Abstract
The recent global resurgence of severe infections caused by the Group A streptococcus (GAS) pathogen, Streptococcus pyogenes, has focused attention on this microbial pathogen, which produces an array of virulence factors, such as the pore-forming toxin, streptolysin O (SOT). Importantly, the interactions of SOT with human neutrophils (PMN), are not well understood. The current study was designed to investigate the effects of pretreatment of isolated human PMN with purified SOT on several pro-inflammatory activities, including generation of reactive oxygen species (ROS), degranulation (elastase release), influx of extracellular calcium (Ca2+) and release of extracellular DNA (NETosis), using chemiluminescence, spectrophotometric and fluorimetric procedures, respectively. Exposure of PMN to SOT alone caused modest production of ROS and elastase release, while pretreatment with the toxin caused significant augmentation of chemoattractant (fMLP)-activated ROS generation and release of elastase by activated PMN. These effects of treatment of PMN with SOT were associated with both a marked and sustained elevation of cytosolic Ca2+concentrations and significant increases in the concentrations of extracellular DNA, indicative of NETosis. The current study has identified a potential role for SOT in augmenting the Ca2+-dependent pro-inflammatory interactions of PMN, which, if operative in a clinical setting, may contribute to hyper-activation of PMN and GAS-mediated tissue injury.
Introduction
Streptococcus pyogenes (or Group A streptococcus [GAS]), is an important cause of pharyngitis and tonsillitis in humans (Walker et al. Citation2014), and may also cause severe invasive infections (Ochi et al. Citation2018). Prior to the antibiotic era, GAS was also a frequent cause of community-acquired pneumonia (CAP) (Akuzawa and Kurabayashi Citation2016; Tamayo et al. Citation2016), but the widespread use of antimicrobial therapy has resulted in fewer cases of CAP due to S. pyogenes. However, a recent global resurgence in the incidence of GAS infections in children has escalated attention to the adverse impact of this important pathogen (WHO Citation2022; Bamford and Whittaker Citation2023). In this context, infection with the influenza virus predisposes to secondary infections with GAS, which may result in necrotizing lung and soft tissue infections. In addition to necrotizing pneumonia, lung abscesses and empyema, GAS may also result in the toxic shock syndrome (Parks et al. Citation2015). Recent concerns related to the resurgence of invasive Group A streptococcal infections are strongly linked to the emergence of the globally distributed highly toxigenic emm1 strains of S. pyogenes such as the M1UK variant (Bamford and Whittaker Citation2023; Brouwer et al. Citation2023; Li et al. Citation2023).
Human neutrophils (PMN) play a vital role in eradicating bacterial pathogens and are important cells during the innate immune response to invading GAS (Limbago et al. Citation2000; Zhu et al. Citation2017). Activated PMN produce an array of mediators that are able to destroy microbial pathogens, including reactive oxygen species (ROS), proteolytic granules, and bioactive lipids. In addition, PMN following exposure to various microbial pathogens, including GAS (Yipp et al. Citation2012), are able to generate extracellular traps composed of chromatin and histones during the process of NETosis. These extracellular chromatin structures contribute to host defense against bacteria by trapping microbes and facilitating their dispersal and, in some cases, destruction.
Importantly, microbial pathogens may escape host defenses via multiple mechanisms, which include inhibition of PMN ROS production and the release of PMN-derived cationic peptides, as well as proteinases such as elastase, enabling evasion of the host immune system, promoting the spread of bacterial pathogens such as GAS. In this context, GAS produces an array of virulence factors, most prominently the pore-forming toxin, streptolysin O (SOT) which harmonizes with NADase to disrupt eukaryotic cell energy metabolism (Zhu et al. Citation2017; Brouwer et al. Citation2023). Via its pore-forming activities, SOT causes structural alterations to cell membranes, which not only enable intracellular penetration of NADase but also inhibit clearance of the pathogen by interfering with PMN-mediated phagocytosis (Sierig et al. Citation2003). These interactions of the two streptococcal virulence factors with PMN also lead to suppression of the anti-microbial reactivities of these cells (Limbago et al. Citation2000; Zhu et al. Citation2017).
In addition to suppression of innate immune mechanisms, microbial pore-forming toxins such as pneumolysin (Ply), have also been reported to promote hyper-activation of PMN (Cockeran et al. Citation2001). The unregulated or exaggerated stimulation of PMN may, in turn, induce tissue damage and organ dysfunction via indiscriminate release of ROS and proteolytic enzymes (Cockeran et al. Citation2001; Letsiou et al. Citation2021). However, potentially cytotoxic effects of SOT on human PMN, which may underpin the severity of disease caused by emergent, highly-toxigenic strains of S. pyogenes, have not been well-explored, but if operative in vivo, may promote poorly-controlled reactivity of PMN with consequent collateral damage to bystander tissues.
Accordingly, the current study was designed to investigate the effects of pretreatment of human PMN with purified SOT on the production of ROS and release of elastase on subsequent activation of the cells with the chemoattractant, N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP), as well as the effects of the toxin on influx of Ca2+ and release of extracellular DNA (NETosis) by human PMN in vitro.
Materials and methods
Ethics approval
Permission to undertake this study and draw blood from healthy, adult human volunteers was granted by the Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria in full compliance with the World Medical Association Declaration of Helsinki, 2013 (Approval #123/2018). Informed consent was obtained from all participants in the study prior to the blood draw.
Streptolysin O toxin
Recombinant streptolysin O toxin (SOT, 8000 ng in 1 ml indicator-free Hanks Balanced Salt Solution [HBSS; pH 7.4] supplemented with 0.1% bovine serum albumin) was purchased from Sigma (St Louis, MO]. Aliquots of stock were prepared as 100-µl volumes and stored at −70°C until use. The final concentration of SOT (80 ng/ml) used in this study was determined in preliminary dose-response experiments to result in influx of Ca2+ into isolated human PMN – indicative of membrane pore formation. With respect to all other chemicals and reagents, these were also purchased from Sigma, unless stated otherwise.
Preparation of neutrophils (PMN)
Cells were isolated from heparinized venous blood (5 U preservative-free heparin/ml blood) that had been drawn from healthy adult volunteers. The PMN were separated from mononuclear leukocytes by centrifugation over Histopaque-1077 (Sigma) cushions at 400 × g for 25 min at room temperature. The resultant pellets were re-suspended in phosphate-buffered saline (PBS, 0.15 M, pH 7.4) and sedimented with 3% gelatin to remove erythrocytes. Following centrifugation (280 × g at 4°C for 10 min), any residual erythrocytes were removed by selective lysis by incubation of the re-suspended cells in 0.83% (w/v) ammonium chloride at 4°C for 10 min. The isolated PMN, which were routinely of high purity (> 90%) and viability (> 95%) as determined by flow cytometric procedures, were then re-suspended to 107 cells/ml in PBS and held on ice until used.
Measurement of reactive oxygen species (ROS)
The production of ROS by PMN was measured using a luminol (5-amino-2,3-dihydro-1,4-phthalazine dione)-enhanced chemiluminescence (CL) procedure that predominantly detects ROS generated by the myeloperoxidase/H2O2/halide system (Minkenberg and Ferber Citation1984). In brief, PMN (2 × 105 cells) were pre-incubated for 10 min at 37 °C in 900 µl of HBSS containing luminol (0.1 mM), followed by sequential addition - 2 min apart - of SOT (80 ng/ml) and the chemoattractant fMLP (1 µM), or equal volumes of HBSS to control systems. All CL responses were then recorded using a Lumac Biocounter (Model 2010, Lumac Systems Inc., Titusville, FL). The final volume in each vial was 1 ml; results are expressed in relative light units (rlu) as the peak values for fMLP-activated systems that were reached 40-50 s after addition of the stimulant.
Elastase release
The extent of PMN degranulation was measured according to the extent of release of the primary granule enzyme, elastase. The PMN were incubated at a concentration of 2 × 106/ml in HBSS for 10 min at 37°C followed by addition of SOT (80 ng/ml) or an equal volume of HBSS and 2 min later by fMLP (0.1 µM) in combination with cytochalasin B (1 µM, final). Tubes were incubated for 10 min at 37°C, then transferred to an ice bath, followed by centrifugation at 400 × g for 5 min to pellet the cells. The PMN-free supernatants were then decanted and assayed for elastase using a micro-modification of a standard colorimetric procedure (Beatty et al. Citation1982). In brief, 125 µL of supernatant was added to the elastase substrate, N-succinyl-l-alanyl-l-alanyl-l-alanine-p-nitroanilide [3 mM in dimethyl sulfoxide (DMSO) in 0.05 M Tris-HCl [pH 8.0]) and elastase activity was then monitored spectrophotometrically at a wavelength of 405 nm and the results expressed as milliunits enzyme/107 cells.
Spectrofluorimetric measurement of extracellular calcium influx
Fura-2-acetomethoxyester (AM) was used as the fluorescent Ca2+-sensitive indicator for these experiments (Grynkiewicz et al. Citation1985). The PMN (107/ml) were incubated with fura-2/AM (2 µM) for 30 min at 37°C, then pelleted by centrifugation and re-suspended in HBSS (pH 7.4) containing 1.25 mM CaCl2. The fura-2-loaded cells were then pre-incubated for 7 min at 37°C and transferred to disposable reaction cuvettes that were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer (Hitachi Ltd, Tokyo, Japan), with excitation and emission wavelengths set at 340 nm and 500 nm, respectively. After a stable baseline was obtained (1 min), SOT (at fixed, final concentration of 80 ng/mL) was added to the cell suspension and alterations in fluorescence intensity monitored over a 5–10 min period. A Ca2+-replete medium was used for these experiments to avoid the enhanced cytotoxicity of pore-forming toxins observed when PMN were suspended in a Ca2+-free medium, which results from defective Ca2+-dependent repair of toxin induced membrane pores (Wolfmeier et al. Citation2015; Nel et al. Citation2016).
In a separate series of experiments, undertaken to confirm the validity of data generated using the dual wavelength Fura-2/AM Ca2+ indicator and the Hitachi 650 10S fluorescence spectrophotometer, the following experiments were performed using the single wavelength fluorescent Ca2+ indicator fluo-8/AM (0.5 µM, Abcam, Cambridge, UK) and a PerkinElmer LS45 luminescence spectrophotometer with excitation and emission wavelengths set at 485 and 525 nm, respectively: (i) influx of extracellular Ca2+ into neutrophils activated with the Ca2+ ionophore A23187 (1 µM, final), in presence and absence of the Ca2+-chelating agent ethylene-diaminetetraacetic acid (EGTA, 10 mM final); and (ii) the effects of the SOT surrogate, Ply (recombinant, 20 ng/ml, final), on Ca2+ influx in the absence and presence of EGTA.
Some of the data are presented as representative traces, including those involving both fura-2/AM and fluo-8/AM, while composite data are expressed as alterations in fluorescence intensity measured as mean metered fluorescence units (MFU) ± SD.
Spectrophotometric detection of NETs
Following pre-incubation for 10 min at 37°C, SOT (80 ng/ml) or phorbol 12-myristate 13-acetate (PMA, 6.25 ng/ml final) - a potent inducer of NETosis, or an equal volume of solvent was added to the PMN (4 × 106 cells in 4 ml HBSS). The tubes were then incubated for 60 min and centrifuged to pellet the cells. To the supernatant fluids (3 mL), 2 μl of the DNA‐binding fluorophore Sytox® Orange (5 µM final; Life Technologies, Eugene, OR) was added, followed by transfer to cuvettes that were placed in the cuvette holder of the Hitachi 650 10S fluorescence spectrophotometer, with excitation and emission wavelengths set at 530 nm and 590 nm, respectively and measurement of fluorescence intensity with the results expressed as MFU.
Expression of results and statistical analysis
Statistical analyses of data from each series of experiments were performed using Prism software (v.5.0, GraphPad, San Diego, CA), with levels of statistical significance calculated using the Mann-Whitney U-test for comparison of non-parametric data [with numbers of different donors (n) clearly indicated]. A p-value < 0.05 was considered significant.
Results
Measurement of ROS
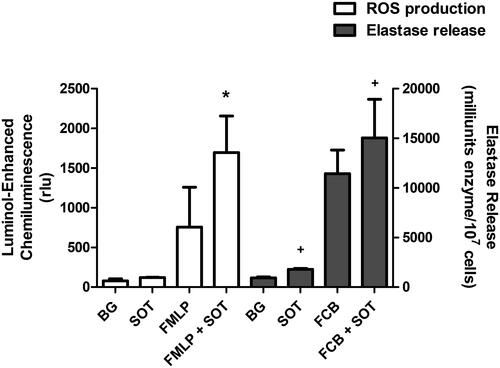
Exposure of PMN to SOT resulted in a modest increase in the generation of ROS (). However, addition of SOT 2 min prior to fMLP resulted in significant augmentation of the chemoattractant-activated responses of PMN. These observations demonstrate that SOT sensitizes PMN for enhanced generation of ROS following activation with fMLP.
Figure 1. Effects of streptolysin O toxin (SOT) on ROS production and elastase release by PMN alone or by cells activated with N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP, 0.1 µM) (unshaded columns), or with the combination of fMLP/cytochalasin B (1 µM) (shaded column). Background (BG) refers to unstimulated control. Results are expressed as means ± SD (n = 5). *p < 0.0007; +p < 0.0001).
Elastase
The effects of SOT on the release of the granule protease, elastase, are shown in . Exposure of PMN to SOT alone resulted in a modest release of elastase. However, pretreatment of PMN with SOT for 2 min prior to addition of fMLP/CB resulted in augmentation of elastase release, which was significantly greater than that observed with cells activated by fMLP/CB alone.
Influx of extracellular Ca2+
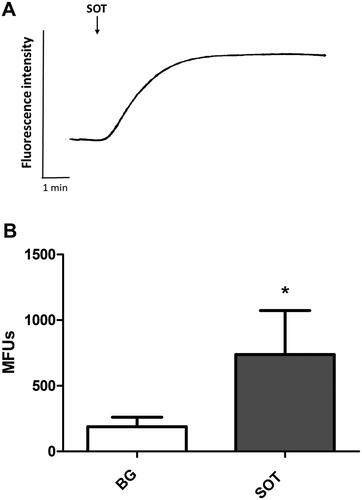
The results shown in are representative traces from six different experiments using cells from three different donors showing alterations in cytosolic Ca2+ (fura-2 fluorescence) in PMN in the presence of SOT (80 ng/ml). Treatment of PMN with SOT was accompanied by a significant elevation of cytosolic Ca2+, which, following a short lag period, increased from basal values of 188 [± 73] MFU to peak values of 739 [± 334]. The peak cytosolic Ca2+ responses remained elevated for the entire duration that fluorescence intensity was monitored.
Figure 2. (A) Effects of streptolysin O toxin (SOT) on the influx of Ca2+ into Fura-2/AM-loaded PMN. Representative traces from a single experiment are shown (n = 6 in the series). SOT added (↓) to PMN suspended in Ca2+-replete medium resulted in a sustained increase in Fura-2 fluorescence following a short lag phase. (B) Mean increases in the fluorescence intensity of PMN loaded with Fura-2/AM following treatment with SOT. Background (BG) refers to the corresponding values for resting cells (in the absence of SOT). Results are expressed as mean MFU ± SD (n = 6). *p < 0.004.
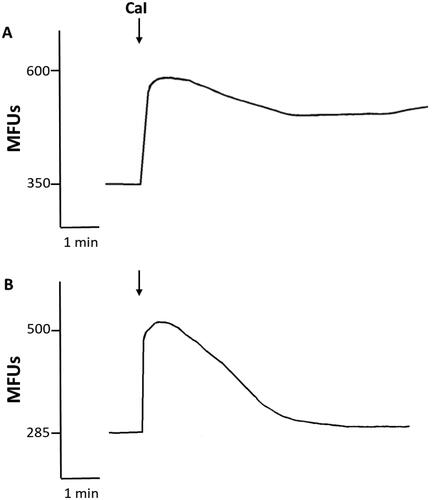
The results of representative experiments using PMN loaded with fluo-8/AM followed by activation with the surrogate activators, A23187 or Ply, in the absence and presence of EGTA (10 mM) are shown in and , respectively. The abrupt influx of Ca2+ observed with both activators and the substantial attenuation of these effects in the presence of EGTA, which were mimicked by Fura-2/AM-loaded neutrophils (data not shown) confirm the reliability of our findings with Fura-2/AM-based Ca2+ influx system.
Figure 3. (A) Effects of pneumolysin (PLY) on influx of Ca2+ into fluo-8/AM-loaded PMN in the presence (a), or absence (B) of EGTA. Representative traces from a single experiment are shown. PLY added (↓) to PMN suspended in Ca2+-replete medium resulted in a sustained increase in fluo-8 fluorescence, while Ca2+ influx peaked rapidly and returned to near basal levels in the presence of EGTA.
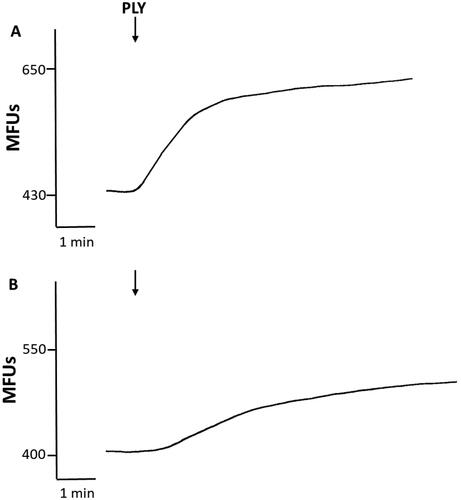
Figure 4. (A) Effects of calcium ionophore (CaI) on influx of Ca2+ into fluo-8/AM-loaded PMN in the presence (a), or absence (B) of EGTA. Representative traces from a single experiment are shown. CaI added (↓) to PMN suspended in Ca2+-replete medium resulted in a sustained increase in fluo-8 fluorescence, while Ca2+ influx peaked rapidly and returned to basal levels in the presence of EGTA.
NETosis
The effects of SOT (80 ng/ml) and PMA (6.25 ng/ml) individually on NETosis were measured using a spectrofluorimetric procedure and a fixed incubation time of 60 min. The data indicate that exposure of PMN to SOT or PMA resulted in significant increases in extracellular DNA to levels of similar magnitude ().
Table 1. Effects of streptolysin O toxin (80 ng/mL) on release of extracellular DNA by PMN in presence of toxin or PMA (6.25 mg/mL).
Discussion
The current study investigated the effects of SOT on the pro-inflammatory activities of human PMN in vitro. Exposure of PMN to SOT alone was associated with a modest increase in the production of ROS and release of the granule protease, elastase. However, pretreatment of PMN with SOT prior to activation of the cells with the chemotactic peptide, fMLP, resulted in significant augmentation of fMLP-activated production of ROS and release of elastase. These observations indicate that SOT sensitizes (‘primes’) the cells for exaggerated generation of ROS and elastase release following subsequent exposure to fMLP, relative to the corresponding responses of fMLP-activated cells in the absence of SOT.
The findings of the current study are in keeping with those of Andersen and Duncan (Citation1980) who observed dose-dependent stimulation of PMN chemiluminescence mediated by SOT, as well as those of Soehnlein et al. (Citation2008), who detected degranulation induced by exposure of PMN to the M1 serotype of S. pyogenes. However, other investigators have reported that these and other indices of PMN anti-bacterial reactivity such as chemotaxis, phagocytosis, ROS production and degranulation are inhibited by SOT (Uchiyama et al. Citation2015). In contrast to the current study, these investigators used a Ca2+-free buffer and PMN activated with PMA or intact bacteria. These aspects of methodology may account for the differences between the current study and that reported by Uchiyama et al. (Citation2015). In this context, it is noteworthy that extra-cellular Ca2+ is essential for the repair of pores inflicted on eukaryotic cells by microbial pore-forming toxins, resulting in substantial augmentation of cytotoxicity (Wolfmeier et al. Citation2015; Nel et al. Citation2016), while PMA does not require mobilization of cytosolic Ca2+ to activate ROS generation. In addition, in the current study elastase release was induced by exposing PMN to a purified form of the toxin, as opposed to intact bacteria. FMLP, a Ca2+-mobilizing chemoattractant, activates ROS production and degranulation of PMN via a Ca2+-dependent mechanism. This suggests that Ca2+ may play an important role in the SOT-mediated priming of PMN. Similar effects have been reported when PMN were exposed to Ca2+-ionophores like ionomycin, which sensitize the cells to subsequent activating stimuli (Vorobjeva and Chernyak Citation2020).
To elucidate the possible involvement of Ca2+ during sensitization of PMN by SOT, the effects of the toxin on influx of Ca2+ into PMN were investigated. In the presence of SOT, an abrupt and sustained increase in fura-2 fluorescence was observed, which is in keeping with increased levels of cytosolic Ca2+ due to the pore-forming activity of the toxin, reaching a mean peak fluorescence intensity of 739 ± 334 MFU. Plasma membrane pore formation facilitates the entry of Ca2+ present in the extracellular medium (1.25 mM) down a concentration gradient into the cytosol, which has a much lower Ca2+ concentration (0.1 µM). Importantly, the reliability of the dual wavelength Fura-2/AM system used in this study was confirmed using the single wavelength Ca2+-sensitive fluorescent dye, fluo-8/AM, together with alternative detection instrument-ation and two surrogate activators of Ca2+ influx.
An increase in Ca2+ influx during PMN activation is also a recognized trigger of NETosis (Nel et al. Citation2016; Letsiou et al. Citation2021). In this context, the current study has demonstrated that following exposure of PMN to SOT, influx of Ca2+ occurs rapidly, resulting in the cells undergoing NETosis, which was detected as an increase in extracellular DNA observed following an incubation period of 60 min. This finding is in agreement with previous reports that showed that exposure of PMN to GAS caused activation of NETosis (Yipp et al. Citation2012; Branzk and Papayannopoulos Citation2013).
The SOT-induced NETosis observed in the current study may promote the clearance of GAS from infected tissues, which would be beneficial to the host in a clinical setting. However, numerous reports have suggested that excessive NETosis may be harmful to the host as NETs may amplify the production of pro-inflammatory cytokines such as interleukin (IL)-8 and tumor necrosis factor (TNF)-α (Hudock et al. Citation2020). Furthermore, the cytotoxic effects of histones and NETosis-induced thrombosis may exacerbate tissue damage (Sørensen and Borregaard Citation2016).
Group A streptococci can cause multiple infections in humans, ranging in severity from mild to severe invasive disease. These microorganisms produce an array of virulence factors, including SOT (Döhrmann et al. Citation2016; Brouwer et al. Citation2023). If operative in vivo, SOT-mediated priming of PMN may result in excessive generation of ROS, release of proteolytic enzymes and NETosis, with associated exacerbation of bystander tissue injury. In this setting, hyper-activation of PMN induced by SOT may contribute to tissue destruction, manifesting clinically as necro-tizing pneumonia, empyema and abscess formation in tissues infected by GAS, especially in the setting of infections caused by emergent highly toxigenic strains of the pathogen (Brouwer et al. Citation2023; Bamford and Whittaker Citation2023). Furthermore, SOT may modulate the inflammatory response by inducing the release of IL-1β via activation of the NLRP3, inflammasome complex (Richter et al. Citation2021). Although the current study focused on the effects of SOT on oxidant production and elastase release by neutrophils, as well as NETosis mediated by SOT, this toxin may also modulate cytokine production and NO release from neutrophils.
The concentration of SOT used in this study (i.e., 80 ng/ml) is likely to be biologically-relevant as the concentrations of this toxin present in supernatants of GAS isolates cultured in vitro have been reported to range from 250 - 600 ng/ml (Clarke et al. Citation2021). Nevertheless, there are still several limitations that the authors wish to acknowledge. These include the in vitro experimental design using recombinant SOT. However, purified SOT facilitates the study of interactions between the toxin and PMN uncomplicated by other cell types and virulence factors secreted by GAS.
In conclusion, the current study has identified SOT-mediated Ca2+ influx as a putative mechanism of augmentation of the pro-inflammatory interactions of PMN, which if operative in a clinical setting, may contribute to hyper-activation of PMN and GAS-mediated tissue injury, underscoring the clinical potential of strategies which neutralize microbial pore-forming toxins (Escajadillo and Nizet Citation2018).
Author contributions
All authors contributed significantly to the conceptualization and planning of the study. DJ, AJT, RA and GRT performed laboratory investigations; DJ, AJT, RA and GRT contributed to analysis of the data and preparation of the figures, while all authors contributed to interpretation of data and compilation of the manuscript. All authors have approved this submitted version of the manuscript.
Informed consent statement
Written informed consent was obtained from all blood donors who partook in the study.
Institutional review board statement
As noted, permission to undertake this study and to draw blood from healthy adult human volunteers was granted by the Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria (Approval number: 123/2018).
Disclosure statement
None of the authors declares a potential conflict of interest. The authors alone are responsible for the content of this manuscript.
Data availability statement
Data can be made available upon reasonable request.
Additional information
Funding
References
- [WHO] World Health Organization. 2022. Increased incidence of scarlet fever and invasive Group A streptococcus infection - multi-country. https://www.who.int/europe/news/item/12-12-2022.
- Akuzawa N, Kurabayashi M. 2016. Bacterial pneumonia caused by Streptococcus pyogenes infection: A case report and review of the literature. J Clin Med Res. 8(11):831–835. doi:10.14740/jocmr2737w.
- Andersen B, Duncan J. 1980. Activation of human neutrophil metabolism by streptolysin O. J Infect Dis. 141(5):680–685. doi:10.1093/infdis/141.5.680.
- Bamford A, Whittaker E. 2023. Resurgence of Group A streptococcal disease in children. BMJ. 380:43. doi:10.1136/bmj.p43.
- Beatty K, Robertie P, Senior R, Travis J. 1982. Determination of oxidized aα-1-proteinase inhibitor in serum. J Lab Clin Med. 192(2):186–192.
- Branzk N, Papayannopoulos V. 2013. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol. 35(4):513–530. doi:10.1007/s00281-013-0384-6.
- Brouwer S, Rivera-Hernandez T, Curren B, Harbison-Price N, de Oliveira D, Jespersen M, Davies M, Walker M. 2023. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat Rev Microbiol. 21(7):431–447. doi:10.1038/s41579-023-00865-7.
- Clarke J, Baltazar M, Alsahag M, Panagiotou S, Pouget M, Paxton W, Pollakis G, Everett D, French N, Kadioglu T. 2021. Streptolysin O concentration and activity is central to in vivo phenotype and disease outcome in Group A Streptococcus infection. Sci Rep. 11(1):19011. doi:10.1038/s41598-021-97866-4.
- Cockeran R, Theron A, Steel H, Matlola N, Mitchell T, Feldman C, Anderson R. 2001. Pro-inflammatory interactions of pneumolysin with human neutrophils. J Infect Dis. 183(4):604–611. doi:10.1086/318536.
- Döhrmann S, Cole J, Nizet V. 2016. Conquering neutrophils. PLoS Pathog. 12(7):e1005682. doi:10.1371/journal.ppat.1005682.
- Escajadillo T, Nizet V. 2018. Pharmacological targeting of pore-forming toxins as adjunctive therapy for invasive bacterial infection. Toxins (Basel). 10(12):542. doi:10.3390/toxins10120542.
- Grynkiewicz G, Poenie M, Tsien R. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 260(6):3440–3450.
- Hudock KM, Collins MS, Imbrogno M, Snowball J, Kramer EL, Brewington JJ, Gollomp K, McCarthy C, Ostmann AJ, Kopras EJ, et al. 2020. Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 319(1):L137–L147. doi:10.1152/ajplung.00144.2019.
- Letsiou E, Alves L, Felten M, Mitchell T, Müller-Redetzky H, Dudek S, Witzenrath M. 2021. Neutrophil-derived extracellular vesicles activate platelets after pneumolysin exposure. Cells. 10(12):3581. doi:10.3390/cells10123581.
- Li H, Zhi X, Viera A, Whitwell H, Schricker A, Jauneikaite E, Li H, Yosef A, Andrew I, Game L. 2023. Characterization of emergent toxigenic M1UK Streptococcus pyogenes and associated sub-lineages. Microb Genom. 9(4):mgen000994.
- Limbago B, Penumalli V, Weinrick B, Scott J. 2000. Role of streptolysin O in a mouse model of invasive Group A Streptococcal disease. Infect Immun. 68(11):6384–6390. doi:10.1128/IAI.68.11.6384-6390.2000.
- Minkenberg I, Ferber E. 1984. Lucigenin-dependent chemiluminescence as a new assay for NADPH-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods. 71(1):61–67. doi:10.1016/0022-1759(84)90206-0.
- Nel J, Theron A, Durandt C, Tintinger G, Pool R, Mitchell T, Feldman C, Anderson R. 2016. Pneumolysin activates neutrophil extracellular trap formation. Clin Exp Immunol. 184(3):358–367. doi:10.1111/cei.12766.
- Ochi F, Tauchi H, Jogamoto T, Miura H, Moritani T, Nagai K, Ishii E. 2018. Sepsis and pleural empyema caused by Streptococcus pyogenes after influenza A virus infection. Case Rep Pediatr. 2018:4509847. doi:10.1155/2018/4509847.
- Parks T, Barrett L, Jones N. 2015. Invasive streptococcal disease: A review for clinicians. Br Med Bull. 115(1):77–89. doi:10.1093/bmb/ldv027.
- Richter J, Monteleone M, Cork A, Barnett T, Nizet V, Brouwer S, Schroder K, Walker M. 2021. Streptolysins are the primary inflammasome activators in macrophages during Streptococcus pyogenes infection. Immunol Cell Biol. 99(10):1040–1052. doi:10.1111/imcb.12499.
- Sierig G, Cywes C, Wessels M, Ashbaugh C. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated Group A Streptococci. Infect Immun. 71(1):446–455. doi:10.1128/IAI.71.1.446-455.2003.
- Soehnlein O, Oehmcke S, Ma X, Rothfuchs AG, Frithiof R, van Rooijen N, Mörgelin M, Herwald H, Lindbom L. 2008. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J. 32(2):405–412. doi:10.1183/09031936.00173207.
- Sørensen OE, Borregaard N. 2016. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest. 126(5):1612–1620. doi:10.1172/JCI84538.
- Tamayo E, Montes M, Vicente D, Pérez-Trallero E. 2016. Streptococcus pyogenes pneumonia in adults: clinical presentation and molecular characterization of isolates 2006-2015. PLoS One. 11(3):e0152640. doi:10.1371/journal.pone.0152640.
- Uchiyama S, Döhrmann S, Timmer AM, Dixit N, Ghochani M, Bhandari T, Timmer JC, Sprague K, Bubeck-Wardenburg J, Simon SI, et al. 2015. Streptolysin O rapidly impairs neutrophil oxidative burst and antibacterial responses to Group A Streptococcus. Front Immunol. 6:581. doi:10.3389/fimmu.2015.00581.
- Vorobjeva N, Chernyak B. 2020. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry (Mosc). 85(10):1178–1190. doi:10.1134/S0006297920100065.
- Walker M, Barnett T, McArthur J, Cole J, Gillen C, Henningham A, Sriprakash K, Sanderson-Smith M, Nizet V. 2014. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. 27(2):264–301. doi:10.1128/CMR.00101-13.
- Wolfmeier H, Schoenauer R, Atanassoff A, Neil D, Kadioglu A, Draeger A, Babiychuk E. 2015. Ca2+-dependent repair of pneumolysin pores: A new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim Biophys Acta. 1853(9):2045–2054. doi:10.1016/j.bbamcr.2014.09.005.
- Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, et al. 2012. Dynamic NETosis is carried out by live neutrophils in human and mouse bacterial abscesses and during severe Gram-positive infection. Nat Med. 18(9):1386–1393. doi:10.1038/nm.2847.
- Zhu L, Randall R, Lee J, Porter A, DeLeo F, Musser J. 2017. Contribution of secreted NADase and streptolysin O to the pathogenesis of epidemic serotype M1 Streptococcus pyogenes infections. Am J Pathol. 187(3):605–613. doi:10.1016/j.ajpath.2016.11.003.

