage of specific cytotoxicity was calculated using the equation: [1 – (live target cells with test articles/live target cells with media only)] × 100. The effector to target cell (E:T) ratio was calculated based on the populations measured with flow cytometry. The E:T ratio for individual donors was calculated by the average of NK cell counts to the average of B-cell counts (positive control) or the average of monocyte cell counts (ordesekimab). Graphs were generated using the Prism software.
To measure CD69 expression on NK cells, an aliquot of human PBMC from Donors 2 and 3 in the ADCC assay was stained with a master mix containing Brilliant Stain Buffer and cell marker antibodies including CD3-BUV395, CD45-BV421, CD16-BV650, CD56-APC, CD69-PE, and Live/Dead Fixable Near-IR, and were acquired in the FACSymphony™ A3 flow cytometer.
Hu714MuXHu cynomolgus macaque toxicology studies
Repeat-dose toxicology studies were conducted to support the conduct of human clinical trials in compliance with the most recent version of the Food and Drug Administration Good Laboratory Practice (GLP) regulations, 21 CFR 58; the Japanese Ministry of Health, Labor, and Welfare, GLP Standards Ordinance 21; the Organization for Economic Cooperation and Development principles of GLP, C(97)186/FINAL; and any other applicable amendments. Additionally, studies in cynomolgus monkeys (M. fascicularis) described below were performed in compliance with the IACUC guidelines, and complied with the Final Rules of the Animal Welfare Act regulations (Code of Federal Regulations, Title 9), the Public Health Service Policy on Humane Care and Use of Laboratory Animals from the Office of Laboratory Animal Welfare (2002), and the Guide for the Care and Use of Laboratory Animals from the National Research Council (1996). Because of the low binding affinity of ordesekimab for macaque IL-15, and the very low inhibitory activity of ordesekimab against macaque IL-15, a human and murine chimeric anti-IL-15 antagonist (Hu714MuXHu) with comparable neutralization of human and cynomolgus IL-15 and the same human IgG1 Fc region as ordesekimab was used as an ordesekimab surrogate to enable the conduct of non-clinical toxicology studies in the cynomolgus macaque.
All studies described below included evaluation of the following study parameters: bioanalysis and toxicokinetic evaluation, mortality check, clinical observations (detailed, cage side, and postdose), body weight, qualitative food evaluation, electrocardiographic, and ophthalmologic examinations, respiration rate, clinical pathology (hematology, coagulation, clinical chemistry, and urinalysis), flow cytometry, cytotoxic T-lymphocyte activity (only evaluated in a 6-months study), organ weights, macroscopic observations, and histopathology. Blood (≈1 ml) was collected by venipuncture into anticoagulant tubes; hematologic parameters were determined on a Advia® 2120 hematology analyzer (Siemens Medical Solutions USA, Malvern, PA, USA) in the testing facility.
A 1-month repeat-dose toxicology study with subcutaneous (SC) or intravenous (IV) injection in the cynomolgus macaque was conducted at Covance Laboratories Inc. (Madison, WI). Male and female cynomolgus macaques of Chinese origin were assigned to five groups of five animals per sex. Each group received an IV injection of the control article, or 30, 60, or 150 mg Hu714MuXHu/kg, or an SC injection of 150 mg Hu714MuXHu/kg once weekly for 4 weeks (five total doses on Days 1, 8, 15, 22, and 29). Dose levels for this study were selected based on results of a pharmacokinetic study in the cynomolgus macaque (data not shown). All animals survived to the scheduled necropsy on Day 30 of the dosing phase, at which point three animals/sex/group were euthanized and necropsied. The reversibility, persistence, or delayed occurrence of any effect of Hu714MuXHu was assessed after a 45-weeks post-treatment period in the remaining two animals/sex/group.
A 3-months repeat-dose toxicology study in the cynomolgus macaque, with a 4-months recovery phase, was conducted at Covance Laboratories Inc. Male and female cynomolgus macaques of Chinese origin were assigned to four groups of six animals/sex. Each group received an SC injection of the control article, or 5, 30, or 150 mg Hu714MuXHu/kg once weekly for 3 months (14 total doses). Dose levels were based on the results of the 1-months toxicology study. Following the dosing phase, four animals/sex/group were euthanized and necropsied. The remaining two animals/sex/group underwent 4 months of recovery period.
A 6-months repeat-dose toxicology study through SC injection in the cynomolgus macaque was conducted at Charles River Laboratories Inc. (Reno, NV). Male and female cynomolgusmacaques of Mauritius origin were assigned to three groups of four animals/sex. Each group received doses of the control article, 30, or 150 mg Hu714MuXHu/kg once weekly for 6 months. Dose levels were based on the results of the 3-months toxicology study.
The mean absolute monocyte counts were derived from the blood samples analyzed on the Advia® analyzer at the testing facility. The values from each group were shown at the indicated time points from pretreatment, dosing, or recovery period in each study.
Ordesekimab clinical studies
Ordesekimab has been studied in an FIH study to investigate safety, tolerability, pharmacokinetics, and pharmacodynamics (PD) in healthy subjects (Phase 1 study 20050193) and in proof-of-concept studies in patients with rheumatoid arthritis (Phase 1 study Hx-IL 15-001, Phase 2 study 20030210) or psoriasis (Phase 1 b/2a study 20060349). Two Phase 2a clinical trials were conducted by Celimmune in NRCD patients (CELIM-NRCD-001, NCT02637141) and Type II refractory celiac disease (RCD-II) patients (CELIM-RCD-002, NCT02633020). Study CELIM-NRCD-001 assessed the efficacy of ordesekimab in attenuating the effects of gluten exposure in adults with celiac disease, as well as the safety and tolerability of ordesekimab when administered to adult patients with celiac disease upon a gluten challenge.
A total of 62 adult patients with NRCD received either one of two dose levels of ordesekimab (150 mg, n = 22; 300 mg, n = 21) or placebo (n = 19), via SC injection once every 2 weeks for a total of six doses over 10 weeks (± 3 days). End-of-study efficacy assessments were performed at study Week 12 and a final study visit was conducted 6 weeks after the last administration of study drug at study Week 16. Study CELIM-RCD-002 evaluated the efficacy and safety of ordesekimab when administered to adult patients with RCD-II (n = 28), received either 8 mg ordeseki-mab/kg (n = 19) or placebo (n = 9) via a 120-min IV infusion for a total of seven doses over 10 weeks. The last study visit was conducted at Week 16. Clinical hematology parameters were assessed, and the mean counts of monocytes (109 cells/L) were analyzed for each visit. All clinical studies were conducted in accordance with the ethical standards set by the respective institutional review boards and the principles stated in the Declaration of Helsinki. Relevant written informed consent was also obtained from all study participants.
Results
IL-15Rα is expressed on subset of monocytes in human and cynomolgus macaque peripheral blood
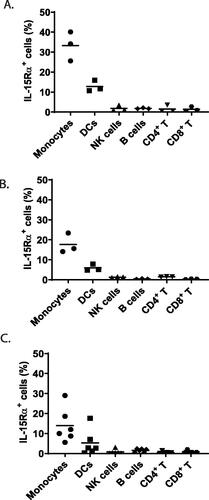
To assess the effector function of ordesekimab, the focus was placed on studying ADCC against a target cell expressing cell surface IL-15Rα with either in vivo or in vitro treatment of ordesekimab or Hu714MuXHu. IL-15Rα expression was assessed by flow cytometry on monocytes, DC, NK cells, B-cells, CD4+, and CD8+ T-cells in PBMC from human and cynomolgus macaque of both Mauritius and Chinese origin. Relative to isotype control, a subset of monocytes and DC in human and cynomolgus macaque expressed IL-15Rα, with monocytes expressing IL-15Rα at a higher frequency than DC in both species, while much lower levels of IL-15Rα were found on other cell populations in both cynomolgus macaque () and human () PBMC. Average frequencies of IL-15Rα+ cells among monocytes from tested donors were 14% for human, and 33.3 and 17.7% for Mauritius and Chinese cynomolgus macaques, respectively. Lower frequencies of IL-15Rα+ DC were observed with averages of 5.3, 12.8, and 5.9% for human, Mauritius-, and Chinese-origin cynomolgus macaques, respectively. In contrast, minimal relative numbers of IL-15Rα+ cells were observed within NK cells, B-cells, CD4+, and CD8+ T-cells, ranging between 0.05 and 3.55% for all human donors and macaques. This is consistent with previously published data that IL-15Rα was primarily expressed on APC, including DCs and monocytes (Dubois et al. Citation2002; Stonier et al. Citation2008). Since monocytes represent a large cell population in PBMC, they were selected as target cells in the subsequent in vivo and in vitro investigations.
Figure 2. IL-15Rα expression in peripheral blood immune cells in the cynomolgus macaque and human. Percent of IL-15Rα-expressing cells in each cell subset was measured by flow cytometry in PBMC from (A) Mauritius-origin cynomolgus macaque (n = 3), (B) Chinese-origin cynomolgus macaque (n = 3), and (C) human (n = 6). IL-15Rα+ cells were determined to be those with IL-15Rα staining greater than mouse IgG2b κ isotype control. Horizontal bars represent average frequency of IL-15Rα+ cells and datapoints represent the individual donors.
Monocyte depletion was not observed in nonclinical toxicology studies using an ordesekimab surrogate
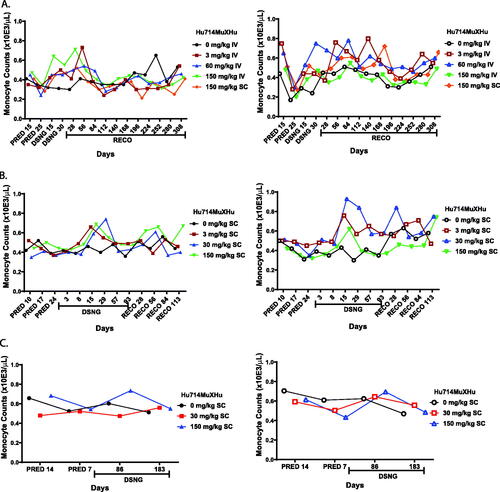
The 1-month and 3-months GLP studies were conducted in cynomolgus macaques of Chinese origin dosed with Hu714MuXHu containing 2.3% HM, while the 6-months toxicology study was conducted in cynomolgus macaques of Mauritius origin with administration of Hu714MuXHu containing 7.9% HM. Because the cynomolgus FcγRIIIα can efficiently bind human IgG1 with even a slightly higher affinity than human FcγRIIIα (Warncke et al. Citation2012; Crowley and Ackerman Citation2019), monocyte counts were analyzed from hematology data collected from individual animals in all three studies, including the recovery phases (). Despite the normal fluctuation in counts over time, no test article-related reduction in monocyte counts were observed in any studies. Moreover, no histopathological findings in organs with IL-15Rα-expressing cells were observed in any studies (data not shown). Thus, results of these toxicology studies demonstrated the lack of Hu714MuXHu-mediated effector function in vivo in cynomolgus macaques.
Figure 3. No reduction of monocyte counts in nonclinical cynomolgus macaque toxicology studies dosed with ordesekimab surrogate Hu714MuXHu. Mean absolute monocyte counts were derived from blood samples from control- or Hu714MuXHu-dosed groups at the indicated days of pretreatment (PRED), dosing (DSNG) and recovery (RECO) study phases. (A) One-month study with Chinese-origin cynomolgus macaque (n = 5/sex/group). (B) Three-months study with Chinese-origin cynomolgus macaque (n = 6/sex/group). (C) Six-months study with Mauritius-origin cynomolgus macaque (n = 4/sex/group). Data from male (left column, filled symbols) and female (right column, open symbols) animals were presented in separate graphs.
Monocyte depletion was not observed in clinical studies of ordesekimab
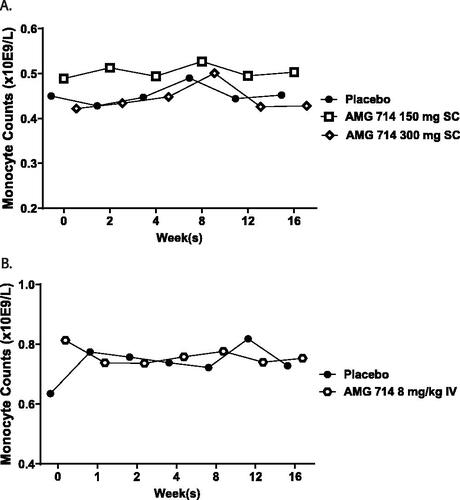
Hematology data from two Phase 2a clinical studies in patients with celiac disease (CELIM-NRCD-001, CELIM-RCD-002) were retrospectively analyzed. The HM level in the ordesekimab lot used in both clinical trials was 3.9%. The average monocyte count from blood tests during and after the dosing periods in both studies were plotted against the timepoints at which the blood was collected (). Compared with the placebo control group, the monocyte counts were similar throughout the 16-weeks collection period in all ordesekimab-treated groups in both studies. A spike appeared at a single timepoint around Week 8 in both placebo and ordesekimab-treated groups in study CELIM-NRCD-001 () was considered to be spurious and not related to ordesekimab. Therefore, no clinical evidence supported ordesekimab-mediated effector function in vivo in humans.
Figure 4. No reduction of peripheral blood monocyte counts in ordesekimab clinical studies. Mean monocyte counts from hematology data collected from placebo- or ordesekimab-administered groups were plotted against the collection timepoints from clinical trials of (A) CELIM-NRCD-001, a Phase 2a study in adult patients with NRCD; placebo (n = 19), 150 mg ordesekimab (n = 22), or 300 mg ordesekimab (n = 21) and (B) CELIM-RCD-002, a Phase 2a study in adult patients with Type II refractory celiac disease; placebo (n = 8) and 8 mg/kg ordesekimab (n = 16).
Ordesekimab is not associated with ADCC activity in vitro
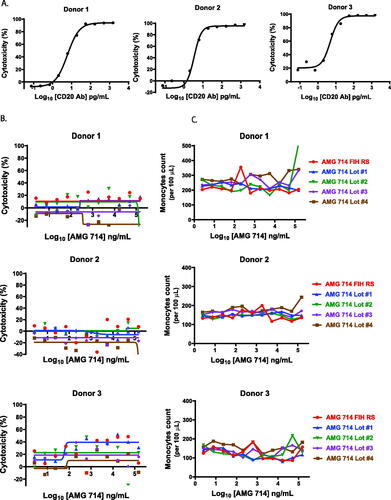
The lack of monocyte depletion in nonclinical and clinical studies indicated ordesekimab surrogate molecule with 2.3% and 7.9% HM, respectively, and ordesekimab with 2.3% HM were not associated with an ADCC activity in vivo. However, the HM level of ordesekimab lots used in clinical trials was lower than that of the lots produced later during manufacturing processes. This led to an expected increase in binding to FcγRIIIα in the new lots (data not shown). To determine if the higher HM content and increased FcγRIIIα binding could lead to ADCC, an in vitro ADCC assay was performed with human PBMC. To promote the formation of the binding complex shown in and to mimic the elevated IL-15 condition in patients, excessive rhIL-15 was added (see Materials and Methods). Primary B-cells and monocytes were used as targets for the positive control anti-CD20 antibody- and ordesekimab-treated samples, respectively. ADCC activity in the positive control was readily apparent against primary B-cells with a top concentration of 1.5 µg/ml (>94% specific target cell lysis for all donors; ). However, in the same assay, ordesekimab lots with varying HM levels ranging from 4.7 to 10.6% did not induce specific cytotoxicity () and did not cause loss of monocytes ().
Figure 5. Lack of ADCC in vitro mediated by ordesekimab expressing a range of HM. ADCC assays were performed with human PBMC in presence of rhIL-15 (500 pg/ml) and serial dilutions of antibodies. (A) Anti-CD20 induced cytotoxicity of primary B-cells (Donor 1: E:T = 1.9, Donor 2: E:T = 15.4, Donor 3: E:T = 1.9). (B) Cytotoxicity, and (C) absolute counts of primary monocytes when cultured with ordesekimab lots (Donor 1: E:T = 1.2, Donor 2: E:T = 4, Donor 3: E:T = 0.7).
Anti-IL-15 activity of ordesekimab was confirmed in the ADCC assay
To confirm the biological activity of ordesekimab in the presence of IL-15 in the ADCC assay, expression of the activation marker CD69 on NK cells was determined by flow cytometry when PBMC were cultured with rhIL-15 and various concentrations of ordesekimab lot #1 (5.0% HM) as in the ADCC assay. In agreement with previous findings, ordesekimab reduced CD69 expression on NK cells in a dose-dependent manner, and the NK cell count was unaffected, confirming the bioactivity of ordesekimab (Supplementary Figure S2; Lebrec et al. Citation2013).
Discussion
Ordesekimab, an anti-IL-15 antagonist antibody, blocks the interaction of IL-15 with the IL-2Rβ and common γ chain of the receptor complex, without interfering with the IL-15/IL-15Rα interaction. This binding of ordesekimab to IL-15 could potentially link immune effector cells (such as NK cells) and IL-15Rα-expressing APC (such as monocytes) together in the presence of endogenous IL-15 to activate ordesekimab-mediated ADCC. Additionally, since previous studies have shown that therapeutic antibodies with HM glycans augmented ADCC effects (Yu et al. Citation2012), different glycan profiles of ordesekimab with varying levels of HM, detected in various manufacturing lots, could in theory further enhance any resulting ADCC, if present.
In this study, a strategy of retrospective analysis of in vivo nonclinical and clinical data followed by in vitro assays was used to evaluate the possibility of ADCC being mediated by different ordesekimab manufacturing lots with varying levels of HM. Overall, target cell (monocyte) depletion was not evident in any of the in vivo or in vitro experiments, demonstrating the lack of an effector function of ordesekimab (or Hu714MuXHu) even with increased HM content. This could be because the simultaneous ligation of NK cells and target cells through ordesekimab () is a rare event, or is not strong enough to trigger ADCC, or the orientation of the antibody in this configuration is not conducive to ADCC. While HM glycans may enhance existing ADCC, it probably will not induce ADCC in an antibody where it is otherwise absent. However, it is important to note that the HM level of ordesekimab administered in previous clinical studies was as low as 3.9%, and clinical data of ordesekimab with greater HM content are not available.
The need to thoroughly investigate the potential for ordesekimab to mediate ADCC arose with the observation of variable HM levels in different production lots. Utilizing the available in vivo data from cynomolgus macaque toxicology studies, it was possible to assess if a mechanism of ADCC, as proposed in , could be observed in toxicology studies as well. Due to low cross-reactivity of ordesekimab in cynomolgus macaques, a surrogate antibody blocking human and cynomolgus IL-15, through the same mechanism as that observed with ordesekimab, was used in cynomolgus macaque studies. Although a slightly different frequency of IL-15Rα expression was detected on monocytes from humans and macaques of Mauritian and Chinese origin, based on the staining data from a limited number of donors, a high frequency of IL-15Rα expression on monocytes permitted the retrospective evaluation of ADCC effect from hematology data collected from in vivo studies. While a lack of monocyte depletion indicated the absence of any ordesekimab- or Hu714MuXHu-mediated ADCC activity, it cannot be completely ruled out because decreases in NK cell counts/activity were observed in cynomolgus studies. Additionally, detection of any ADCC activity could have been confounded by the effects of ordesekimab-mediated anti-IL-15 activity on NK cells. Indeed, decreased NK cell counts were observed throughout the dosing period at all dose levels, but the effect was thought to be a consequence of a PD response, given the known role of IL-15 in NK cell biology (Burkett et al. Citation2004; Zhang et al. Citation2018). However, since the reduction of NK cells by IL-15 antagonist antibodies is not translatable to humans (Lebrec et al. Citation2013), the observation of stable monocyte counts in ordesekimab clinical studies confirmed the absence of ordesekimab-mediated ADCC in vivo.
As illustrated in , putative ordesekimab-mediated ADCC would require the presence of soluble IL-15. While the serum of healthy donors is known to have an endogenous IL-15 level of ∼3 pg/ml, the levels increase to ∼10 pg/ml in patients with celiac disease (Vorobjova et al. Citation2019). This low level of endogenous IL-15 could therefore underestimate the effect when concluding a lack of effector function of ordesekimab from data collected in naïve human donors or animals. Yet, the lack of ADCC activity based on monocyte counts, as observed in previous clinical trials of adult patients with NRCD or RCD-II, provides strong evidence that ordesekimab is not associated with effector function even in the presence of elevated IL-15 levels.
To directly compare ADCC by ordesekimab with varying levels of HM content, an in vitro assay was performed with human PBMCs to assess whether ordesekimab manufacturing lots with various levels of HM have an impact on ADCC activity. Under conditions where the anti-IL-15 activity of ordesekimab was confirmed by the inhibition of NK cell activation in the presence of IL-15, ordesekimab lots with different levels of HM (4.7 − 10.6%) did not reveal any ADCC activity against monocytes. However, the inhibition of NK cell activation by ordesekimab may indicate that the lack of ADCC is due to impairment of NK cell activation as demonstrated by CD69 expression (Supplementary Figure S2). Nevertheless, the purpose of this study was to assess potential effector function represented by ADCC induced by ordesekimab with different glycan profiles. Overall, a strategy including in vivo and in vitro investigations was able to address any concerns related to ADCC, mediated by ordesekimab with varying glycan compositions.
Supplemental Material
Download PDF (835.4 KB)Acknowledgments
Medical writing support was provided by Kate Smigiel, PhD, of Amgen Inc., and Meenakshi Mukherjee, PhD, of Cactus Life Sciences on behalf of Amgen Inc. The authors would like to thank Dr. Rupa Padaki, Dr. Lubna Abuqayyas, Dr. Le Zhang, Dr. Meredith Jones, and Dr. Raymond Doss for helpful discussions.
Disclosure statement
All authors except H. L. are current employees and stockholders of Amgen Inc. H. L. is a former employee of Amgen Inc. No potential conflict of interest was reported by the author(s).
Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete details are found at: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/
Additional information
Funding
References
- Abadie V, Jabri B. 2014. IL-15: A central regulator of celiac disease immunopathology. Immunol Rev. 260(1):221–234.
- Burkett P, Koka R, Chien M, Chai S, Boone D, Ma A. 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T-cell homeostasis. J Exp Med. 200(7):825–834.
- Crowley A, Ackerman M. 2019. Mind the Gap: How interspecies variability in IgG and its receptors may complicate comparisons of human and non-human primate effector function. Front Immunol. 10:697.
- Dubois S, Mariner J, Waldmann T, Tagaya Y. 2002. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 17(5):537–547.
- Gogesch P, Dudek S, van Zandbergen G, Waibler Z, Anzaghe M. 2021. The role of Fc receptors on the effectiveness of therapeutic monoclonal antibodies. IJMS. 22(16):8947.
- Golay J, Manganini M, Facchinetti V, Gramigna R, Broady R, Borleri G, Rambaldi A, Introna M. 2003. Rituximab-mediated antibody-dependent cellular cytotoxicity against neoplastic B-cells is stimulated strongly by IL-2. Haematologica. 88(9):1002–1012.
- Green P, Cellier C. 2007. Celiac disease. N Engl J Med. 357(17):1731–1743.
- Lebrec H, Horner MJ, Gorski KS, Tsuji W, Xia D, Pan W-J, Means G, Pietz G, Li N, Retter M, et al. 2013. Homeostasis of human NK cells is not IL-15-dependent. J Immunol. 191(11):5551–5558.
- Lee H, Im W. 2017. Effects of N-Glycan composition on structure and dynamics of IgG1 Fc and their implications for antibody engineering. Sci Rep. 7(1):12659.
- Penny H, Baggus E, Rej A, Snowden J, Sanders D. 2020. Non-responsive celiac disease: A comprehensive review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients. 12(1):216.
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, et al. 2003. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 278(5):3466–3473.
- Stonier S, Ma L, Castillo E, Schluns K. 2008. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 trans-presentation. Blood. 112(12):4546–4554.
- Vorobjova T, Tagoma A, Oras A, Alnek K, Kisand K, Talja I, Uibo O, Uibo R. 2019. Celiac disease in children, particularly with accompanying type 1 diabetes, is characterized by substantial changes in the blood cytokine balance, which may reflect inflammatory processes in the small intestinal mucosa. J Immunol Res. 2019:6179243.
- Wang X, Mathieu M, Brezski R. 2018. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 9(1):63–73.
- Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. 2012. Different adaptations of IgG effector function in human and non-human primates and implications for therapeutic antibody treatment. J Immunol. 188(9):4405–4411.
- Yu M, Brown D, Reed C, Chung S, Lutman J, Stefanich E, Wong A, Stephan J, Bayer R. 2012. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs. 4(4):475–487.
- Zhang M, Wen B, Anton O, Yao Z, Dubois S, Ju W, Sato N, DiLillo D, Bamford R, Ravetch J, et al. 2018. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci USA. 115(46):E10915–E10924.
