ABSTRACT
Research on human functioning is notoriously difficult. This particularly holds for the study of light effects, at least if one wants to go beyond establishing that changes in light “have an effect” and understand why this effect occurs—in other words, if one wants to make causal inferences about the mechanism behind it. The latter is, of course, crucial for generalizing insights and being able to use them effectively in other contexts. The culmination of many decades of research has taught us that light affects psychological functioning in numerous ways and through various pathways. This implies that, regardless of the investigator’s particular interests in either of those mechanisms, generally all will be at play, simultaneously, for participants in any lighting study. The present tutorial aims to address this complexity and how to deal with it by concisely describing the most important pathways that we currently are aware of. Such awareness is important both in contemplating the design and methodology of a study and in interpreting results from other studies and generalizing them to a particular application or light design.
Learning outcomes
It is our intent that, after studying this article, the reader will:
understand the complexity of the processing of light and the basic mechanisms underlying light’s effects on psychological functioning.
be able to explain the essence of and difference between image-forming (IF) and non-image-forming (NIF) pathways.
be aware of the multiple mechanisms potentially at play within the IF pathway, including those related to
visual performance; for instance, as a result of a gradual buildup of fatigue and/or frustration due to eyestrain or insufficient contrast, which may impact alertness, mood, and cognitive performance,
visual (dis)comfort; for instance, in cases of disability glare, discomfort glare, or dazzling glare or insufficient contrast, which also may impact psychological functioning.
visual experience, which, through appraisal, motivational, or associative mechanisms, may impact our mindset and behaviors as diverse as performance on tasks, helping behavior, cooperation, and aggression.
be aware of the multiple mechanisms potentially at play within the NIF pathway, including
circadian mechanisms, driving sleep timing, duration, and quality, which influence wakefulness and alertness during the day as well as mood and cognitive performance.
acute effects, which are less well understood than circadian effects but largely follow similar neural pathways and may impact important aspects of psychological functioning.
understand that the implication of these different pathways is that research designs should be developed and scrutinized thoroughly before one can draw conclusions on causal mechanisms.
1. Introduction
“Nothing so practical as good theory.”—Kurt Lewin (Greenwood & Levin, Citation1998, p. 19)
Undoubtedly, the lighting study best known to the general audience is the landmark Hawthorne illumination experiment. This set of studies conducted in a Western Electric factory in the 1920s investigated the relationship between amount of light and worker productivity. Perhaps surprising, the data were never formally analyzed or reported in the scientific literature, but informal accounts of the results made their way to the public nonetheless. The unexpected positive performance outcomes of this study, regardless of the direction or size of the light intensity manipulation, opened the world’s eyes to some of the important pitfalls of doing research on humans.Footnote1
Research on human functioning is notoriously difficult. This particularly holds for the study of light effects, at least if one wants to go beyond establishing that changes in light “have an effect” and understand why this effect occurs—in other words, if one wants to make causal inferences about the mechanism behind it. The latter is, of course, crucial for generalizing insights and being able to use them effectively in other contexts. For instance, if one wants to transfer the findings from a particular study to lighting practice.
In the present article we will limit ourselves to light’s impact on psychological functioning, which encompasses important outcomes such as alertness, mood, cognitive performance, social behavior, and mental health and well-being. Many decades of research have taught us that light affects psychological functioning in numerous ways and through various pathways. Today, these pathways are generally clustered in two basic categories—that of visual, or image-forming, mechanisms and that of nonvisual, or non-image-forming, mechanisms. It is important to note, however, that these two categories also denote two quite separate domains of research, performed by scholars in quite disparate scientific disciplines and often published in different disciplinary journals. However, regardless of the investigator’s particular interests in either of those classes of mechanisms, generally all will be at play, simultaneously, for participants in any lighting study and, of course, for users of any lit—or unlit—space. It is therefore important that lighting researchers and lighting designers are aware of all mechanisms potentially driving light effects, regardless of whether they are planning studies themselves or interpreting the results and implications of others. The present tutorial aims to address this complexity by concisely describing the most important pathways that we currently are aware of.
2. Multiple pathways
Mechanisms driving effects of light on psychological functioning are generally categorized in one of two major groups labeled image-forming (IF) and non-image-forming (NIF) pathways.Footnote2 Both classes include a host of mechanisms discussed—though far from comprehensively—in more detail below.Footnote3 To help structure the theories and mechanisms underlying IF and NIF pathways, de Kort and Veitch (Citation2014) presented the scheme depicted in .
Fig. 1. Pathways of light relevant to psychological functioning. Adapted from de Kort and Veitch (Citation2014).
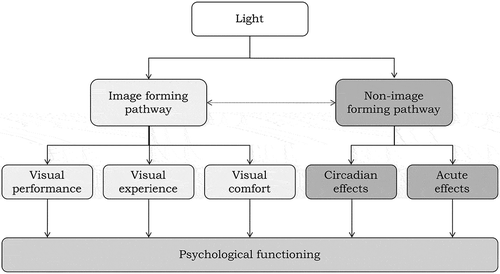
2.1. Psychological functioning
First, we should perhaps explain what the term “psychological functioning” refers to. In the scheme presented in , psychological functioning refers to behavior and the mental processes that drive it, such as perception, attention and cognition, emotion (affect), and motivation (conation). Behavior basically refers to any type of response but in lighting research often pertains to social or performance behavior (e.g., cooperation, aggression, reaction time on tasks or productivity at work) and, of course, general well-being and mental health are important outcomes in and of themselves. As we will see, both classes of mechanisms discussed below exert powerful impacts on mental processes and behavior.
2.2. Image-forming mechanisms
The IF pathway refers to the processing of light information via the visual system. In other words, it pertains to what we actually see. This visual pathway presents important routes to individuals’ well-being and psychological functioning. Visual lighting needs have received much attention for decades, particularly in the domain of psychophysics, and we know that light conditions influence task visibility, visual performance and comfort, and the visual impressions of spaces and the persons and objects in that space (e.g., Boyce Citation2014).
Vision starts in the eye and requires light. Photoreceptors in the outer layer of the retina—the well-known rods and cones—absorb light particles and transduce photic information to neural signals that travel to the optic chiasm via the optic nerve. The retina consists of multiple layers of neurons that are tightly interconnected. These interconnections are the means through which light processing starts immediately, including, for instance, contrast detection, color processing, and very quick adaptation to local and ambient light levels. Typically such processing continues deeper in the brain, but the initiation in the retina implies that, rather than transferring direct pixel-based information like a digital camera, the optic nerve transports preprocessed light information, having already converted individual photoreceptor inputs into, for instance, light- and color channel–based data and accentuating color and light contrasts (both spatial and temporal), which are important for fast downstream (deeper in the brain) detection of, for instance, borders and shapes; that is, object detection. From there, light information takes different routes into the brain. The pathway mainly responsible for vision is that from the optic chiasm, via the lateral geniculate nucleus to the visual cortex. Once the signals reach the cortex, we become consciously aware of them; in other words, we see.
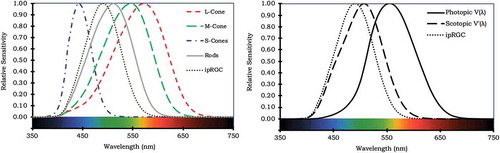
Under dim conditions, we rely mostly on rod receptors for vision, which are more sensitive to light, whereas under brighter conditions, vision is driven most by the cone receptors. Rod receptors and the three types of cone receptors—S, M, and L, for short, medium, and longer wavelengths—each have a slightly different peak sensitivity in terms of the wavelength of light (see ). In bright conditions, the combined (differential) input of the three types of cones provides us color information, in addition to a lightness sensation. The human spectral sensitivity curve V(λ) describes the combined (S, M, and L) sensitivity for cone vision in bright (>3 cd/m2, termed photopic) conditions as a function of the wavelength of light (see ). All photometric measures (luminous flux, luminous intensity, luminance, illuminance) are basically derived from their radiometric counterparts (radiant flux, intensity, radiance, and irradiance) by weighting them according to this curve. Similarly, V′(λ) is the established sensitivity for rod vision in dim (<0.03 cd/m2, termed scotopic) conditions. The ranges of the luminous conditions in which rods and cones are sensitive overlap. Importantly, in this intermediate (mesopic) range, both types of photoreceptors are active, and both sensitivity curves should be considered.
Fig. 2. Relative sensitivity curves: (a) individual photoreceptors and (b) photopic sensitivity curve V(λ), scotopic sensitivity curve V′(λ), and, for comparison purposes, the ipRGC is also included.
2.2.1. Visual performance
The first thing we consider is the process of making sense of what is seen—the construction of the image. Lighting plays an important part in this, because we need light to see—as Boyce (Citation2014) boldly puts it: “With light we can see; without light we cannot” (p. 43). We humans can process light information over an enormous range of ambient light levels, stretching from conditions in starlight to those in bright sunlight—that is, more than eight orders of magnitude—but the visual system does need to adapt to this. It does so through various adaptation mechanisms (only one of which is the transition from rod to cone vision described above) at the level of the pupil, the retina, and the cortex (Hood and Finkelstein Citation1986; for a more complete model of light adaptation, see Gloriani et al. Citation2016). In addition, the contrast resulting from characteristics of the visual stimulus, its background, and lighting conditions needs to be sufficient. Therefore, interestingly, for functional vision in real-life conditions, visual contrast is a function of the available lighting in combination with object characteristics of both the stimulus and its background but, at the same time, an individual’s contrast sensitivity is influenced by adaptation to the level of surround luminance (Aparicio et al. Citation2010).
Visual performance is generally defined as the speed and accuracy of performing a visual task. Rea and Ouellette (Citation1988) developed an empirical model of visual performance in adapted state in terms of reaction time as a function of target area, target contrast, and adaptation luminance for achromatic stimuli. The model basically implies that one perceives more detail as more light reaches the retina, but nonlinearly. At low light levels, visual performance improves substantially with even a small increase in light, but at high light levels the effect levels off and becomes almost negligible. Color vision further facilitates the perception and recognition of objects, particularly in conditions of low luminance contrast, but color was only included in such models very recently (O’Donell et al. Citation2011), illustrating the complexity of vision and the fact that we still have not reached full understanding, even after decades of intensive research on this topic.
2.2.2. Visual comfort
Visual comfort is a phenomenon that has proven hard to define with strong consensus. Some scholars subscribe to its characterization as “the absence of visual discomfort,” but apart from this being merely a tautological statement, this excludes the perspective on the potential positive, desirable aspects of lighting conditions (Boyce Citation2014). Most research in this domain has, however, focused on visual discomfort and particularly on glare, the “hindrance to vision by too much light” (Vos Citation2003). Perhaps somewhat counterintuitive, visual comfort does not necessarily show a strong relation with visual performance, but it does often predict or correlate with satisfaction with lighting conditions (Borisuit Citation2013; Rea Citation2000; Roufs and Boschman Citation1991). Glare can cause discomfort, which may be experienced as ocular pain and muscle strain (Berman et al. Citation1994) or induce avoidance behaviors (Rea et al. Citation1985). The mechanisms behind discomfort are not fully understood (Boyce Citation2014). Multiple types of glare have been suggested; a convenient classification consisting of three categories and partially based on the hypothesized underlying mechanisms was forwarded by Vos (Citation2003), including disability glare—“the masking effect caused by light scattered in the ocular media which produces a veiling luminance over the field of view” (p. 164)—discomfort glare—the distracting or irritating effect of peripheral light sources in the field of view—and dazzling glare—conditions of light overexposure to which one squints one’s eyes, which may be even painful.
There are models available to predict the degree of discomfort in certain circumstances. The predominant model for interior lighting currently is the Unified Glare Rating (CIE Citation1995), but the general consensus is that most models hold only within restricted conditions or circumstances, and none of the models available are completely accurate. Recent considerations, for instance, pertain to nonuniformity of the source (e.g., Cai and Chung Citation2012; Donners et al. Citation2016; Geerdinck Citation2012; Geerdinck et al. Citation2014; Scheir et al. Citation2015; Yang et al. Citation2017) or sources that are not static (Issolio and Colombo Citation2006). Understanding glare caused by nonuniform and nonstatic sources is, of course, becoming more and more important given the increasing application of light emitting diode lighting. In addition, no current indices of visual discomfort can properly explain the high variability existing between individuals’ discomfort glare perceptions. Interpersonal differences in, for instance, age, culture, or chronotype and many factors besides objectively measurable light characteristics (e.g., time of day or season, task difficulty, whether the light source is artificial or natural) may be at play (e.g., Borisuit Citation2013; Galasiu and Veitch Citation2006; Van Den Wymelenberg et al. Citation2010 for a review on this matter, see Pierson et al. Citation2018).
Through mechanisms underlying visual (dis)comfort and visual performance, light conditions may impact alertness, mood, and cognitive performance acutely; for instance, in cases of disability glare or insufficient contrast or perhaps as a result of a gradual buildup of fatigue and/or frustration due to eyestrain. It is hard to stay concentrated on a task and perform well while one is hindered by low readability or bothered by discomfort. But we also should not underestimate the effects that lighting conditions induce via what has been labeled as the visual experience (see ).
2.2.3. Visual experience
Beyond helping us to discern objects and faces, light adds a lot more to a scene of course. In 1973, Flynn and colleagues recognized that rather than registering the objective environment, persons search for meaningful information in their environment and that light had an important role in this process: “light can be discussed as a vehicle that facilitates the selective process and alters the information content in the visual field” (p. 87). Since the early works of Flynn and colleagues, numerous studies have shown that light—particularly brightness and luminance distribution—influences the impression of a space in terms of the visual appearance in terms of, for instance, its spaciousness (e.g., Durak et al. Citation2007; Flynn et al. Citation1973; Veitch et al. Citation2011). Light also impacts the affective appraisal or perceived atmosphere of a space and has the potential to change the same space from cosy to tense or from lively to detached (Durak et al. Citation2007; Stokkermans et al. Citation2018; Vogels et al. Citation2008). Light affects the appreciation of a space (Flynn et al. Citation1973; Hawkes et al. Citation1979; Houser et al. Citation2002), sometimes even to the point of inducing pleasure and/or arousal in the visitor (Kuijsters et al. Citation2015). At night, light influences perceptions of safety (e.g., Haans and de Kort Citation2012), as well as perceived restorativeness of and fascination with urban streetscapes (Nikunen and Korpela Citation2012). Both of these latter studies suggest that indeed light changes the way in which we filter information from the environment to build an overall impression of it. So light may change the way we perceive a space, how much we like it, and how we feel. In office conditions, light has been shown to impact satisfaction—not only with the light but beyond the visual realm; for instance, also with climatic conditions (e.g., Te Kulve Citation2018) and the entire workplace (Veitch et al. Citation2011)—and via this appraisal route to also impact mood (Küller et al. Citation2006) and the motivation with which we perform our work and our productivity (Veitch et al. Citation2008, Citation2011).
But light also guides and directs attention and awareness (Steidle and Werth Citation2014; Veitch Citation2001) and has meaning even to the point where it induces cognitive associations (e.g., Elliot and Maier Citation2014; Schietecat et al. Citation2018a, Citation2018b). For instance, quite strong associations exist between brightness and goodness (morality) but also activity and liveliness, in contrast to how darkness is generally associated with immorality and, to a lesser extent, inactivity (Schietecat et al. Citation2018a). Acting like contextual cues that impact information processing, such cognitive associations may result in approach or avoidance behaviors (e.g., Mehta and Zhu Citation2009), more positive or negative appraisals of objects or scenes (Beute and de Kort Citation2013; Lakens et al. Citation2013), different styles of information processing (Steidle et al. Citation2011), and different social perceptions and moral behavior (e.g., Baron et al. Citation1992; Schietecat Citation2018; Zhong et al. Citation2010), resulting, for instance, in more positive assessments of written personnel folders of fictive employees under dimmer conditions but also more dishonest reporting of one’s performance on a task and persons photographed in dimmer conditions being judged less positive and more aggressive than the same individuals photographed in bright conditions. These studies imply that, through associative pathways, light not only changes the way we perceive spaces and the objects and persons in them but also how we assess those objects, tasks, and other persons and how we relate to them. Via this route, light may impact our mindset and behaviors as diverse as performance on tasks, helping behavior, cooperation, and aggression.
2.3. Non-image-forming mechanisms
The NIF pathway has received a lot of attention in the lighting literature over the past decade. Since the discovery of a fifth type of photoreceptor, non-rod, non-cone, in the human eye (Berson et al. Citation2002; Hattar et al. Citation2002), labs across the globe have contributed substantially to our understanding of the mechanisms that are thought to underlie these photobiological effects of light exposure. These photoreceptors, named intrinsically photoreceptive retinal ganglion cells (ipRGC), are also found in the retina, though in a different (inner) layer than the rods and cones. They appear to be the main driver behind light effects directly impacting our internal clock and alertness-related regions in the central brain and brainstem (Cajochen et al. Citation2014). Their signals travel a different route than those of the traditional photoreceptors: instead of targeting the visual cortex, they target more central parts of the brain, among them our central circadian pacemaker (the biological clock). The exact pathways are still being defined, a process involving very fundamental work in non-human studies. However, we know that these signals do not contribute to our conscious visual experience; hence the label “non-image-forming.”
Importantly, ipRGCs transduce photic energy similar to the way rods and cones do, but they contain a different photopigment—melanopsin—and hence have a different spectral sensitivity curve with a peak sensitivity at about 470 nm, which does not align with either V(λ) for cone vision in bright conditions or V’(λ) for rod vision in dim conditions (see ). It does, however, receive input from both rods and cones and may hence also be indirectly sensitive to light in those parts of the spectrum (Lucas et al. Citation2014). The ipRGC-driven responses differ from rod- and cone-driven responses in several other ways. Particularly relevant is, for instance, that spatial information is only minimally retained in the signal (Lucas Citation2013; Münch and Kawasaki Citation2013), that its temporal dynamic is substantially slower, in both onset and offset (Lucas Citation2013; Münch and Kawasaki Citation2013), so it requirtes a longer light stimulus to be activated but its signal is also more persistent. Its photic information is sent not to the visual cortex but, as mentioned above, to central brain areas, in particular the central circadian pacemaker in the hypothalamus and regions in the brainstem implicated in alertness and sleep behavior (Vandewalle et al. Citation2009).
2.3.1. Circadian effects
In the literature, the NIF effects are generally categorized as circadian or acute. The first category pertains to entrainment of our internal clock to the Earth’s 24-hour rhythm and the induction of phase shifts in which the timing of the central endogenous pacemaker relative to external clock time is changed. Literature shows that light is crucial for a healthy entrainment of our biological clock. Experts in the field agree that it affects circadian rhythms “more powerfully than any drug” (Czeisler Citation2013, p. S13). Light exposure can shift and entrain the internal clock but, importantly, these effects depend on many characteristics of the exposure. First, because these effects are driven mainly (though not entirely; e.g., see Gooley et al. Citation2010) by melanopic (ipRGC) activation, the intensity and spectrum of the light falling on the retina are crucial determinants. Responses typically follow a compressive nonlinear function, where the shift induced by exposure initially rises fast with increasing light levels and then saturates (Boivin et al. Citation1996; Zeitzer et al. Citation2000) and where light near the peak sensitivity of the ipRGCs exerts greater effects than light outside their sensitive range (Warman et al. Citation2003). Effects are mildly, and nonlinearly, dependent on the duration of light exposure (Duffy and Wright Citation2005), but much more important is the timing of exposure: circadian shifts vary not only in size but even in direction depending on whether light exposure occurs early or late in one’s subjective morning, afternoon, or evening. The dynamics of this phenomenon are captured in the so-called phase-response curve (Khalsa et al. Citation2003; Minors et al. Citation1991), which sketches whether a bout of light exposure will result in a phase advance, resulting in earlier wake and sleep propensity, or a phase delay, causing one to become sleepy later in the day and also wanting to rise later the following day.
As such, light has an enormous influence on our physical and mental health (e.g., Alvarez and Ayas Citation2004; Alvaro et al. Citation2013). Healthy sleep and well-entrained sleep–wake cycles impact recovery from surgery and depression, and light therapy is used as a curative treatment for most types of depression (both seasonal and nonseasonal) and is currently suggested also for (adjunctive) treatment of ADHD, dementia, bulimia, and numerous other psychiatric disorders (Terman and Terman Citation2005). Naturally, healthy sleep is also a major prerequisite for wakefulness and alertness during the day as well as cognitive performance (Dijk et al. Citation1992).
2.3.2. Acute NIF effects
Through NIF pathways, light may also acutely impact brain activity, physiology, and endocrinology. This means that these responses emerge upon or soon after light exposure commences and subsequently dissipate after the light exposure has ended. Brighter or more blue-enriched light sometimes results in better mood (Stephenson et al. Citation2012), different emotional processing (Vandewalle et al. Citation2010), and higher alertness, concentration, and performance on cognitive tasks (Smolders et al. Citation2012, Citation2013; Vandewalle et al. Citation2009), as well as effort expenditure (Lasauskaite and Cajochen Citation2018), even among healthy, day-active persons. Although reported effects are perhaps not as robust as one would hope (e.g., see Huiberts et al. Citation2016, Citation2017; Smolders and de Kort Citation2014, Citation2017; Smolders et al. Citation2018; for recent reviews see Souman et al. Citation2017; Lok et al. Citation2018) and are certainly less well understood than circadian effects, similar neural pathways and ipRGC involvement are implied for acute light exposure effects on important aspects of psychological functioning. As for circadian effects, acute effects of light appear to vary with intensity, spectrum, duration, and timing (Cajochen Citation2007).
2.4. Integration
Through the abovementioned IF mechanisms—visual performance, visual (dis)comfort, and via appraisal, awareness, and associative processes—the literature reports that light can influence numerous aspects of psychological functioning, including mood, alertness, cognitive performance, and social behavior. Moreover, light exerts powerful effects on humans via NIF pathways. It is important to note that although some refer to this nonvisual route as the “biological” or “physiological” pathway, many NIF effects eventually contribute to the same mental processes as IF effects do: perception, attention and cognition, affect and motivation; in other words, psychological functioning.
If one is merely interested in establishing that light exerts an influence on human functioning, this need not be problematic, but if one wants to understand underlying mechanisms and be able to generalize research findings or translate them to the lighting practice, the overlap in effects of IF and NIF mechanisms on mental processes may pose challenges. Physiological mechanisms may induce psychological effects and vice versa: psychological processes may induce physiological effects. In essence, physiological and psychological phenomena may be identical but assessed and considered at a different grain level. Therefore, establishing an effect on a physiological or psychological indicator in itself does not prove which mechanism was at play.
Alertness, for instance, measured through self-report or performance on a vigilance task, may be influenced by light via NIF or melanopsin-driven mechanisms, acutely through the suppression of melatonin production, activation of alertness-related regions in the brainstem (Cajochen Citation2007), an increase in core body temperature (Te Kulve et al. Citation2016), or circadian effects on sleep quality and duration (Duffy and Wright Citation2005), but performance may also be influenced via perceived comfort and satisfaction (Küller et al. Citation2006; Veitch et al. Citation2011), and higher wall luminance may induce subjective alertness via the visual pathway (de Vries et al. Citation2018) or may be modulated by top-down attention guided by visual processes.
For this reason, one should be cautious in “blind” generalization of findings from a specific domain to the lighting practice. As an example, alerting and circadian effects via the NIF route are more powerful for light with more power in the blue part of the light spectrum. This may be seen as a way of helping users concentrate and perform better in an office environment. But adding power in this part of the spectrum will often result in a more blue appearance of the light or much higher light levels, both of which are often disliked by office users, resulting perhaps in demotivation and underperformance. Likewise, researchers have to be aware that in manipulating one element—for instance, melanopic activation—they are generally also manipulating the appearance of the stimulus, which creates a potential confound in their studies, perhaps leading to misattribution of the established effect. Both for practice and for research, prevention of unfortunate applications and designs starts with a solid awareness of the various pathways and their implications.
3. Implications and conclusions
Light has the potential to affect psychological functioning and health. To cite one of our nestors in the field: “There is still much to learn about the impact of light on human health but what is known is enough to ensure that the topic requires the attention of all those concerned with the lighting of buildings” (Boyce Citation2010, p. 8). The fact that light acts in various ways and, importantly, through various mechanisms on psychological functioning does tend to complicate matters for both researchers and practitioners in the field, and establishing robust causal mechanisms is paramount for the advancement of the domain. Unfortunately, many of the researchers who performed studies into psychological effects of light in the 1970s and 1980s, and actually sometimes to date, ignored—or perhaps one should say “were insufficiently aware of”—the photobiological (NIF) pathways impacting and hence potentially confounding their studies. Moreover, it appears that current researchers investigating NIF studies sometimes remain unaware of the potential role of visually driven mechanisms confounding theirs.
This article aimed to underline the case for a strong role of theory in lighting research and to broaden the scope of researchers and practitioners to theoretical and methodological considerations that may not be their initial or primary interest. The lighting field became famous—or perhaps infamous—for discovering just how sensitive human research is to study designs and protocols and, as a result, most researchers to date are now keenly aware of demand characteristics, placebo effects, observer effects, and novelty effects. Specific domains may have developed gold standards for robust research, such as the constant routine and forced desychrony protocols in chronobiology (Wirz-Justice Citation2007). But the new challenge in front of us involves dealing with the complexity that comes with the realization that lighting affects us through multiple pathways—several of which have not even been mentioned or discussed in the present text.
Lighting has always been an interdisciplinary domain, but over recent years we have seen new domains, new theories, and new methods entering the field. This holds great promise and is, in fact, essential for the lighting domain. We have seen tremendous transitions due to technological innovations. At the same time, insights on light’s effects on humans have both deepened and diversified. But doing good research in lighting has not gotten easier and perhaps all of us should try to be a little more aware of important insights and theories from others’ perspectives. As was argued in the Introduction, just because one wants to study biological effects of light does not mean that psychological, visibility, or comfort effects of one’s manipulations will not play a role or vice versa. There may not be a way to totally prevent or circumvent the confounding and ambiguity issues raised above, but a thorough awareness of such effects may help us to contemplate better designs and better strategies in research for quantifying, controlling, and manipulating light and for selecting the best parameters, mediators, and moderators. Embracing such strategies should incrementally advance our insights and, importantly, promote the integration of theory in the lighting domain. Hence, an understanding of the theoretical structure outlined in (and detailed in Section 2) should inform future lighting research. Similarly, awareness may sensitize light designers to the multiformity of light effects and the optimal selection of design parameters in a given application.
Acknowledgments
The author thanks the editor, the guest editor, and two anonymous reviewers for their thoughtful comments and suggestions on earlier versions of this article.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes
1. The debate regarding the causal mechanism behind these findings is still ongoing—some attribute them to observer effects, novelty effects, or demand characteristics (Olson et al. Citation2004); a fairly recent analysis of the original data even claims that the data patterns were not really as remarkable as often reported and showed “Monday morning effects” rather than what is generally implied with Hawthorne effects (Levitt and List Citation2011).
2. A third important class of mechanisms through which light exposure affects functioning pertains to light falling on the skin. Extreme exposure to ultraviolet, visible, and infrared radiation can damage the skin through both thermal and photochemical mechanisms (e.g., see Boyce Citation2010). But, at the positive end, light on the skin is also responsible for the production of vitamin D and serotonin. Via this route it potentially affects mood, cognitive performance, and health (Wacker and Holick Citation2013), but in the present text we limit ourselves to effects of light information received and processed by the eyes.
3. Admittedly, photic information is also processed through additional neural pathways not belonging to either group, but we will limit ourselves to these two broad classes (Ward Citation2015).
References
- [CIE] Commission Internationale de l’Eclairage. 1995. Discomfort glare in interior lighting. Vienna (Austria): CIE. Publication No: CIE 117–1995.
- Alvarez GG, Ayas NT. 2004. The impact of daily sleep duration on health: A review of the literature. Prog Cardiovasc Nurs. 19:56–59.
- Alvaro PK, Roberts RM, Harris JKA. 2013. Systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 36:1059–68.
- Aparicio JA, Arranz I, Matesanz BM, Vizmanos JG, Padierna L, González VR, Mar S, Menéndez JA, Issolio L. 2010. Quantitative and functional influence of surround luminance on the letter contrast sensitivity function. Ophthalmic Physiol Opt. 30(2):188–99.
- Baron RA, Rea MS, Daniels SG. 1992. Effects of indoor lighting (illuminance and spectral distribution) on the performance of cognitive tasks and interpersonal behaviors: the potential mediating role of positive affect. Motiv Emot. 16(1):1–33.
- Berman SM, Bullimore MA, Jacobs RL, Bailey IL, Gandhi. 1994. An objective measure of discomfort glare. J Illum Eng Soc. 23(2):40–49.
- Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 295:1070–73.
- Beute F, de Kort YAW. 2013. Let the sun shine! Measuring explicit and implicit preference for environments differing in naturalness, weather type and brightness. J Environ Psychol. 36:162–78.
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. 1996. Dose-response relationships for resetting of human circadian clock by light. Nature. 379(6565):540.
- Borisuit A. 2013. The impact of light including non-image forming effects on visual comfort [ Dissertation]. Lausanne (Switzerland): École Polytechnique Fédérale de Lausanne.
- Boyce PR. 2010. The impact of light in buildings on human health. Indoor Built Environ. 19(1):8–20.
- Boyce PR. 2014. Human factors in lighting. 3rd ed. London (UK): Crc Press.
- Cai H, Chung T. 2012. Evaluating discomfort glare from non-uniform electric light sources. Light Res Technol. 45(3):267–94.
- Cajochen C. 2007. Alerting effects of light. Sleep Med Rev. 11(6):453–64.
- Cajochen C, Chellappa SL, Schmidt C. 2014. Circadian and light effects on human sleepiness-alertness. In: Garbarino S, editor. Sleepiness and human impact assessment. Milan (Italia): Springer. p. 9–22.
- Czeisler CA. 2013. Perspective: casting light on sleep deficiency. Nature. 497:S13.
- Dijk DJ, Duffy JF, Czeisler CA. 1992. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1(2):112–17.
- Donners MAH, Vissenberg MCJM, Geerdinck LM, van den Broek-Cools JHF, Buddemeijer-Lock A. 2016. A psychophysical model of discomfort glare in both outdoor and indoor applications. CIE 2016 “Lighting Quality and Energy Efficiency”. Melbourne, Australia. March 3–5, 2016.
- Duffy JF, Wright KP Jr. 2005. Entrainment of the human circadian system by light. J Biol Rhythms. 20(4):326–38.
- Durak A, Camgöz Olguntürk N, Yener C, Güvenç D, Gürçinar Y. 2007. Impact of lighting arrangements and illuminances on different impressions of a room. Build Environ. 42:3476–82.
- Elliot AJ, Maier MA. 2014. Color psychology: effects of perceiving color on psychological functioning in humans. Annu Rev Psychol. 65:95–120.
- Flynn JE, Spencer TJ, Martyniuk O, Hendrick C. 1973. Interim study of procedures for investigating the effect of light on impression and behavior. J Illum Eng Soc. 3:87–94.
- Galasiu AD, Veitch JA. 2006. Occupant preferences and satisfaction with the luminous environment and control systems in daylit offices: A literature review. Energy Build. 38(7):728–42.
- Geerdinck LM. 2012. Glare perception in terms of acceptance and comfort [ Master’s thesis]. Eindhoven (The Netherlands): Eindhoven University of Technology (TUE). 74p.
- Geerdinck LM, Van Gheluwe JR, Vissenberg MCJM. 2014. Discomfort glare perception of non-uniform light sources in an office setting. J Environ Psychol. 39:5–13.
- Gloriani AH, Matesanz BM, Barrionuevo PA, Arranz I, Issolio L, Mar S, Aparicio JA. 2016. Influence of background size, luminance and eccentricity on different adaptation mechanisms. Vision Res. 125:12–22.
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 12;2(31):31ra33.
- Greenwood DJ, Levin M. 1998. Introduction to action research: Social research for social change. Thousand Oaks (CA): Sage.
- Haans A, de Kort YAW. 2012. Light distribution in dynamic street lighting: two experimental studies on its effects on perceived safety, prospect, concealment, and escape. J Environ Psychol. 32(4):342–52.
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 295:1065–71.
- Hawkes RJ, Loe DL, Rowlands E. 1979. A note towards the understanding of lighting quality. J Illum Eng Soc. 8:111–20.
- Hood DC, Finkelstein MA. 1986. Sensitivity to light. In: Boff K, Kaufman L, Thomas J, editors. Handbook of perception and human performance, vol. 1: sensory processes and perception, chapter 5. New York (NY): John Wiley and Sons. p. 5–1 to 5–3.
- Houser KW, Tiller DK, Bernecker CA, Mistrick RG. 2002. The subjective response to linear fluorescent direct/indirect lighting systems. Light Res Technol. 34:243–64.
- Huiberts LM, Smolders KCHJ, de Kort YAW. 2016. Non-image forming effects of illuminance level: exploring parallel effects on physiological arousal and task performance. Physiol Behav. 164:129–39.
- Huiberts LM, Smolders KCHJ, de Kort YAW. 2017. Seasonal and time-of-day variations in acute non-image forming effects of illuminance level on performance, physiology and subjective well-being. Chronobiol Int. 34(7):827–44.
- Issolio L, Colombo EM. 2006. Brightness for different surround conditions: the effect of transient glare. Percept Psychophys. 68(4):702–09.
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. 2003. A phase response curve to single bright light pulses in human subjects. J Physiol. 549(3):945–52.
- Kuijsters A, Redi J, De Ruyter B, Heynderickx I. 2015. Lighting to make you feel better: improving the mood of elderly people with affective ambiences. PLoS One. 10(7):e0132732.
- Küller R, Ballal S, Laike T, Mikellides B, Tonello G. 2006. The impact of light and colour on psychological mood: a cross-cultural study of indoor work environments. Ergonomics. 49(14):1496–507.
- te Kulve M. 2018. Interactions between light and temperature perception [ Dissertation]. Maastricht, The Netherlands: Maastricht University.
- te Kulve M, Schellen L, Schlangen LJ, van Marken Lichtenbelt WD. 2016. The influence of light on thermal responses. Acta Physiol. 216(2):163–85.
- de Kort YAW, Veitch JA. 2014. From blind spot into the spotlight. J Environ Psychol. 39:1–4.
- Lakens D, Fockenberg DA, Lemmens KPH, Ham J, Midden CJH. 2013. Brightness differences influence the evaluation of affective pictures. Cognition Emotion. 27(7):1225–46.
- Lasauskaite R, Cajochen C. 2018. Influence of lighting color temperature on effort-related cardiac response. Biol Psychol. 132:64–70.
- Levitt SD, List JA. 2011. Was there really a Hawthorne effect at the Hawthorne plant? An analysis of the original illumination experiments. Am Econ J Appl Econ. 3(1):224–38.
- Lok R, Smolders KCHJ, Beersma DGM, de Kort YAW. 2018. Light, alertness, and alerting effects of white light: a literature overview. J Biol Rhythms. 33(6):589–601.
- Lucas RJ. 2013. Mammalian inner retinal photoreception. Current Biol. 23(3):R125–33.
- Lucas RJ, Peirson SN, Berson DM, Brown T, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, et al. 2014. Measuring and using light in the melanopsin age. Trends Neurosci. 37:1–9.
- Mehta R, Zhu RJ. 2009. Blue or red? Exploring the effect of color on cognitive task performances. Science. 323(5918):1226–29.
- Minors DS, Waterhouse JM, Wirz-Justice A. 1991. A human phase-response curve to light. Neurosci Lett. 133(1):36–40.
- Münch M, Kawasaki A. 2013. Intrinsically photosensitive retinal ganglion cells: classification, function and clinical implications. Curr Opin Neurol. 26(1):45–51.
- Nikunen H, Korpela KM. 2012. The effects of scene contents and focus of light on perceived restorativeness, fear and preference in nightscapes. J Environ Plann Manage. 55(4):453–68.
- O’Donell BM, Colombo EM, Boyce PR. 2011. Colour information improves relative visual performance. Light Res Technol. 43(4):423–38.
- Olson R, Verley J, Santos L, Salas C. 2004. What we teach students about the Hawthorne studies: A review of content within a sample of introductory IO and OB textbooks. Industrial-Organizational Psychol. 41(3):23–39.
- Pierson C, Wienold J, Bodart M. 2018. Review of factors influencing discomfort glare perception from daylight. Leukos. 14(3):111–48.
- Rea MS. 2000. IESNA lighting handbook: reference & application. 9th ed. New York (NY): Illuminating Engineering Society of North America.
- Rea MS, Ouellette MJ, Kennedy ME. 1985. Lighting and task parameters affecting posture, performance and subjective ratings. J Illum Eng Soc. 14(2): 231-38.
- Rea MS, Ouellette MJ. 1988. Visual performance using reaction times. Light Res Technol. 20(4):139–53.
- Roufs JAJ, Boschman MC. 1991. Visual comfort and performance. In: Roufs JAJ, editor. The man-machine interface. London (UK): MacMillan Press. p. 24–40.
- Scheir GH, Hanselaer P, Bracke P, Deconinck G, Ryckaert WR. 2015. Calculation of the unified glare rating based on luminance maps for uniform and non-uniform light sources. Build Environ. 84:60–67.
- Schietecat AC. 2018. Cross-modal associations between aggression and light [ Doctoral dissertation]. The Netherlands: Eindhoven University of Technology.
- Schietecat AC, Lakens D, de Kort YAW, IJsselsteijn WA. 2018a. Predicting context-dependent cross-modal associations with dimension-specific polarity attributions. Part 2—red and valence. Collabra Psychol. 4(1):21.
- Schietecat AC, Lakens D, IJsselsteijn WA, de Kort YAW. 2018b. Predicting context-dependent cross-modal associations with dimension-specific polarity attributions. Part 1 - Brightness and aggression. Collabra Psychol. 4(1):14.
- Smolders KCHJ, de Kort YAW. 2014. Bright light and mental fatigue: effects on alertness, vitality, performance and physiological arousal. J Environ Psychol. 39:77–91.
- Smolders KCHJ, de Kort YAW. 2017. Investigating daytime effects of correlated colour temperature on experiences, performance, and arousal. J Environ Psychol. 50:80–93.
- Smolders KCHJ, de Kort YAW, Cluitmans PJM. 2012. A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol Behav. 107:7–16.
- Smolders KCHJ, de Kort YAW, van Den Berg S. 2013. Daytime light exposure and feelings of vitality: results of a field study during regular weekdays. J Environ Psychol. 36:270–79.
- Smolders KCHJ, Peeters ST, Vogels IMLC, de Kort YAW. 2018. Investigation of dose-response relationships for effects of white light exposure on correlates of alertness and executive control during regular daytime working hours. J Biol Rhythms. 33(6):649–661.
- Souman JL, Borra T, de Goijer I, Schlangen LJ, Vlaskamp BN, Lucassen MP. 2018. Spectral tuning of white light allows for strong reduction in melatonin suppression without changing illumination level or color temperature. J Biol Rhythms. Jul 1;0748730418784041.
- Souman JL, Tinga AM, Te Pas SF, Van Ee R, Vlaskamp BN. 2017. Acute alerting effects of light: A systematic literature review. Behav Brain Res. 337: 228–39.
- Steidle A, Werth L. 2014. In the spotlight: brightness increases self-awareness and reflective self-regulation. J Environ Psychol. 39:40–50.
- Steidle A, Werth L, Hanke EV. 2011. You can’t see much in the dark. Soc Psychol. 42(3):174–84.
- Stephenson KM, Schroder CM, Bertschy G, Bourgin P. 2012. Complex interaction of circadian and non-circadian effects of light on mood: shedding new light on an old story. Sleep Med Rev. 16(5):445–54.
- Stokkermans M, Vogels I, de Kort YAW, Heynderickx I. 2018. Relation between the perceived atmosphere of a lit environment and perceptual attributes of light. Light Res Technol. 50(8): 1164–78.
- Terman M, Terman JS. 2005. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 10(8):647–63.
- Van Den Wymelenberg K, Inanici M, Johnson P. 2010. The effect of luminance distribution patterns on occupant preference in a daylit office environment. J Illum Eng Soc. 07(02):103–22.
- Vandewalle G, Maquet P, Dijk DJ. 2009. Light as a modulator of cognitive brain function. Trends Cogn Sci. 13(10):429–38.
- Vandewalle G, Schwartz S, Grandjean D, Wuillaume C, Balteau E, Degueldre C, Schabus M, Phillips C, Luxen A, Dijk DJ, et al. 2010. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci. 107(45):19549–54.
- Veitch JA. 2001. Psychological processes influencing lighting quality. J Illum Eng Soc. 30(1):124–40.
- Veitch JA, Newsham GR, Boyce PR, Jones CC. 2008. Lighting appraisal, well-being and performance in open-plan offices: A linked mechanisms approach. Light Res Technol. 40(2):133–51.
- Veitch JA, Stokkermans MG, Newsham GR. 2011. Linking lighting appraisals to work behaviors. Environ Behav. 45(2):198–214.
- Vogels IMLC, de Vries M, van Erp TAM. 2008. Effect of coloured light on atmosphere perception. Association Internationale de la Couleur (AIC). Stockholm (Sweden): Scandinavian Colour Institute. Paper 60, p. 1–4.
- Vos JJ. 2003. Reflections on glare. Light Res Technol. 35(2):163–75.
- de Vries A, Souman JL, de Ruyter B, Heynderickx I, de Kort YAW. 2018. Lighting up the office: the effect of wall luminance on room appraisal, office workers’ performance, and subjective alertness. Build Environ. 142:534–43.
- Wacker M, Holick MF. 2013. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinology. 5(1):51–108.
- Ward J. 2015. The student’s guide to cognitive neuroscience. 3rd ed. New York (NY): Psychology Press.
- Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. 2003. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 342(1–2):37–40.
- Wirz-Justice A. 2007. How to measure circadian rhythms in humans. Medicographia. 29(1):84–90.
- Yang Y, Ronnier Luo M, Ma SN. 2017. Assessing glare. Part 2: modifying unified glare rating for uniform and non-uniform led luminaires. Light Res Technol. 49:727–42.
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. 2000. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 526(3):695–702.
- Zhong CB, Bohns VK, Gino F. 2010. Good lamps are the best police: darkness increases dishonesty and self-interested behavior. Psychol Sci. 21(3):311–14.
