 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Architectural lighting has potent biological effects but applied lighting practices that capitalize on this potential have been limited. In this review, we endeavor to consolidate and synthesize key references that will be useful for lighting professionals, with the goal of supporting knowledge translation into pragmatic lighting strategies. Specifically, we explain relevant terminology, outline basic concepts, identify key references, provide a balanced overview of the current state of knowledge, and highlight important remaining questions. We summarize the physiological effects of light on human health and well-being, including a description of the processes underlying the photic regulation of circadian, neuroendocrine, and neurobehavioral functions. We review seminal work elucidating the elements mediating the potency of light for these physiological responses, with specific attention to factors critical for interpreting those findings. In parallel, we explain and endorse melanopic Equivalent Daylight Illuminance () as the preferred measure to quantify the biological potency of light. Ultimately, while future studies are necessary to further facilitate the translation of laboratory knowledge to domestic and workplace settings, the immediate potential for applied lighting to better support human health is clear. Aiming for integrative lighting solutions that have biologically high potency light during the day and low potency during the night is perhaps the most immediate improvement to be made in order to better support applications for humans.
1. Introduction
Architectural lighting is largely engineered to support visual performance, maintain visual comfort, appropriately render colors, address esthetic and psychological considerations, and maximize energy efficiency (DiLaura et al. Citation2011). In the past forty years, new scientific research has dramatically expanded our understanding of human responses to light, including a variety of biological effects that are distinct from vision and that have a profound influence on human physiology and behavior. Specifically, numerous studies have demonstrated that light also modulates sleep, circadian rhythms, alertness, fatigue, body temperature, neuroendocrine function, neurocognitive responses, and mood.
Our understanding of the specific physiology underlying these non-image forming functions has rapidly increased in the past two decades and continues to evolve, with constant discoveries. In this paper, we will first summarize key findings relating to the regulation of the circadian timing system, melatonin suppression, and acute alerting properties, as these nonvisual responses to light are among the most well characterized and have direct implications for human health and performance. It is important, however, to recognize that light affects many physiological functions, and is known, for example, to have direct effects on sleep (Prayag et al. Citation2019), mood (Bedrosian and Nelson Citation2017; Fernandez et al. Citation2018), and pupillary response (Gooley et al. Citation2012; Spitschan Citation2019). In addition, seminal work on the various parameters of light that mediate its potency for biological and behavioral responses will be presented, with a bias toward studies in humans. It is noteworthy that most fundamental properties of the circadian system are highly conserved in mammals, and animal research is referenced when information can only be derived in model systems or when it directly supports evidence from human studies.
In describing research findings, those that appear most ready for translation into domestic and working environments are highlighted. We discuss what is known and what is unknown, with a further effort to classify the information presented into three basic categories along a spectrum of certainty. In the first case, we detail what we believe to be well-established phenomena due to extensive and converging lines of evidence, including consistency of findings from research conducted in model systems, controlled laboratory studies of humans and/or applied work under naturalistic conditions. A second category includes largely agreed upon understandings for which there remains some uncertainty due to inconsistencies and/or scarcity in the literature. In such cases, we suggest possible reasons for lack of concordance between studies and propose future work that may serve to reconcile these differences. Some domains we cover are relatively under-explored in terms of empirical research; however, the potential impacts along with findings to date suggest that further studies are warranted.
Our review only touches briefly on the physiological and behavioral effects of light as they relate to clinical populations, children and adolescents, and those working nonstandard schedules, since we view these as unique populations. Focusing on adult day workers allows us to make more specific recommendations than if we were to develop guidelines that also apply to these special cases. Finally, we conclude with a summary that reviews the main points of the paper. Here, we describe immediate improvements that may be implemented as well as future studies that are necessary in order to develop novel lighting strategies that better support human physiological health and well-being.
1.1. Neuroanatomy and physiology underlying biological and behavioral responses to light
The physiological effects of light are mediated by the eye in humans. Light entering the eye stimulates retinal photoreceptors that convert photic information into neuronal signals, which get transmitted via ganglion cells to various regions of the brain (). For many years, it was thought that there were only two classes of photoreceptors in the human eye—the rods and cones; however, another very different photoreceptor type was discovered in the mammalian eye about two decades ago. These retinal photoreceptors are specialized ganglion cells that contain the photopigment melanopsin and are intrinsically sensitive to light, and were therefore called intrinsically photosensitive retinal ganglion cells (ipRGCs) (Berson et al. Citation2002; Hattar et al. Citation2002; Provencio et al. Citation1998, Citation2000).
Fig. 1. Schematic illustration of the neuroanatomical underpinnings of physiological effects of light. The intrinsically photosensitive retinal ganglion cells (ipRGCs) transmit environmental light information via the retinohypothalamic tract (RHT) to the central clock in the brain (SCN, suprachiasmatic nuclei); other direct projections of ipRGCs include thalamic and other brain regions. The response will depend on the light characteristics and/or other mediating factors. LGN: lateral geniculate nucleus; IGL: intergeniculate leaflet.
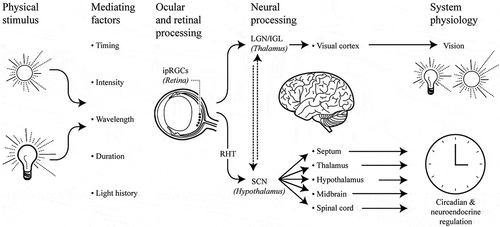
From the retina, light information is transmitted to multiple targets in the human brain via two major pathways. The visual pathway employs the optic nerve, chiasm and tract, which sends information to structures involved in image formation, including the lateral geniculate nucleus (LGN), intergeniculate leaflet (IGL) and visual cortex of the occipital lobe. The retinohypothalamic tract (RHT) is responsible for carrying light information from the retina to the suprachiasmatic nuclei (SCN) in the hypothalamus. The SCN serves as the biological clock in mammals and has numerous downstream connections with other central nervous system structures, including the spinal cord and brain (e.g. septum, thalamus, midbrain and other regions of the hypothalamus). The RHT also projects to other nonvisual nuclei and regulatory centers of the brain that are independent of the circadian pacemaker (Gooley et al. Citation2003; Hattar et al. Citation2006).
1.2. Action spectra
The method for determining the spectral sensitivity of a photoreceptor is via the construction of an action spectrum, which is by definition, the relative response of a living organism to different wavelengths of visible and near-visible electromagnetic radiation (Horspool and Lenci Citation2003). Action spectra studies have served to elucidate underlying physiology while simultaneously providing practical guidance for optimizing the spectral quality of light for human health.
There are two basic types of action spectra: polychromatic and analytic (Coohill Citation1999). Generally, when investigators started exploring light-sensitive biological reactions, they began by determining polychromatic action spectra using either narrow bandwidth light sources (with a FWHM of >/=16 nm) or broad bandwidth light sources that can span the entire visible spectrum (Brainard and Hanifin Citation2005). These are practically useful, as free-living individuals are exposed to polychromatic light in domestic and work settings. While polychromatic action spectra may have translational value, they are less informative in terms of elucidating underlying mechanisms.
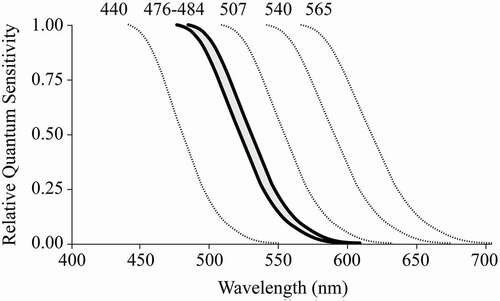
Analytic action spectra are developed using monochromatic light with FWHM of 15 nm or less. In addition to the absolute peak, which is often reported, the action spectrum curve provides the relative sensitivity of the photoreceptors across various wavelengths of light. For example, across action spectra studies of various physiological responses to light, the potency of a 555 nm photic stimulus is relatively higher than a 560 nm stimulus, which is slightly higher than 565 nm, and so on and so forth. Separate action spectra studies have demonstrated that the photopigment melanopsin is most sensitive to short-wavelength light, with a peak response at ~480 nm in vivo (Bailes and Lucas Citation2013; Panda et al. Citation2005; Torii et al. Citation2007), which is distinct from the peak spectral sensitivities of the classical rod and cone photoreceptors (Stockman and Sharpe Citation1999) (see ). Human rods peak at 507 nm; M- and L-cones peak at longer wavelengths (540 and 565 nm); and S-cones peak at shorter wavelengths of ~ 440 nm. This peak sensitivity is a relative sensitivity, meaning that receptors will respond to shorter and longer wavelength stimulation, but to a lesser extent than when stimulated at peak spectral sensitivity. The short-wavelength sensitivity of the ipRGCs is, at least in part, reflected in the circadian, neuroendocrine and neurobehavioral responses to light described in humans (Brainard et al. Citation2001; Nowozin et al. Citation2017; Prayag et al. Citation2019b, Thapan et al. Citation2001, see also Table S1). Recent work by Spitschan et al. (Citation2019b), using silent substitution methods, is consistent with prior work that suggests the spectral sensitivity of neuroendocrine and alerting responses is primarily attributed to melanopsin-containing ipRGCs rather than short-wavelength cones (which peak at 440 nm).
Fig. 2. Long wavelength limb of the spectral sensitivity functions for rod, cones, and melanopsin photopigment. Illustration includes the range of results (upper and lower bounds) for the melanopsin absorption spectra, based on Bailes and Lucas, Citation2013, Panda et al., Citation2005, and Torii et al., Citation2007.
In general, we now know that all four rod and cone human photoreceptors also contribute to the firing rate of ipRGCs. Studies to date demonstrate a complicated interplay between the classical photoreceptors and ipRGCs, with the relative contribution of each photoreceptor hinging on both the specific photic response and the context. For example, there is no single spectral sensitivity function that can account for all aspects of the pupillary response (Gooley et al. Citation2012), which represents one of the simpler physiological effect of light to observe. Consequently, Lucas et al. (Citation2014) urged that our knowledge about the differential activation of photoreceptors by a given light source(s) be incorporated into the measurement and reporting of light. The toolbox proposed by Lucas et al. (Citation2014) thus considers the spectral power distribution of incident light within the context of the five photopigment-specific sensitivity functions displayed in .
1.3. Quantifying the biological potency of light
The approach to measuring and reporting light by Lucas and colleagues was echoed by the Commission Internationale de l’Éclairage (CIE) in a technical note (Citation2015), which was revised and formalized via an internationally-balloted consensus within CIE S 026/E:2018 CIE System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light (CIE Citation2018). This international standard represents a significant step in providing guidance to the broader lighting and scientific communities as to how best to quantify light exposure. For clarification purposes, the 10 nm differential between the aforementioned 480 nm peak spectral sensitivity for melanopsin and the 490 nm peak described in the CIE technical note is contextual, as the former is a function of photon flux whereas the latter accounts for pre-receptoral filtering and stimulus energy. When incident flux density is most relevant, photic stimuli for nonvisual physiological responses should not be quantified using illuminance measures in photopic lux, but rather as melanopic equivalent daylight illuminance, abbreviated as melanopic EDI or , also in units of lux. The “photometric equivalent” concept can be applied to quantities other than illuminance, such as the luminous energy (lm-s), luminous intensity (cd or lm/sr) and luminance (cd/m2 or lm/sr-m2). Translating previously reported lux values into melanopic EDI (or another photometric equivalent illuminance) is often hampered by incomplete information about the spectral composition of the light sources employed, which limits replication and comparison across studies.
The consensus-based metrics from CIE S 026/E:2018 provide a systematic way of characterizing light spectra based on photoreceptor responses, and we encourage use of this system. Ultimately, it is important to keep in mind that spectrally-derived measures are subject to future revision, especially during this rapid period of knowledge acquisition. We therefore join others (e.g. Knoop et al. Citation2019, Spitschan et al. Citation2019a) in requesting that researchers report the spectral power distributions (SPDs) of all photic stimuli experienced by study participants. The reporting of absolute SPDs will facilitate replication and comparisons across studies as well as back-testing of future models.
1.4. Physiological effects of light: description and basic methods
Light can markedly influence a host of physiological functions, including circadian entrainment and phase shifting of the circadian system, neuroendocrine regulation, sleep, alertness, learning and memory, mood, and pupillary responses. In this manuscript, we mainly focus on studies of phase shifting, melatonin suppression, and alertness, as these functions have been the most extensively studied in both animal models and humans. It is important to keep in mind, however, that outside of the laboratory, each physiological effect of light does not occur in isolation; the same light may simultaneously influence all or some of these various functions. Thus, integrative lighting strategies must consider the broader effects of light on human health and performance. Multiple-outcome studies are limited to date, but will be useful to further guide future applications.
1.4.1. Phase shifting and entrainment
In humans, nearly every cell of the body contains molecular level rhythms, generated by cellular circadian clock machinery, which regulate cell metabolism, immune responses, DNA repair, and mitochondrial function (Sulli et al. Citation2018). Recent work has shown that beyond this clock in the cell, the SCN along with a network of clocks in peripheral organs, coordinate various physiological functions that result in circadian (“circa” meaning about and “dies” meaning a day) peaks and troughs in physiology, including core body temperature (CBT), hormone levels (e.g. melatonin, cortisol), energy metabolism patterns, reproductive cycles, and immune function variability across the day (see for example Pilorz et al. Citation2018, for an in-depth review). On a behavioral level, feeding-fasting, sleep-wake and rest-activity cycles, as well as fluctuations in cognitive function, are modulated by the circadian clock. Under ideal conditions, cellular, physiological and behavioral events are integrated and coherent at every level (Vetter Citation2020).
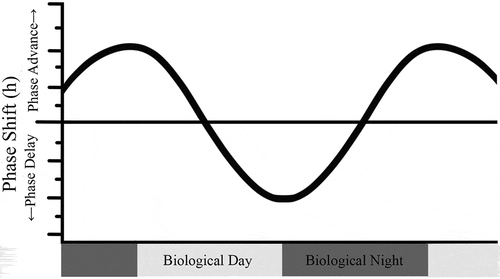
Light is the primary regulator of human circadian rhythms and a robust timekeeping signal, which together with a functional circadian system, will lead to stable entrainment (Roenneberg et al. Citation2013). Under normal conditions, circadian entrainment occurs via daily light-induced shifts that adjust for the difference between the endogenous period of the rhythm and the more precise 24-hour period of the solar light-dark cycle. Even just a single brief pulse of light is enough to shift the clock, and both the magnitude and direction of that phase shift is dependent on the timing of photic administration (). For example, light of sufficient potency early in the biological night elicits a delay in rhythms whereas light much later in the night will cause an advancing shift to an earlier time. Such shifts reflect the differential effects of light on the SCN at different phases of the clock in mammals, which are often described via phase response curves (PRCs) (Boivin et al. Citation1996; Czeisler et al. Citation1989; Honma and Honma Citation1988; Khalsa et al. Citation2003; Klein et al. Citation1991; Minors et al. Citation1991; Moore Citation1995; Pittendrigh et al. Citation1984).
Fig. 3. Phase Response Curve (PRC) for Light (Type 1). This schematic PRC illustrates that the magnitude of phase shifting by a light pulse will depend on the time of administration, and more specifically, on an individual’s biological (or circadian) time, which is typically based on the timing of the melatonin rhythm. Biological night refers to the time when melatonin levels are high. Figure credit: UC San Diego BioClock Studio, modified from Rueger et al., Citation2013.
1.4.2. Neuroendocrine regulation
In addition to light’s capacity to shift the circadian phase of various human hormones, light exposure during the biological night can also acutely reduce the high nocturnal levels of circulating melatonin, which is produced and secreted by the pineal gland. While there are substantial inter-individual differences in absolute melatonin levels, nocturnal melatonin is robustly suppressed by bright light in most individuals (Arendt Citation2006; Bojkowski et al. Citation1987; Lewy et al. Citation1980; McIntyre et al. Citation1989). Laboratory studies of melatonin suppression by light in humans involve collection of blood, saliva or urine at night, when the hormone typically rises in healthy individuals. Human studies employing the acute melatonin suppression response as a primary dependent variable are generally less labor- and time-intensive than the protocols required for assessment of circadian phase shifting, allowing for more powerful within-subjects experimental designs and increased replication within and between labs.
1.4.3. Alertness
Light also has acute alerting properties similar to that observed after caffeine consumption (Wright et al. Citation1997). Specifically, light has been shown to significantly reduce reaction time and attentional lapses, decrease subjective sleepiness, improve alertness, and enhance performance on some neurocognitive tests (Badia et al. Citation1991; Cajochen Citation2007; Cajochen et al. Citation2011; Rahman et al. Citation2014; Souman et al. Citation2018). Laboratory studies suggest that the alerting effects of light may vary by time of day, though this response has largely been characterized during the night (Rahman et al. Citation2014), and improvements in alertness may be minimal in well-rested individuals during daytime (Lok et al. Citation2018). It is also important to note that not all studies have identified a consistent enhancement of all measures of alertness and neurocognitive responses (Rahman et al. Citation2017; Segal et al. Citation2016; Sletten et al. Citation2017; Souman et al. Citation2018). Alertness is most commonly characterized via validated subjective alertness measures (e.g. Karolinska Sleepiness Scale, KSS, Akerstedt and Gillberg Citation1990) and the objective assessment of sustained attention (psychomotor vigilance test, PVT, Dorrian et al. Citation2004). Such measures have the advantage of being sensitive to sleep deprivation with a large literature for comparison, though the generalizability to more practical outcomes is debated. Alerting and cognitive effects of light are often of special interest in the context of educational institutions and workplaces seeking to improve performance. Around-the-clock organizations, in particular, may aim to enhance nighttime alertness levels of employees, and thereby reduce error rates and increase safety. Few studies, however, have tested the alerting or performance benefits of intentionally designed architectural lighting solutions in relation to operational outcomes.
2. Factors that mediate the physiological responses to light: Key findings from laboratory studies
Broadly, there are two categories of elements that mediate human biological and behavioral responses to light: 1) the physical characteristics of the light stimuli and 2) the ocular/neural physiology that modifies and responds to the stimuli. The physical characteristics of light stimuli include light intensity, spectrum, timing and duration. The ocular/neural physiology that modifies or responds to light stimuli includes conscious and reflexive behaviors of the eye, transduction through the ocular media, pupillary dilation, photoreceptor sensitivity and distribution, neural integration over time and space, and state of retinal and neural adaptation (photic history). Some of these elements have been more extensively studied than others (Brainard et al. Citation1997; Lucas et al. Citation2014).
Most of the work that has characterized the mediating elements of light in terms of nonvisual physiological responses has necessarily been conducted under highly controlled conditions. The general findings of these studies are likely to apply to real world environments; however, the specifics may differ. This is because most of the research includes methodology that controls for gaze behavior and/or pharmacologically dilates pupils. Such controlled conditions serve to maximize the proportion of measured light that hits the retina, which is appropriate for isolating variables of interest but limits direct translation since outside of the laboratory, pupils will normally adjust to light levels and gaze behavior will vary across time. Specifically, light levels reported to obtain a particular response in controlled laboratory studies likely represent an underestimation of the light levels required to elicit a comparable response under more naturalistic conditions. Empirical evidence from studies in applied settings is scarce, presumably due to multiple factors, including the complexity of the photic stimulus and the difficulty of measuring individual-level light exposure in the field (e.g. see Adamsson et al. Citation2018). In addition, obtaining state-of-the art physiological measures outside of the laboratory can be challenging.
2.1. Intensity
Phase shifting, melatonin suppression, and alertness all demonstrate a characteristic light intensity-dependent response, typically measured for a fixed exposure duration. Dose-response data may be fit to a parametric model and provide a dynamic range of responsiveness, including irradiances within the boundaries of threshold and maximum response. The threshold is the amount of light required to elicit a detectable difference from 0, and the maximum response dose represents irradiances at which saturation is achieved (i.e. additional light will not increase the response further). The half-saturation constant (ED50) is the photic dose eliciting a half maximum response and is often used as a point of comparison for determining relative sensitivity, with relatively lower ED50 values indicating greater sensitivity to light. In other words, increased sensitivity is inferred when lower light levels are capable of eliciting an equivalent (ED50 or half-saturation) response.
Relevant factors and key parameters from landmark studies of dose-response to light for melatonin suppression, phase resetting, and acute alerting are summarized in Table S2. In general, results demonstrate a characteristic non-linear, sigmoid function across various physiological responses in both humans and model systems. This means that for all the studied physiological effects of light (even in different species), there is a similar steep increase in the response to light with increasing intensities, which eventually hits a point of saturation wherein adding more light cannot further increase the response.
In comparing the ED50 values between studies (Table S2), there appear to be species differences in photic sensitivity, as indicated by significantly lower values of ED50 for work in hamsters (Brainard et al. Citation1983; Glickman et al. Citation2012; Nelson and Takahashi Citation1991) versus humans (Brainard et al. Citation2001, Citation2015, Citation2008; Cajochen et al. Citation2000; Gooley et al. Citation2010; Thapan et al. Citation2001; West et al. Citation2011; Zeitzer et al. Citation2000). Furthermore, two of the listed human studies reported the need for significantly greater intensities of light in order to achieve a half-saturation response as compared to other human work, across output measures (Cajochen Citation2007; Zeitzer et al. Citation2000). This is likely due to the following methodological factors: 1) the use of polychromatic light (and thus a less targeted, optimized stimulus) and 2) freely responsive pupils, meaning less light will reach the retina, as light also causes pupil constriction when the eyes are not artificially dilated via pharmacological agents. The suggestion that the differences in sensitivity are attributable to differences in methodology is supported by the fact that when focusing on human studies with more similar lighting specifications and pupillary conditions, the ED50 range becomes relatively narrow (3.47x1012 to 2.0 × 1013 photons cm−2s−1). In line with this, when the spectrum is not optimized and the pupils are not dilated, the ED50 occurs at generally higher intensities (7.94x1013 to 1.0 × 1014 photons cm−2s−1).
2.2. Spectral quality
One way to reduce the intensity of the light needed to induce a particular physiological effect is by optimizing spectrum. Comparable phase resetting, melatonin suppression, and alerting responses are obtained with lower levels of short-wavelength light as compared to all other tested wavelengths. Indeed, short wavelength radiation is the most potent region of the spectrum for stimulating a variety of biological effects across species (Tables S1 and S2, Bailes and Lucas Citation2013; Berson et al. Citation2002; Brainard et al. Citation2001; Hankins and Lucas Citation2002; Hattar et al. Citation2003; Lucas et al. Citation2001; Thapan et al. Citation2001). In addition, the duration and magnitude for which a response is sustained after the light spectrum is changed and/or is altogether extinguished may vary, as there is evidence for longer lasting physiological effects of light with short versus relatively longer wavelengths (Gooley et al. Citation2010; Hanifin et al. Citation2019; Rahman et al. Citation2014).
It is important to note that the analytic action spectra listed in Table S1 employ monochromatic wavelengths of light, a standard technique in photobiology which we described earlier. Monochromatic light sources, however, are impractical for most domestic living and workplace applications. Studies with polychromatic light provide a closer approximation to what can be more realistically implemented. For example, Smith and Eastman compared high CCT with a relatively lower CCT white light source (17,000 K, 4000 lux vs. 4100 K, 5000 lux) for phase shifting, and both light sources were equally effective at delaying (Smith and Eastman Citation2009) and advancing an individual’s circadian clock (Smith et al. Citation2009b); however, this comparison without varying photic intensity limits comparisons and conclusions that can be made. Overall, work to date examining the physiological effects of polychromatic light of different spectra is limited and has yielded seemingly inconsistent results that are somewhat more difficult to interpret (Hanifin et al. Citation2019; Revell et al. Citation2010; Revell and Skene Citation2007; Sletten et al. Citation2017; Souman et al. Citation2018b).
A recent systematic review of the literature reports extensively on the influence of photic spectral quality on acute alerting properties (Souman et al. Citation2018). Alerting effects of light demonstrate a dose-response effect akin to that observed with phase resetting and melatonin suppression (Cajochen Citation2007, Table S2), and most laboratory studies using narrow bandwidth or monochromatic light stimuli show increased objective (e.g. reductions in reaction time on the psychomotor vigilance test, PVT) and subjective alertness (e.g. Karolinska Sleepiness Scale, KSS) with short versus longer wavelength photic stimuli (e.g., Cajochen Citation2007; Cajochen et al. Citation2005; Lockley et al. Citation2006; Revell et al. Citation2006).
Differences in the alerting effects of light as a function of spectral quality are not, however, consistently observed across laboratory and field studies employing polychromatic white light. In one controlled laboratory study, the effectiveness for increasing alertness by light was examined with different correlated color temperature (CCT) lamps (6500 K vs. 2500 K vs. 3000 K), and results showed enhanced subjective alertness, faster reaction times, and increased melatonin suppression in the 6500 K condition (Chellappa et al. Citation2011). Ye et al. (Citation2018) employed dynamic light with different cycle times and different ranges of CCT. They found that cycle time had little effect but that participants performed better and were more alert under lighting of higher CCT. In two other laboratory studies, afternoon light exposures yielded less consistent effects of spectrum on alerting (Sahin and Figueiro Citation2013; Segal et al. Citation2016). Further research is warranted to identify whether these differences between study findings are due to the time of day of photic exposure, the chosen outcome measures, differences in the light source and/or other methodological factors. Many past studies have also used light levels in the upper range of the dose-response curve, suggesting the lack of effect may be due to a ceiling response (Souman et al. Citation2018).
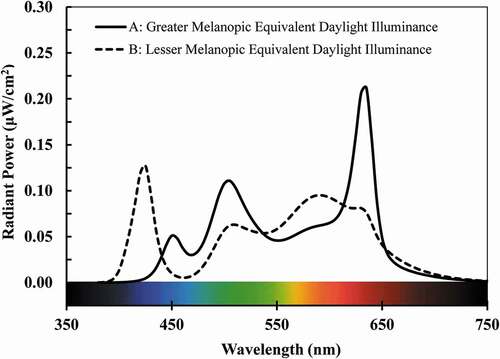
It is noteworthy that metameric polychromatic stimuli (i.e., perceived as the same color and luminance to a standard observer but with distinct spectral power distributions) may produce different physiological responses. More specifically, two metameric light sources can appear to be equivalent when characterized by chromaticity, CCT and/or luminance, while being very dissimilar when characterized using spectral power distributions (SPDs) and thus, biological potency. shows two exemplary metameric SPDs. They have equivalent CIE 1931 2° (x, y) chromaticity (0.405, 0.390) (CIE Citation2019), corresponding to a CCT of 3500 K and a Duv of zero (Ohno Citation2014, CIE 1931). If viewed directly, both sources would appear identical to the CIE 1931 2° standard observer (CIE Citation2019). They also have identical average color-rendering scores, with IES/CIE Rf values of 70 and IES Rg values of 100 (CIE Citation2017; ANSI/IES Citation2020IES). This implies that, on average, both SPDs will introduce the same magnitude of color shift within a polychromatic environment, in comparison to a reference illuminant. Yet, the sources differ in their biological potency. At 500 photopic lux, source A (solid line) provides a melanopic EDI value of 450 lux and source B provides melanopic EDI of 425 lux, a difference of about 7% (CIE Citation2018). Much larger differences in biological potency are possible if photometric and colorimetric criteria are intentionally manipulated, as is commonly done to support human visual needs (e.g. Esposito and Houser Citation2019; Royer et al. Citation2017b; Wei and Houser Citation2016).
Fig. 4. Two spectral power distributions (SPDs) at 3500 K that are a metameric match to the CIE 1931 2° Standard Observer. Melanopic content refers to the short-wavelength proportion of the light source and its relative efficacy to stimulate melanopsin, the light-sensitive biochemical in the retinal ganglion cells.
CCT alone is thus an inadequate, derived measure to estimate physiological effects of light (e.g., see also Houser Citation2017), as there is an infinite variety of spectral power distributions (SPDs) at any given CCT. Souman et al. (Citation2018b) exposed individuals to light of equivalent illuminance and CCT, but varied spectral quality. Results showed that short-wavelength enriched polychromatic white light (i.e., higher power between 450 and 500 nm) suppressed melatonin levels, while a metameric polychromatic white light source depleted in the 450 to 500 nm part of the spectrum did not result in melatonin suppression. Another recent experiment showed metameric movies, generating stimuli that differed up to threefold in their excitation of melanopsin. These participants reported feeling less tired and showed increased melatonin suppression when the movies emitted were enriched in the short-wavelength region of the spectrum, despite no differences in self-reported visual appearance (Allen et al. Citation2018). Because of the complexity of spectrally-derived quantities, care is needed to conceptualize, realize, characterize and optimize light spectra for both physiological and visual functions (Lucas et al. Citation2014; Veitch et al. Citation2019).
As noted in section 1.2., absolute SPDs should be reported. The current accepted standard for reporting illuminance is now α-opic equivalent daylight illuminance (EDI), (CIE Citation2018, Citation2019), where there is one α-opic quantity for each of the long- medium- and short-wavelength sensitive cones, rods, and the melanopsin-containing ipRGCs.
is derived from an illuminant’s absolute SPD. Future work will benefit from such universal reporting standards that include biological potency in the consideration of the lighting stimulus, as characterized with
, the α-opic quantity related to melanopsin. Spectral performance beyond biological potency is especially important for studies that endeavor to produce results with ecological validity in real-world settings, where image and non-image forming effects are both pertinent. Complete reporting will also include derived colorimetric quantities, including CCT and Duv or chromaticity (ANSI/NEMA 2017) and measures of color rendering performance based on TM-30-18 (IES Citation2018).
2.3. Timing
As described earlier, the timing of photic administration can significantly alter both the magnitude and direction of circadian phase shifting by light (see ). Similarly, the rise of melatonin and acute alerting properties are also time-dependent (Arendt Citation2006; Brainard et al. Citation1997; Cajochen Citation2007), with the largest effects during the biological night. In interpreting results across studies, as well as when developing lighting applications that address these physiological responses, consideration of an individual’s internal, biological timing is of utmost importance. Most of the research described throughout this paper administered the light stimulus in the biological night. Daylight research is, while receiving increasing attention, still currently understudied (Münch et al. Citation2020), and future multi-outcome studies examining the physiological effects of light during both night and day will be crucial to inform integrative lighting solutions for home and workplace settings.
2.4. Duration
Physiological responses to light are also influenced by the duration of photic exposure, and these effects of duration can further interact with some of the other factors that are also known to alter light response. For example, the effect of exposure duration is well illustrated by the study conducted by Gooley et al. (Citation2010), where the magnitude of melatonin suppression for various durations within a 6.5 h exposure was systematically examined for 460 and 555 nm monochromatic light, and the effect of photic duration was differentially impacted by wavelength. Specifically, 460 nm light elicited a more sustained melatonin suppression response as compared to the relatively longer wavelength stimulus. The relationship between duration and intensity is non-linear, so that shorter pulses of light have been reported to result in larger physiological effects per minute of exposure. For example, a 4100 K light source generated a one-hour phase delay with just 0.2 h of light exposure (at 7,669 lux) and a 2.6 h phase delay with 4 h of similar intensity (8,396 lux), suggesting that the shorter duration stimulus was approximately five times more effective at shifting the circadian rhythm (Chang et al. Citation2012).
Within the range of responsiveness, there are practical and physiological cost-benefit considerations to increasing intensity versus duration of photic exposure. Shorter exposures may be more convenient and implementable; yet, higher photic intensities may then be required to obtain a particular response and may result in undesirable side-effects such as glare, headache, eyestrain, and nausea (Terman and Terman Citation1999). Such side-effects generally remit spontaneously or with photic reduction but can alter acceptance and/or compliance (Glickman et al. Citation2006). For architectural lighting applications in educational, workplace and residential settings, the duration of exposure is likely to be extended whereas there may be other scenarios where shorter exposures are more typical. Thus, exposure duration represents another factor that must be considered when attempting to optimize the physiological effects of light in practice.
2.5. Light history
While the influence of prior photic exposure on subsequent response to light is generally appreciated, the duration of time that is relevant remains uncertain. Consequently, human laboratory research will often attempt to control for light history effects by maintaining study participants in dimly lit or completely dark conditions prior to administration of a light pulse, though the duration of that period of adaptation varies across studies (see Table S2, for some examples). Within the small body of work characterizing the effects of light history, most studies that have begun to tackle the issue have reported rather robust effects. For example, animal studies examining phase resetting by light have found that the magnitude of a phase shift is significantly reduced after pre-exposure to a non-saturating stimulus (Nelson and Takahashi Citation1999). Stability of entrainment to 22 h light-dark (LD) cycles in Syrian hamsters also appears to depend on prior lighting conditions (Chiesa et al. Citation2006). Some of these results could be explained by strong early effects of light on the circadian system leading to a reduction in photic sensitivity due to light adaptation, as proposed in a methodological examination of phase sensitivity to 12 light pulses of different durations (Comas et al. Citation2006). In terms of human studies, Chang et al. (Citation2011) had participants spend three days in the laboratory in 1 lux (“dim light”) or 90 lux (“brighter light”) while awake. Simulated nighttime light levels were very dim (<0.1 lux) for all, and participants were later exposed to a 6.5 h light pulse (90 lux) at ~1 h prior to habitual bedtime. The phase of the melatonin rhythm was delayed by ~40 min later when participants were pre-exposed to dim versus brighter light. Similar findings have been reported in earlier human studies, even with significant methodological differences (e.g., Hébert et al. Citation2002; Jasser et al. Citation2006; Smith et al. Citation2004; Zeitzer et al. Citation2011).
In contrast to experiments that control for previous light exposure, studies that instead extend the duration of darkness prior to administration of a light pulse demonstrate increased photic sensitivity of neurons in the SCN (Aggelopoulos and Meissl Citation2000). Similarly, phase-shifts in rodents are enhanced after many days in continuous darkness (Refinetti Citation2003; Shimomura and Menaker Citation1994). This extended period is well beyond any known photoreceptor adaptation timeframe and thus suggests prior state of entrainment can also alter the photic response. Limited work suggests that prior daylength (i.e., photoperiod) may exert an influence on the circadian system. In seminal work by Wehr et al. (Citation1991, Citation1993, Citation1997), seasonal differences in melatonin secretion patterns have been observed in some, but not all, individuals. Likewise, animal models, such as hamsters, show increases in the duration of activity and melatonin secretion under the longer nights of winter (Goldman Citation2001). In addition, hamsters show a 40-fold increased sensitivity to light for phase resetting when previously maintained under a short winter day versus a longer summer photoperiod (Glickman et al. Citation2014, Citation2012); however, this does not appear to hold true for the melatonin suppression response in this same model system (Glickman et al. Citation2014). Studies in humans have reported a similar pattern of results, though the photocycle was not explicitly controlled or monitored. For example, in one human laboratory study, sleep duration (and consequently, photoperiod) was altered between 6 or 9 hours, with the 9-hour sleep time resulting in a significantly greater phase shifting response (Burgess and Eastman Citation2006). In any given study that administers a single light pulse under different photoperiods, however, it is impossible to control both the duration of time in darkness prior to a test pulse and to match the circadian timing of the stimulus.
Little is currently known about the role of light history on physiology and behavior outside the laboratory. One of the earliest human experiments to examine the influence of photic history in real world settings found increased melatonin suppression by light in winter versus summer in a small population of men in Antarctica (Owen and Arendt Citation1992). More recent work in Antarctica suggests that a month-long period of daylight deprivation increases retinal sensitivity to bright, short wavelength light (generated by narrow bandwidth LEDs, Kawasaki et al. Citation2018). Another study of indoor and outdoor dayworkers only found the expected correlation between evening light exposure and later circadian timing of the melatonin rhythm when they considered the ratio between daytime and evening light, rather than daytime or evening light exposure alone (Dumont and Beaulieu Citation2007). Finally, similar to findings on phase shifting and melatonin suppression, effects of light on alertness also seem to depend on prior light history (Chang et al. Citation2013).
Taken together, it is absolutely clear that photic history will affect subsequent response to light; however, the very definition of light history, the magnitude of the effect, and which responses are affected is less clear, especially in real world settings. Thus, the influence of prior light history on the physiological effects of light, and its translation to application, remain a wide-open area of study that warrants further investigation.
2.6. Comparison of findings from studies characterizing physiological effects of light
Though there are some similarities between response characteristics of melatonin suppression and phase shifting by light, preliminary evidence in humans suggests that these two effects are not superimposable (Rahman et al. Citation2018; Sletten et al. Citation2009), which is consistent with animal work (e.g., Glickman et al. Citation2014). In experiments using short pulses of light, the SCN’s network and structure appear to add a non-linear component to the response that can “fill in” short periods of darkness, an effect that is not observed with acute melatonin suppression (Najjar and Zeitzer Citation2016). Photic sensitivity, in particular, may vary by light response. For example, in the golden hamster, melatonin suppression was induced at lower light levels than phase resetting (Nelson and Takahashi Citation1991). Comparisons between dose response curves for photic phase shifting and melatonin suppression in humans is less clear, since the curves intersect and consequently, suggest opposite conclusions in terms of relative sensitivity, depending on the parameter of interest (Gooley et al. Citation2010; Zeitzer et al. Citation2000). Future research that examines the elements mediating the effects of light across multiple responses within the same experimental set up and in the same participants would allow for a deeper understanding of the generalizability of findings across different outcome measures.
3. Effects of light on sleep, productivity, health, and well-being: a brief summary of findings from observational and intervention studies
The invention of electric lighting, the development of commercial aircraft, the ubiquity of electronic displays and the requirement of work schedules at all times of the day and night (such as in the healthcare and security sectors) have profoundly altered the photic exposure patterns of humans (Lunn et al. Citation2017). Today, the human experience of light is determined not only by season, geographical location, weather and time-of-day, but also by habitat, workplace, travel, social and lifestyle habits, access to lighting technology and as a determinant of several of these aspects, socio-economic status. Light exposure can influence not only visual functions but also all physiological and behavioral processes under the control of the circadian timing system as well as its direct effects on alertness, cognition, mood, and behavior. Thus, electric lighting may adversely impact health and safety when these physiological effects of light are not considered. Yet, there is enormous potential to improve human health, performance and well-being via the development of innovative lighting technologies and strategies that address these effects.
While the promise for lighting interventions to enhance human health seems evident from the strong, converging lines of evidence, several aspects inherent to laboratory protocols hamper our ability to translate and design effective field-based interventions. Some of these have been described in greater detail earlier in the paper. Briefly, field studies cannot always completely control the light stimuli, including its intensity, duration, spectral power distribution, or position relative to the eyes. They also cannot control for relevant individual behaviors, such as involuntary pupillary responses, movement between locations and gaze behaviors. In addition, field studies cannot control individual light history, and light exposures will be limited to a range that may not map onto those tested in laboratory settings. The next section will mainly focus on the role of light exposure patterns and health in the general population, and will briefly touch on select findings in clinical patient populations.
3.1. Light, rhythms, and sleep
Nine out of 10 Americans report regular use of an electronic display in the hour prior to sleep onset (Gradisar et al. Citation2013). Mimicking this situation while examining sleep and circadian rhythms in the laboratory has shown that phone, tablet and computer use can shift circadian rhythms, suppress melatonin, and lead to shortened and/or disrupted sleep (Cajochen et al. Citation2011; Chang et al. Citation2015; Chinoy et al. Citation2018; Gooley et al. Citation2011), even if the light source is attenuated in the short-wavelength region of the spectrum (Rahman et al. Citation2017). These results highlight that light intensity is one of the key drivers of physiological responses to light. As discussed in greater detail earlier, laboratory studies have shown that relatively higher levels of daytime light exposure can serve to reduce the magnitude of physiological responses to light in the later evening and nighttime (Chang et al. Citation2011; Hébert et al. Citation2002; Zeitzer et al. Citation2011). Together, these findings suggest moderate evidence for a potentially maladaptive role of light exposure in the evening or night but more research is warranted to better understand the mediating effect of daytime light history on responses to evening and nighttime light exposure.
Field studies examining the effects of light on sleep are currently scarce. Limited available evidence suggests that sleep timing, quality, and architecture may be influenced by individual light exposure patterns encountered in real world settings (Boubekri et al. Citation2014; Stothard et al. Citation2017; Vetter et al. Citation2011; Wams et al. Citation2017; Wright et al. Citation2013). These findings are relevant, as sleep and circadian disturbances are well recognized as predictors of health and longevity. Lighting could therefore serve as a powerful prevention and intervention tool, albeit such evidence in the general population is currently limited (Mason et al. Citation2018). Large-scale, well-powered observational and intervention studies are necessary next steps to further advance evidence-based recommendations.
3.2. Can light in workplaces and classrooms increase alertness?
While laboratory studies have characterized the influence of timing, intensity and wavelength on the alerting response to light, applied work examining the effects of light on productivity and performance are a relatively underexplored area of research. One large study of 104 office workers observed beneficial effects of fluorescent light at 17,000 K compared to 4000 K on self-reported performance, subjective alertness, and daytime sleepiness (Viola et al. Citation2008) but others have not reported the same benefits (Souman et al. Citation2018).
In night shift workers, nighttime decreases in alertness and performance can threaten the health and safety of employees as well as the quality of operations. With permanent night shift work schedules, circadian adaptation to the night shift schedule may be beneficial to workers. Studies examining circadian adaptation in shift work settings are limited, at least partly, due to the difficulty of assessing circadian phase shifts outside of the laboratory; however, the effects of bright light on sleep and performance measures have been assessed. Indeed, bright light exposure during a night worker’s shift can reduce sleepiness and improve alertness at night (Griepentrog et al. Citation2018; Kakooei et al. Citation2010; Lowden et al. Citation2004; Sadeghniiat-Haghighi et al. Citation2011; Yoon et al. Citation2002). These effects occur in conjunction with reductions in nighttime melatonin levels (Kakooei et al. Citation2010; Lowden et al. Citation2004) and improvements in daytime sleep (Boivin et al. Citation2012; Lowden et al. Citation2004; Ross et al. Citation1995; Yoon et al. Citation2002).
In rotating schedules, though, attempts to physiologically adapt to an inverted schedule are not necessarily desirable, as it may lead to a state of chronic jetlag, often referred to as circadian disruption (Vetter Citation2020). Thus, an ideal light regimen might be one that can specifically improve alertness and reduce fatigue during the night shift, while minimizing the effects of light on the circadian system. In the laboratory, van de Werken et al. (Citation2013) showed that nighttime, short-wavelength attenuated polychromatic light exposure only marginally suppressed melatonin levels, while performance was comparable to a bright light condition. This suggests the potential utility of spectrally-tuned lighting solutions for shift workers that take into account several physiological and behavioral effects of light; however, further studies are needed to demonstrate the efficacy of such strategies in an actual work environment.
Another approach that may prove useful for improving alertness and performance, particularly for heterogeneous populations, would be to manipulate individual light intensity and spectral quality rather than simply designing a one-size-fits-all architectural lighting solution. Dark sunglasses that can reduce the potency of light for circadian resetting and/or melatonin suppression (Burgess et al. Citation2002; Smith and Eastman Citation2012; Smith et al. Citation2009a; van der Lely et al. Citation2015) might be a particularly effective tool for this individualization, although compliance, especially in the longer term, may represent a challenge. Since these glasses are likely to simultaneously reduce alertness, their safety while driving and/or performing work activities must also be evaluated (Lockley et al. Citation2006). To our knowledge, individually-controlled light sources, such as desk lamps, have not been systematically evaluated in large-scale studies for efficacy in regulating circadian rhythms nor for user compliance or long-term feasibility, though such technologies may be another possible strategy. Ultimately, shift workers may require multi-component interventions that can refine photic exposure patterns on an individual level. In addition to optimizing workplace lighting, further benefit may come from the use of tools that individualize photic exposure based on a worker’s circadian phase, alertness levels and/or preferred sleep schedule. As interventions become more complicated, education will be a critical component to increase knowledge, adherence, and motivation to achieve optimal implementation of these strategies.
Unfortunately, field studies examining the efficacy of lighting for health applications often suffer from insufficient sample sizes and limited statistical power to detect an effect, particularly in situations where populations are more diverse, the signal is less well measured, and the exposure cannot be controlled to a great extent. In many studies, light exposure is poorly characterized, sometimes using only photopic quantities (e.g., lux, lumens) that are known to poorly correlate with physiological responses. There is a strong scientific consensus about optimum reporting of lighting metrics for both laboratory and field studies examining the effects of light on human physiology, health and performance (CIE Citation2018; Lucas et al. Citation2014; Spitschan et al. Citation2019a; Knoop et al., Citation2019). Such reporting is feasible now and will advance this field significantly. In addition, future work will benefit from technological advances in individual-level photic exposure measures, including increased accuracy and precision, allowing investigators to generate findings with greater generalizability (Adamsson et al. Citation2018; Markvart et al. Citation2015, Webler et al. Citation2019).
3.3. Can light also influence long-term health?
Irregular light exposure, dim light environments during the day, and excess light exposure in the evening weaken circadian entrainment and/or lead to a later or delayed circadian phase, which often results in circadian disturbances. So-called circadian disruption is considered an emerging risk factor for metabolic and cardiovascular disease and has been associated with an increased risk for certain types of hormone-sensitive cancers and mortality in both human and animal studies (Abbott et al. Citation2018; Arble et al. Citation2010; Evans and Davidson Citation2013; Karatsoreos et al. Citation2011; Rea et al. Citation2008; Roenneberg and Merrow Citation2016; Smolensky et al. Citation2016; Stevens et al. Citation2014; Sulli et al. Citation2018; Vetter Citation2020). Exposure to light at night is also thought to lead to disturbed rhythms (Dominoni et al. Citation2016), and its effects have been studied through ecological assessments of light (such as satellite-based light exposure estimates) or at the individual level, using questionnaires, wearable sensors or an installed sensor in the domestic or workplace setting (or a combination thereof). Recent simulation work using light measurements collected in outdoor metropolitan areas suggests that nighttime pedestrian exposure to ambient outdoor light may be able to suppress melatonin (Chen et al. Citation2020). It is important to note, however, that predictions from such simulations need to be followed up with empirical testing.
Exposure to light at night, which typically refers to illumination from electrical light sources, has been proposed as a risk factor for breast cancer (Lunn et al. Citation2017; Stevens et al. Citation2007, Citation2014), possibly mediated by melatonin suppression, downstream alterations of the immune system, and/or other types of endocrine disruption (Bedrosian et al. Citation2016; Dominoni et al. Citation2016; Russart and Nelson Citation2018; Stevens and Rea Citation2001). Empirical data on human tumorigenesis supports the hypothesis that such nighttime light exposure suppresses melatonin, which fosters the development of breast cancer (Blask et al. Citation2005). Additional animal studies have shown that grafted human breast cancer tissue is more resistant to chemotherapy treatments when rats are exposed to light at night (Dauchy et al. Citation2014), and that rapidly cycling shifts of the light/dark cycle, which result in photic exposure during the biological night, will exacerbate tumor growth in mice that are genetically more prone to develop breast cancer (Van Dycke Kirsten et al. Citation2015). Such lines of evidence led the World Health Organization (WHO) to identify long-term shiftwork as a probable cause of cancer in 2007 (IARC Citation2010), an evaluation that was maintained in the WHO 2019 monograph (IARC Citation2019). Although there are uncertainties in identifying the specific roles of circadian disruption, melatonin suppression, and sleep disturbance in the health consequences of exposure to light at night, the American Medical Association (AMA) published a position statement on the adverse health effects of nighttime lighting in 2012. Specifically, the AMA recognized a need for “further multidisciplinary research on occupational and environmental exposure to light-at-night, the risk of cancer, and effects on various chronic diseases” (AMA Citation2012).
Epidemiological studies designed to examine the relationship between light at night and cancer have shown mixed results, possibly due to difficulties in accurately assessing light at night (Davis et al. Citation2001; Hurley et al. Citation2014; James et al. Citation2017; Johns et al. Citation2018; Portnov et al. Citation2016; White et al. Citation2017). Daytime light exposure patterns and cancer endpoints have not yet been assessed to our knowledge. In general, most studies consider only one light variable in their analyses, which represents a major limitation for translation, given that laboratory studies have clearly demonstrated the influence of multiple dimensions of light on physiology.
There is also some indication that exposure to light at night is associated with obesity and impaired glucose tolerance in both animals and humans (Fonken and Nelson Citation2014; Fonken et al. Citation2010; Obayashi et al. Citation2013; Park et al. Citation2019; Stenvers et al. Citation2016). In a recent study, morning and evening light exposure with a peak wavelength of 468 ± 8 nm reduced insulin sensitivity as compared to a dim light condition (Cheung et al. Citation2016). Although the exact mechanisms underlying this association are currently unclear, they suggest a role for light exposure in metabolic health. The effects of light on metabolic health are, at least in part, likely to be mediated by the effects of light on circadian rhythms and sleep, which— when disrupted— are both risk factors for metabolic disorders (Broussard and Van Cauter Citation2016; Depner et al. Citation2014). Favorable light exposure profiles are thought to strengthen circadian rhythms and sleep, and could thereby also improve glucose tolerance and insulin sensitivity. In addition, improved sleep may be associated with a healthier lifestyle, such as improved dietary choices, as recently reported by a pilot study of sleep extension (e.g., Al Khatib et al. Citation2018). Evidence, however, for a link between light and metabolic health is currently scarce, both in the animal and human literature, and further work in larger samples is warranted before evidence-based recommendations are possible.
Often, the discussion of nighttime light exposure mixes night shift work and light conditions. Because night shift work usually requires individuals to be awake and active during the night, it is not only associated with light exposure during the night, but also with alterations in timing and quality of food intake, melatonin suppression, and disrupted sleep (Vetter and Scheer Citation2019). Light levels experienced by shift workers at night are also typically brighter than outdoor nighttime illumination, which is an important factor to consider when drawing conclusions from studies on exposure to outdoor light at night and also, in night shift workers. Daugaard et al. (Citation2017) have indeed shown that night shift workers show transient and partly light-mediated reductions in melatonin levels on workdays; however, this observation does not hold true on days off, wherein melatonin levels are comparable to those observed in day workers. Recent work by Razavi et al. (Citation2019) further demonstrated that inter-individual differences in chronotype influence the relationship between shift work and melatonin levels. Future studies that differentiate between light at night and shift work (and all associated risk factors) are needed to better grasp the public health relevance of these distinct light exposure patterns.
Taken together, the link between light exposure and health is strong, particularly for health conditions that are mediated via the effects of light on the circadian system, neuroendocrine regulation, and sleep; more direct effects of light on metabolic health, for example, are less certain. Future studies will require longer-term recordings of individual-level photic exposure patterns, given the known effects of light history on subsequent physiological responses to light, along with putative health outcomes (Mason et al. Citation2018). Lighting design for the general working population can be immediately improved upon with some key agreed-upon principles, which are informed by both laboratory and field studies, and which we summarize below.
4. Key findings: agreement, controversies, challenges, and translation to current lighting practices
For the general population following a daytime-oriented schedule, a simple rule of thumb is “bright days, dark nights”. The local light/dark cycle should serve as the main point of orientation. Daylight exposure is crucial for resetting the circadian clock, meaning that individuals should be encouraged to seek daylight or indoor light environments with high melanopic EDI whenever possible during the biological day. This is necessary because indoor light levels have historically focused narrowly on visual optimization rather than supporting other light-mediated biological and behavioral functions.
The spectral composition of indoor lighting typically tends toward “warmer” color temperatures and less short wavelength emissions. As numerous studies now demonstrate that non-image forming physiological responses to light are most sensitive to short-wavelengths, current indoor lighting during daytime hours often does not provide sufficient melanopic EDI. Taken together, low light levels with “warm” color temperatures result in photic conditions that may not provide a clear daytime signal to our circadian timing system, a situation which has been shown to lead to a later phase of entrainment in urban populations (Stothard et al. Citation2017; Wright et al. Citation2013). Daytime lighting with high melanopic EDI has also been associated with enhanced mood, improved nighttime sleep, and better daytime performance (Münch et al. Citation2020), so that daytime light exposure with high melanopic EDI and dark nights appear to be a useful guideline for integrative lighting solutions that support both visual and nonvisual effects of light.
The extensive body of research laid out in this review provides a strong rationale for predicting improvements in physiological function with a shift in lighting design practices toward the use of high melanopic EDI lighting in built environments with daytime occupancy; however, the specifics of suggested practices are likely subject to change as we learn more. It will be critical to assess whether and to what extent following new guidelines, such as those recommended by the Well Building Standard Circadian Lighting Design criterion (IWBI, Citation2020), will lead to better health. Despite anticipated health and safety benefits, high melanopic EDI lighting may be less well accepted by building occupants due to esthetic or visual comfort issues (e.g., Wei et al. Citation2014). Advances in technology, however, now allow for the potential to design lighting systems that prioritize and balance vision, health, and subjective experience. The granular control of light spectrum made possible by solid-state lighting technologies enables a wide range of visual outcomes and biological potency.
It is also clear that the timing of light exposure is of critical importance. In general, light exposure after dusk, in the hours leading up to and following bedtime, pushes the circadian clock later and alerts the brain, making it difficult to fall asleep and reducing sleep duration as well as quality. To promote health and reduce sleep disruption in most individuals on a typical daytime work or school schedule, morning light (at work and home) should have high melanopic EDI, while evening light in the domestic context should be relatively dimmer, and of low melanopic EDI, with a spectrum minimizing short-wavelength light. High melanopic EDI light can be provided during the day at the workplace or school and will be naturally provided for individuals working outdoors. For alternative schedules, such as shiftwork, irregular schedules and/or evening work or activities, individual lighting control will be more important to support unique individual needs. Research on such individualized lighting solutions, however, is currently scarce. Light in the morning generally shifts the clock earlier, maintaining circadian entrainment, and can help individuals to keep an earlier schedule. If working a regular daytime schedule (e.g. a 9–5 schedule), an early schedule is desirable, as it will reduce the mismatch between the work schedule and an individual’s biological rhythm, and thus minimize circadian misalignment (Vetter Citation2020).
In some cases, automated lighting designs or strategies may be necessary or more effective than solutions that require individuals to remember to adjust lighting to appropriate levels. Individual-level characteristics may determine the effect of light on physiology as well. For example, children may be more sensitive to light prior to bedtime (Akacem et al. Citation2016), so strategies that limit short wavelength light exposure during the hours leading up to and during bedtime are especially important in this population. In addition, older individuals have been reported to have different physiological responses to light than younger, middle-aged participants, reinforcing the notion that age is another, albeit under-studied, factor to consider when optimizing integrative lighting solutions (Chellappa et al. Citation2019; Daneault et al. Citation2012; Herbst et al. Citation2012; Herljevic et al. Citation2005; Najjar et al. Citation2014; Sletten et al. Citation2009). Individuals taking antidepressant medications also appear more sensitive to light, as compared to controls (McGlashan et al. Citation2018). Recent laboratory work suggests that there is a greater than 50-fold difference in evening light sensitivity, as assessed by melatonin suppression, even between healthy, young individuals (Phillips et al. Citation2019). Identifying the factors contributing to these individual differences in light sensitivity will be of utmost importance for developing optimized lighting strategies. Individuals who spend a lot of time indoors (such as in nursing homes) will also benefit from lighting systems that are bright and/or contain a lot of short-wavelength light during the daytime combined with dimmer, short wavelength-depleted light pre-sleep, as this helps to mimic outdoor lighting conditions, and thus strengthens circadian entrainment (e.g., with an increase in rhythm amplitude) and has been shown to reduce sleep fragmentation and cognitive decline (e.g., Ancoli-Israel et al. Citation2003; Riemersma-van der Lek et al. Citation2008; Royer et al. Citation2012; but see Hopkins et al. Citation2017). There is also evidence that genetic polymorphisms and biological sex may modify the effects of light on physiology (Chellappa et al. Citation2017, Citation2012, Citation2014; Roecklein et al. Citation2009).
The ever-changing nature of light exposure patterns throughout a day, a season, and over a lifetime, combined with the individual behavioral and physiological responses to light, create substantive challenges to the study of the effects of light on health. These factors result in a wide variety of light exposure profiles and photic histories that have not yet been well replicated in the laboratory. For example, laboratory studies often control pupil dilation and/or gaze direction, both determinants of photic dose at the level of the retina. In typical living and working conditions, these two factors would not be controlled and are therefore likely contributing to divergence in findings between controlled laboratory research and intervention, field, and epidemiological studies.
From this review, the importance of monitoring 24 h light exposure profiles and light history is evident, suggesting that individual-level sensor usage with continuous and longer-term recording potential may be useful when estimating the health consequences of lighting strategies. Current reporting of light exposure patterns, especially outside of the laboratory setting, still often reverts to illuminance in terms of lux, now well established to be an inappropriate measure for quantifying the physiological effects of light (Brown Citation2020; CIE Citation2015, Citation2018; Lucas et al. Citation2014). While our understanding of the mechanisms underlying these effects of light has dramatically evolved, and while consensus-based suggestions for how to best assess and report light exposure have been put forward (Lucas et al. Citation2014), practice lags behind, both in research and application. In non-laboratory settings, sensor quality and resolution have hampered some of these efforts (Figueiro et al. Citation2013; Markvart et al. Citation2015; Price et al. Citation2017). It is important to note that despite these difficulties, individual photic exposure patterns in real life have often been associated with the dim light melatonin onset as well as sleep structure, quality, duration and timing, suggesting ecological validity of the sensor data collected in some field studies (e.g., de la Iglesia et al. Citation2015; Dijk et al. Citation1987; Stebelová et al. Citation2014; Stothard et al. Citation2017; Wams et al. Citation2017; Woelders et al. Citation2017; Wright et al. Citation2013).
The best methods for assessing cumulative and chronic light exposure patterns, as are relevant to chronic disease outcomes, currently remain less well understood. Additional differences between laboratory and real-world settings include the fact that laboratory studies are typically conducted in highly screened populations, which do not reflect the diversity in age, ethnicity, sex, health status, medication intake, and income of the general population. It is also likely that specific subgroups may respond to light in unique ways (see Phillips et al. Citation2019 for a recent example). Future work is needed to identify determinants of individual-level light responses, potential effect modifiers, and their role in long-term health.
Ultimately, the empirical, epidemiological, and observational discoveries related to how light regulates the human circadian, neuroendocrine, and neurobehavioral systems need to be translated into standard lighting practice (DiLaura et al. Citation2011). The Illuminating Engineering Society (IES) is the recognized authority for establishing lighting application standards in North America. Founded in 1906, the IES maintains approximately 70 consensus standards in the IES Lighting Library (IES Citation2020). The IES maintains that all lighting practices in North America, including the ones related to light and health, should be based on a consensus document developed through an accredited American National Standards Institute (ANSI) process. The IES published a technical memorandum on this field in 2008 and more recently reconfirmed it under both IES and ANSI consensus processes (ANSI/IES Citation2018). A recommended practice for this field is currently being developed under the aegis of the IES.
5. Summary
The science of understanding human physiological responses to light has advanced greatly over the past two decades. Converging research findings clearly show that physiological functions relevant to human health and safety, such as circadian, alerting and neuroendocrine responses, are most sensitive to short-wavelength light and at intensities greater than are currently recommended in typical lighting design guidelines (with standard fixtures). It is also clear that the majority of lighting products and design practices are not yet optimized for human health and well-being, as these are dramatically influenced by the physiological responses to light. Current understandings can guide lighting practice for daytime, pre-sleep and nighttime environments. The most immediate nonvisual, physiological responses to light include acute alerting effects (especially during the nighttime), which can impact job or learning performance as well as sleep timing and quality. More research is necessary to reach the next level of understanding of the details relating to physiological responses to light, mediating factors in everyday lighting environments, and to establish convincing validation of the benefits of integrative lighting solutions in domestic and workplace settings.
In terms of the physiological effects of light, the relationship between spectral quality (with possible interacting wavelength effects from polychromatic light), intensity, duration, timing, and photic history needs to be clarified with respect to the specific response of interest. For translation into practice, though, outcome-wide research will be beneficial to generalize the effects of light on humans (and their ecosystems). More studies are also necessary for better understanding the impact of mediating factors that affect the actual light levels reaching the retina. These include pupil responses, gaze and aversion behaviors and individual practices (e.g. use of directly viewed electronic displays). Finally, there needs to be translational research where objective physiological measures are assessed and compared in response to different photic stimuli in realistic lighted applications. As this research is performed and with convergence of findings, more precise lighting guidance and best practices may be developed.
It is important to recognize that the term “circadian” light is a misnomer; light through the eye provides input to both the visual system as well as a host of nonvisual physiological processes, which we aim to convey via the term “integrative lighting”. The goal should be to optimize light exposure to beneficially impact the wide plethora of functions known to be affected by light, as highlighted in a recent CIE position statement (CIE Citation2019). The position statement reinforces melanopic EDI as the metric of choice to quantify polychromatic light exposure in everyday situations of individuals with day-active schedules. In addition, the CIE endorses bright days (as evidenced by high melanopic EDI), and dim evening and nights (low melanopic EDI levels), similar to the public outreach brief released by the Society for Research on Biological Rhythms (Vetter et al. Citation2019). Both statements suggest that this rule of thumb would improve daytime function, support circadian entrainment, and promote a good night’s sleep.
In parallel with new understandings of physiological responses to light, LED technology has advanced to the point where it can efficiently and effectively provide almost any light stimulus that might be deemed necessary to support human health. It should be noted, however, that just as the light that reaches the retina is influenced by various factors, the light emitted from installed lighting is mediated by many other aspects of the environment. Lighting layouts within the room, surface colors and textures, room geometry, the view and the inclusion of daylight from windows, will all contribute to the effectiveness of the installed lighting for providing physiological impacts. Thus, alongside further research on the physiological responses to light, there needs to be further investigation into lighting technology and deployment in order to achieve the full benefit of these more recent understandings, especially with the longer lifespan of new LED technologies.
The potential value of improved lighting applications that are optimized for beneficial human physiological responses is significant. While individual health benefits may be modest, small benefits accrued across the entire population can potentially result in extensive society-wide health gains (Pattison et al. Citation2018). Although this manuscript does not detail the therapeutic applications of light, it is noteworthy that light therapy can have high clinical efficacy, and research into these applications may further inform our understanding of human physiological responses to light for healthy individuals. In conclusion, there is a tremendous opportunity to understand and deploy lighting products and practices that leverage our understanding of the effects of light on human physiology to improve health, safety and well-being.
Disclaimer
One of the authors (GG) is an employee of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C.105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ The views expressed in this article are those of the authors and do not reflect the official views or position of the Department of the Army/Navy/Air Force, Department of Defense, or U.S. Government. This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Supplemental Material
Download MS Word (26.7 KB)Acknowledgments
We would like to thank T. Phan and S.S. Golden of the UC San Diego BioClock Studio for contributing to ; L. Hunt (CU Boulder) for assistance with the Tables 1 and 2; and R. Lucas (University of Manchester) for helpful and constructive feedback on an earlier version of the manuscript. The material in the report is based upon work supported by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE), under award number DE-FE0025912.
Disclosure statement
CV is part of the Circadian Light Therapy (Inc.) Scientific Advisory Board. GLG reports that she has received equipment, advice and/or financial support, and/or served as a consultant to Philips, Litebook, BIOS Lighting, f.lux, PennWell Corporation, LightShow West, Well Building Institute, and that she holds two currently issued patents (USPTO 7,678,140 and 8,366,755) and three continuing patent applications (USPTO 16/252,300, 15/085,522 and 14/273,971). DBB provides conferences and legal expert advices on various shift work-related cases; is co-inventor to one patent (US 2010/0138379 A1); and has received free use of equipment from Somno-Art. MH is co-founder and President of f.lux Software LLC. DJS is co-inventor on two issued patents (EP 1614441A1 and EP3055022A). GCB has no conflicts of interest relative to the scientific content of this manuscript. In the spirit of open disclosure, however, he reports having issued (USPTO 7,678,140 and 8,366,755) and pending (USPTO 16/252,300, 15/085,522 and 14/273,971) patents related to the photoreceptor system for melatonin regulation. That intellectual property has been licensed by Litebook Company Ltd. In addition, he and his research program have received financial, material and travel support from a range of federal, industrial, legal and philanthropic organizations in the past and present. KWH has no conflicts of interest relative to the scientific content of this manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Abbott SM, Malkani RG, Zee PC. 2018. Circadian disruption and human health: a bidirectional relationship. Eur J Neurosci. 51(1):567–583. doi:https://doi.org/10.1111/ejn.14298.
- Adamsson M, Laike T, Morita T. 2018. Comparison of static and ambulatory measurements of illuminance and spectral composition that can be used for assessing light exposure in real working environments. LEUKOS. 15(2–3):181–194. doi:https://doi.org/10.1080/15502724.2017.
- Aggelopoulos NC, Meissl H. 2000. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 523(Pt1):211–222. doi:https://doi.org/10.1111/j.1469-7793.2000.t01-1-00211.x.
- Akacem LD, Wright KP, LeBourgeois MK. 2016. Bedtime and evening light exposure influence circadian timing in preschool-age children: a field study. Neurobiol Sleep Circadian Rhythms. 1(2):27–31. doi:https://doi.org/10.1016/j.nbscr.2016.11.002.
- Akerstedt T, Gillberg M. 1990. Subjective and objective sleepiness in the active individual. Int J Neurosci. 52:29–37. doi:https://doi.org/10.3109/00207459008994241.
- [AMA] American Medical Association. 2012. AMA Policy H-135.932. Light pollution: adverse health effects of night-time lighting. Chicago (IL): AMA.
- Al Khatib HK, Hall WL, Creedon A, Ooi E, Masri T, McGowan L, Harding SV, Darzi J, Pot GK. 2018. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. Am J Clin Nutr. 107(1):43–53. doi:https://doi.org/10.1093/ajcn/nqx030.
- Allen AE, Hazelhoff EM, Martial FP, Cajochen C, Lucas RJ. 2018. Exploiting metamerism to regulate the impact of a visual display on alertness and melatonin suppression independent of visual appearance. Sleep. 41:8. doi:https://doi.org/10.1093/sleep/zsy100.
- Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L. 2003. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 1(1):22–36. doi:https://doi.org/10.1207/S15402010BSM0101_4.
- [ANSI/IES] American National Standards Institute / Illuminating Engineering Society. 2018. IES TM-18-18: Light and human health: An overview of the impact of optical radiation on visual, circadian, neuroendocrine, and neurobehavioral responses. New York (NY): IES .
- [ANSI/IES] American National Standards Institute / Illuminating Engineering Society. 2020. IES method for evaluating light source color rendition. New York (NY): IES .
- Arble DM, Ramsey KM, Bass J, Turek FW. 2010. Circadian disruption and metabolic disease: findings from animal models. Clin Endocrinol Metab. 24(5):785–800.
- Arendt J. 2006. Melatonin and human rhythms. Chronobiol Int. 23(1–2):21–37. doi:https://doi.org/10.1080/07420520500464361.
- Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. 1991. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 50(3):583–588. doi:https://doi.org/10.1016/0031-9384(91)90549-4.
- Bailes HJ, Lucas RJ. 2013. Human melanopsin forms a pigment maximally sensitive to blue light (λmax≈ 479 nm) supporting activation of Gq/11 and Gi/o signaling cascades. Proc R Soc B. 280(1759):20122987. doi:https://doi.org/10.1098/rspb.2012.2987.
- Bedrosian TA, Fonken LK, Nelson RJ. 2016. Endocrine effects of circadian disruption. Annu Rev Physiol. 78:109–131. doi:https://doi.org/10.1146/annurev-physiol-021115-105102.
- Bedrosian TA, Nelson RJ. 2017. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 7(1):e1017. doi:https://doi.org/10.1038/tp.2016.262.
- Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 295(5557):1070–1073. doi:https://doi.org/10.1126/science.1067262.
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 65(23):11174–11184. doi:https://doi.org/10.1158/0008-5472.CAN-05-1945.
- Boivin DB, Boudreau P, James FO, Kin NM. 2012. Photic resetting in night-shift work: impact on nurses’ sleep. Chronobiol Int. 29(5):619–628. doi:https://doi.org/10.3109/07420528.2012.675257.
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. 1996. Dose-response relationships for resetting of human circadian clock by light. Nature. 379(6565):540–542. doi:https://doi.org/10.1038/379540a0.
- Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, Arendt J. 1987. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 19(9):437–440. doi:https://doi.org/10.1055/s-2007-1011846.
- Boubekri M, Cheung I, Reid KJ, Wang C, Zee PC. 2014. Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. J Clin Sleep Med. 10(6):603–611. doi:https://doi.org/10.5664/jcsm.3780.
- Brainard GC, Hanifin JP. 2005. Photons, clocks, and consciousness. J Biol Rhythms. 20(4):314–325. doi:https://doi.org/10.1177/0748730405278951.
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 21(16):6405–6412. doi:https://doi.org/10.1523/JNEUROSCI.21-16-06405.2001.
- Brainard GC, Hanifin JP, Warfield B, Stone MK, James ME, Ayers M, Kubey A, Byrne B, Rollag M. 2015. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res. 58(3):352–361. doi:https://doi.org/10.1111/jpi.12221.
- Brainard GC, Richardson BA, Reiter RJ, Matthews SA, King TS. 1983. The suppression of pineal melatonin content and n-acetyltransferase activity by different light irradiances in the Syrian hamster: a dose-response relationship. Endocrinology. 113(1):293–296. doi:https://doi.org/10.1210/endo-113-1-293.
- Brainard GC, Rollag MD, Hanifin JP. 1997. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. 12(6):537–546. doi:https://doi.org/10.1177/074873049701200608.
- Brainard GC, Sliney D, Hanifin JP, Glickman G, Byrne B, Greeson JM, Jasser S, Gerner E, Rollag MD. 2008. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms. 23(5):379–386. doi:https://doi.org/10.1177/0748730408323089.
- Broussard JL, Van Cauter E. 2016. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 23(5):353–359. doi:https://doi.org/10.1097/MED.0000000000000276.
- Brown TM. 2020. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J Pineal Res. 69:e12655. doi:https://doi.org/10.1111/jpi.12655.
- Burgess HJ, Eastman CI. 2006. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 395(3):191–195. doi:https://doi.org/10.1016/j.neulet.2005.10.082.
- Burgess HJ, Sharkey KM, Eastman CI. 2002. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 6(5):407–420. doi:https://doi.org/10.1053/smrv.2001.0215.
- Cajochen C. 2007. Alerting effects of light. Sleep Med Rev. 11(6):453–464. doi:https://doi.org/10.1016/j.smrv.2007.07.009.
- Cajochen C, Frey S, Anders D, Spati J, Bues M, Pross A, Mager R, Wirz-Justice A, Stefani O. 2011. Evening exposure to a light-emitting diodes (led)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 110(5):1432–1438. doi:https://doi.org/10.1152/japplphysiol.00165.2011.
- Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 90(3):1311–1316. doi:https://doi.org/10.1210/jc.2004-0957.
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. 2000. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 115(1):75–83. doi:https://doi.org/10.1016/S0166-4328(00)00236-9.
- Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting e-readers negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 112(4):1232–1237. doi:https://doi.org/10.1073/pnas.1418490112.
- Chang A-M, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE, Czeisler CA. 2012. Human responses to bright light of different durations. J Physiol. 590(Pt13):3103–3112. doi:https://doi.org/10.1113/jphysiol.2011.226555.
- Chang AM, Scheer FA, Czeisler CA, Aeschbach D. 2013. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep. 36(8):1239–1246. doi:https://doi.org/10.5665/sleep.2894.
- Chang A-M, Scheer FAJL, Czeisler CA. 2011. The human circadian system adapts to prior photic history. J Physiol. 589(5):1095–1102. doi:https://doi.org/10.1113/jphysiol.2010.201194.
- Chellappa SL, Bromundt V, Frey S, Steinemann A, Schmidt C, Schlote T, Goldblum D, Cajochen C. 2019. Association of intraocular cataract lens replacement with circadian rhythms, cognitive function, and sleep in older adults. JAMA Ophthalmol. 137(8):878–885. doi:https://doi.org/10.1001/jamaophthalmol.2019.1406.
- Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. 2011. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One. 6(1):e16429. doi:https://doi.org/10.1371/journal.pone.0016429.
- Chellappa SL, Steiner R, Oelhafen P, Cajochen C. 2017. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci Rep. 7(1):14215. doi:https://doi.org/10.1038/s41598-017-13973-1.
- Chellappa SL, Viola AU, Schmidt C, Bachmann V, Gabel V, Maire M, Reichert CF, Valomon A, Gotz T, Landolt HP, et al. 2012. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene per3. J Clin Endocrinol Metab. 97(3):E433–437. doi:https://doi.org/10.1210/jc.2011-2391.
- Chellappa SL, Viola AU, Schmidt C, Bachmann V, Gabel V, Maire M, Reichert CF, Valomon A, Landolt HP, Cajochen C. 2014. Light modulation of human sleep depends on a polymorphism in the clock gene period3. Behav Brain Res. 271:23–29. doi:https://doi.org/10.1016/j.bbr.2014.05.050.
- Chen S, Wei M, Dai Q, Huang Y. 2020. Estimation of possible suppression of melatonin production caused by exter-ior lighting in commercial business districts in metropolises. LEUKOS. 16(2):137–144. doi:https://doi.org/10.1080/15502724.2018.
- Cheung IN, Zee PC, Shalman D, Malkani RG, Kang J, Reid KJ. 2016. Morning and evening blue-enriched light exposure alters metabolic function in normal weight adults. PLoS One. 11(5):e0155601. doi:https://doi.org/10.1371/journal.pone.0155601.
- Chiesa JJ, Angles-Pujolras M, Díez-Noguera A, Cambras T. 2006. History-dependent changes in entrainment of the activity rhythm in the Syrian hamster (mesocricetus auratus). J Biol Rhythms. 21(1):45–57. doi:https://doi.org/10.1177/0748730405283654.
- [CIE] Commission Internationale de l’Eclairage. 2015. Report on the first international workshop on circadian and neurophysiological photometry, 2013. CIE 003:2015. Vienna (Austria): CIE.
- [CIE] Commission Internationale de l’Eclairage. 2017. Colour fidelity index for accurate scientific use. CIE 224:2017. Vienna (Austria): CIE.
- [CIE] Commission Internationale de l’Eclairage. 2018. CIE system for metrology of optical radiation for ipRGC-influenced responses to light. CIE S 026/E:2018. Vienna (Austria): CIE.
- [CIE] Commission Internationale de l’Eclairage. 2019. Position statement on non-visual effects of light - recommendating proper light at the proper time, 2nd edition. Vienna (Austria): CIE. 4 p. https://cie.co.at/publications/position-statement-non-visual-effects-light-recommending-proper-light-proper-time-2ndhttp://www.cie.co.at/files/CIE%20Position%20Statement%20-%20Proper%20Light%20at%20the%20Proper%20Time%20%282019%29_0.pdf.
- Chinoy ED, Duffy JF, Czeisler CA. 2018. Unrestricted evening use of light-emitting tablet computers delays self-selected bedtime and disrupts circadian timing and alertness. Physiol Rep. 6(10):e13692. doi:https://doi.org/10.14814/phy2.13692.
- Comas M, Beersma DGM, Spoelstra K, Daan S. 2006. Phase and period responses of the circadian system of mice (mus musculus) to light stimuli of different duration. J Biol Rhythms. 21(5):362–372. doi:https://doi.org/10.1177/0748730406292446.
- Coohill TP. 1999. Photobiological action spectra: what do they mean? In: Matthes R, Sliney D, DiDomenico S, Murray P, Phillips R, Wengraitis S, editors. Measurements of optical radiation hazards. Munich: International Commission on Non-Ionizing Radiation Protection. p. 27–39.
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. 1989. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 244(4910):1328–1333. doi:https://doi.org/10.1126/science.2734611.
- Daneault V, Vanderwalle G, Hébert M, Teikari P, Mure LS, Doyon J, Gronfier C, Cooper HM, Dumont M, Carrier J. 2012. Does pupil constriction under blue and green monochromatic light exposure change with age? J Biol Rhythms. 27(3):257–264. doi:https://doi.org/10.1177/0748730412441172.
- Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, et al. 2014. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 74(15):4099–4110. doi:https://doi.org/10.1158/0008-5472.CAN-13-3156.
- Daugaard S, Garde AH, Bonde JPE, Christoffersen J, Hansen AM, Markvart J, Schlunssen V, Skene DJ, Vistisen HT, Kolstad HA. 2017. Night work, light exposure and melatonin on work days and days off. Chronobiol Int. 34(7):942–955. doi:https://doi.org/10.1080/07420528.2017.1327867.
- Davis S, Mirick DK, Stevens RG. 2001. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 93(20):1557–1562. doi:https://doi.org/10.1093/jnci/93.20.1557.
- de la Iglesia HO, Fernandez-Duque E, Golombek DA, Lanza N, Duffy JF, Czeisler CA, Valeggia CR. 2015. Access to electric light is associated with shorter sleep duration in a traditionally hunter-gartherer community. J Biol Rhythms. 30(4):342–350. doi:https://doi.org/10.1177/0748730415590702.
- Depner CM, Stothard ER, Wright KP Jr. 2014. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 14(7):1–9. doi:https://doi.org/10.1007/s11892-014-0507-z.
- Dijk DJ, Visscher CA, Bloem GM, Beersma DGM, Daan S. 1987. Reduction of human sleep duration after bright light exposure in the morning. Neurosci Lett. 73(2):181–186. doi:https://doi.org/10.1016/0304-3940(87)90014-0.
- DiLaura DL, Houser KW, Mistrick RG, Steffy GR. Editors. 2011. The lighting handbook: reference and application, 10th edition. New York (NY): Illuminating Engineering Society.
- Dominoni DM, Borniger JC, Nelson RJ. 2016. Light at night, clocks and health: from humans to wild organisms. Biol Lett. 12(2):20160015. doi:https://doi.org/10.1098/rsbl.2016.0015.
- Dorrian J, Rogers NL, Dinges DF. 2004. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep deprivation: clinical issues pharmacology, and sleep loss effects. New York (NY): CRC Press. p. 67–98.
- Dumont M, Beaulieu C. 2007. Light exposure in the natural environment: relevance to mood and sleep disorders. Sleep Med. 8(6):557–565. doi:https://doi.org/10.1016/j.sleep.2006.11.008.
- Ebihara S, Tsuji K. 1980. Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a zeitgeber in the retinal degenerate c3h mouse and the normal c57bl mouse. Physiol Behav. 24(3):523–527. doi:https://doi.org/10.1016/0031-9384(80)90246-2.
- Esposito T, Houser KW. 2019. Models of colour quality over a wide range of spectral power distributions. Lighting Res Technol. 51(3):331-352. doi:10.1177/1477153518765953.
- Evans JA, Davidson AJ. 2013. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 119:283-323. doi:https://doi.org/10.1016/B978-0-12-396971-2.00010-5.
- Fernandez DC, Foggerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, et al. 2018. Light affects mood and learning through distinct retina-brain pathways. Cell. 175(1):71–84. doi:https://doi.org/10.1016/j.cell.2018.08.004.
- Figueiro MG, Hamner R, Bierman A, Rea MS. 2013. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Lighting Res Technol. 45(4):421–434. doi:https://doi.org/10.1177/1477153512450453.
- Fonken LK, Nelson RJ. 2014. The effects of light at night on circadian clocks and metabolism. Endocrinol Rev. 35(4):648–670.
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 107(43):18664–18669. doi:https://doi.org/10.1073/pnas.1008734107.
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. 1991. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol A. 169(1):39–50. doi:https://doi.org/10.1007/BF00198171.
- Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. 2006. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 59(6):502–507. doi:https://doi.org/10.1016/j.biopsych.2005.07.006.
- Glickman G, Webb IC, Elliott JA, Baltazar RM, Reale ME, Lehman MN, Gorman MR. 2012. Photic sensitivity for circadian response to light varies with photoperiod. J Biol Rhythms. 27(4):308–318. doi:https://doi.org/10.1177/0748730412450826.
- Glickman GL, Harrison EM, Elliott JA, Gorman MR. 2014. Increased photic sensitivity for phase resetting but not melatonin suppression in Siberian hamsters under short photoperiods. Horm Behav. 65(3):301–307. doi:https://doi.org/10.1016/j.yhbeh.2014.01.002.
- Goldman BD. 2001. Mammalian photoperiodic system: formal properties and neuroendocrine mechanism of photoperiodic time measurement. J Biol Rhythms. 16(4):283–301. doi:https://doi.org/10.1177/074873001129001980.
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. 2011. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 96(3):E463–E472. doi:https://doi.org/10.1210/jc.2010-2098.
- Gooley JJ, Ho Mien I, St. Hilaire M, Yeo S-C, Chua EC-P, Van Reen E, Hanley CJ, Hull JT, Czeisler CA, Lockley SW. 2012. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 32(41):14242–14253. doi:https://doi.org/10.1523/JNEUROSCI.1321-12.2012.
- Gooley JJ, Lu J, Fischer D, Saper CB. 2003. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 23(18):7093–7106. doi:https://doi.org/10.1523/JNEUROSCI.23-18-07093.2003.
- Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2(31):31ra33–31ra33. doi:https://doi.org/10.1126/scitranslmed.3000741.
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. 2013. The sleep and technology use of Americans: findings from the national sleep foundation’s 2011 sleep in America poll. J Clin Sleep Med. 9(12):1291–1299. doi:https://doi.org/10.5664/jcsm.3272.
- Griepentrog JE, Labiner HE, Gunn SR, Rosengart MR. 2018. Bright environmental light improves the sleepiness of nightshift ICU nurses. Crit Care. 22(1):295. doi:https://doi.org/10.1186/s13054-018-2233-4.
- Hanifin J, Lockley S, Cecil K, West K, Jablonski M, Warfield B, James M, Ayers M, Byrne B, Gerner E. 2019. Randomized trial of polychromatic blue-enriched light for circadian phase shifting, melatonin suppression, and alerting responses. Physiol Behav. 198:57–66. doi:https://doi.org/10.1016/j.physbeh.2018.10.004.
- Hankins MW, Lucas RJ. 2002. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a non-classical photopigment. Curr Biol. 12(3):191–198. doi:https://doi.org/10.1016/S0960-9822(02)00659-0.
- Hannibal J, Christiansen AT, Heegaard S, Fahrenkrug J, Kiilgaard JF. 2017. Melanopsin expressing human retinal ganglion cells: subtypes, distribution, and intraretinal connectivity. J Comp Neurol. 525(8):1934–1961. doi:https://doi.org/10.1002/cne.24181.
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. 2006. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 497(3):326–349. doi:https://doi.org/10.1002/cne.20970.
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 295(5557):1065–1070. doi:https://doi.org/10.1126/science.1069609.
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 424(6944):76–81. doi:https://doi.org/10.1038/nature01761.
- Hébert M, Martin SK, Lee C, Eastman CI. 2002. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 33(4):198–203. doi:https://doi.org/10.1034/j.1600-079X.2002.01885.x.
- Herbst K, Sander B, Lund-Andersen H, Broensted AE, Kessel L, Hansen MS, Kawasaki A. 2012. Intrinsically photosensitive retinal ganglion cell function in relation to age: a pupilometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol. 12:4. doi:https://doi.org/10.1186/1471-2415-12-4.
- Herljevic M, Middleton B, Thapan K, Skene DJ. 2005. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 40(3):237–242. doi:https://doi.org/10.1016/j.exger.2004.12.001.
- Honma K, Honma S. 1988. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 42:167–168.
- Hopkins S, Morgan PL, Schlangen LM, Williams P, Skene DJ, Middleton B. 2017. Blue-enriched lighting for older people living in care homes: effect on activity, actigraphic sleep, mood, and alertness. Curr Alzheimer Res. 14(10):1053–1062. doi:https://doi.org/10.2174/1567205014666170608091119.
- Horspool WM, Lenci F. 2003. CRC handbook of organic photochemistry and photobiology. Vol. 1 & 2. 2nd ed. Chapter 52. New York (NY): CRC press. p. 1–31.
- [IARC] International Agency for Research on Cancer. 2010. Monograph 98: painting, firefighting, and shiftwork. Lyon (France): IARC Publication.
- [IARC] International Agency for Research on Cancer. 2019. Monograph 124: night shift work. Lyon (France): IARC Publication.
- Houser K. 2017. The AMA’s misguided report on human and environmental effects of led lighting. LEUKOS. 13(1):1–2. doi:https://doi.org/10.1080/15502724.2016.1247566.
- Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, Reynolds P. 2014. Light at night and breast cancer risk among California teachers. Epidemiology. 25(5):697–706. doi:https://doi.org/10.1097/EDE.0000000000000137.
- IES. 2018. IES method for evaluating light source color rendition. IES-TM-30-18. Illuminating Engineering Society of North America New York
- [IES] Illuminating Engineering Society. 2020. Lighting library. New York (NY): IES. [accessed 2020 Aug 19]. https://www.ies.org/lighting-library/.
- James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F. 2017. Outdoor light at night and breast cancer incidence in the nurses’ health study II. Environ Health Perspect. 125(8):087010. doi:https://doi.org/10.1289/EHP935.
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. 2006. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 21(5):394–404. doi:https://doi.org/10.1177/0748730406292391.
- Johns LE, Jones ME, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ. 2018. Domestic light at night and breast cancer risk: a prospective analysis of 105,000 UK women in the generations study. Br J Cancer. 118:600. doi:https://doi.org/10.1038/bjc.2017.359.
- Kakooei H, Ardakani ZZ, Ayattollahi MT, Karimian M, Saraji GN, Owji AA. 2010. The effect of bright light on physiological circadian rhythms and subjective alertness of shift work nurses in Iran. Int J Occup Saf Ergon. 16(4):477–485. doi:https://doi.org/10.1080/10803548.2010.11076860.
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. 2011. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 108(4):1657–1662. doi:https://doi.org/10.1073/pnas.1018375108.
- Kawasaki A, Wisniewski S, Healey B, Pattyn N, Kunz D, Basner M, Münch M. 2018. Impact of long-term daylight deprivation on retinal light sensitivity, circadian rhythms and sleep during the Antarctic winter. Sci Rep. 8(1):16185. doi:https://doi.org/10.1038/s41598-018-33450-7.
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. 2003. A phase response curve to single bright light pulses in human subjects. J Physiol. 549(3):945–952. doi:https://doi.org/10.1113/jphysiol.2003.040477.
- Klein DC, Moore RY, Reppert SM. 1991. Suprachiasmatic nucleus: the mind’s clock. New York (NY): Oxford University Press.
- Knoop M, Broszio K, Diakite A, Liedtke C, Niedling M, Rothert I, Rudawski F, Weber N. 2019. Methods to describe lighting conditions in experiments on non-image-forming aspects. LEUKOS. 15(2–3):163-179.doi:10.1080/15502724.2018
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. 1980. Light suppresses melatonin secretion in humans. Science. 210(4475):1267–1269. doi:https://doi.org/10.1126/science.7434030.
- Li JY, Schmidt TM. 2018. Divergent projection patterns of m1 ipRGC subtypes. J Comp Neurol. 526(13):2010–2018. doi:https://doi.org/10.1002/cne.24469.
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. 2006. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. SLEEP. 29(2):161–168.
- Lok R, Woelders T, Gordijn MCM, Hut RA, Beersma DGM. 2018. White light during daytime does not improve alertness in well-rested individuals. J Biol Rhythms. 33(6):637–648. doi:https://doi.org/10.1177/0748730418796036.
- Lowden A, Akerstedt T, Wibom R. 2004. Suppression of sleepiness and melatonin by bright light exposure during breaks in night work. J Sleep Res. 13(1):37–43. doi:https://doi.org/10.1046/j.1365-2869.2003.00381.x.
- Lucas RJ, Douglas RH, Foster RG. 2001. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 4(6):621–626. doi:https://doi.org/10.1038/88443.
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, et al. 2014. Measuring and using light in the melanopsin age. Trends Neurosci. 37(1):1–9. doi:https://doi.org/10.1016/j.tins.2013.10.004.
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, et al. 2017. Health consequences of electric lighting practices in the modern world: a report on the national toxicology program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 607–608(Supplement C):1073–1084. doi:https://doi.org/10.1016/j.scitotenv.2017.07.056.
- Markvart J, Hansen ÅM, Christoffersen J. 2015. Comparison and correction of the light sensor output from 48 wearable light exposure devices by using a side-by-side field calibration method. LEUKOS. 11(3):155–171. doi:https://doi.org/10.1080/15502724.2015.1020948.
- Mason IC, Boubekri M, Figueiro MG, Hasler BP, Hattar S, Hill SM, Nelson RJ, Sharkey KM, Wright KP, Boyd WA, et al. 2018. Circadian health and light: a report on the national heart, lung, and blood institute’s workshop. J Biol Rhythms. 33:748730418789506. doi:https://doi.org/10.1177/0748730418789506.
- McGlashan EM, Nandam LS, Vidafar P, Mansfield DR, Rajaratnam SMW, Cain SW. 2018. The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacology. 235(11):3201–3209. doi:https://doi.org/10.1007/s00213-018-5019-0.
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. 1989. Human melatonin suppression by light is intensity dependent. J Pineal Res. 6(20):149–156. doi:https://doi.org/10.1111/j.1600-079X.1989.tb00412.x.
- Minors DS, Waterhouse JM, Wirz-Justice A. 1991. A human phase-response curve to light. Neurosci Lett. 133:36–40. doi:https://doi.org/10.1016/0304-3940(91)90051-T.
- Moore RY. 1995. Organization of the mammalian circadian system. Ciba Found Symp. 1995;183:88-99.
- Münch M, Wirz-Justice A, Brown SA, Kantermann T, Martiny K, Stefani O, Vetter C, Wright KP Jr., Wulff K, Skene DJ. 2020. The role of daylight for humans: gaps in current knowledge. Clocks Sleep. 2:61–85. doi:https://doi.org/10.3390/clockssleep2010008.
- Mure L, Vinberg F, Hanneken A, Panda S. 2019. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science. 366(6470):1251–1255. doi:https://doi.org/10.1126/science.aaz0898.
- Najjar RP, Chiquet C, Teikari P, Cornut PL, Blaustrat B, Denis P, Cooper HM, Gonfier C. 2014. Aging of non-visual spectral sensitivity to light in humans: ompensatory mechanisms? PLoS One. 9(1):e85837. doi:https://doi.org/10.1371/journal.pone.0085837.
- Najjar RP, Zeitzer JM. 2016. Temporal integration of light flashes by the human circadian system. J Clin Invest. 126(3):938–947. doi:https://doi.org/10.1172/JCI82306.
- Nelson DE, Takahashi JS. 1991. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res. 554(1):272–277. doi:https://doi.org/10.1016/0006-8993(91)90200-f.
- Nelson DE, Takahashi JS. 1999. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol Regul Integr Comp Physiol. 277(5):R1351–R1361. doi:https://doi.org/10.1152/ajpregu.1999.277.5.R1351.
- Nowozin C, Wahnschaffe A, Rodenbeck A, de Zeeuw J, Hadel S, Kozakov R, Schopp H, Munch M, Kunz D. 2017. Applying melanopic lux to measure biological light effects on melatonin suppression and subjective sleepiness. Curr Alzheimer Res. 14(10):1042–1052. doi:https://doi.org/10.2174/1567205014666170523094526.
- Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. 2013. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the Heijo-Kyo study. J Clin Endocrinol Metab. 98(1):337–344. doi:https://doi.org/10.1210/jc.2012-2874.
- Ohno Y. 2014. Practical use and calculation of CCT and duv. Leukos. 10(1):47–55. doi:https://doi.org/10.1080/15502724.2014.839020.
- Owen J, Arendt J. 1992. Melatonin suppression in human subjects by bright and dim light in Antarctica: time and season-dependent effects. Neurosci Lett. 137(2):181–184. doi:https://doi.org/10.1016/0304-3940(92)90399-R.
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. 2005. Illumination of the melanopsin signaling pathway. Science. 307(5709):600–604. doi:https://doi.org/10.1126/science.1105121.
- Park Y-M-M, White AJ, Jackson CL, Weinberg CR, Sandler DP. 2019. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med. 179(8):1061–1071. doi:https://doi.org/10.1001/jamainternmed.2019.0571.
- Pattison PM, Tsao JY, Brainard GC, Bugbee B. 2018. LEDs for photons, physiology and food. Nature. 563:493–500. doi:https://doi.org/10.1038/s41586-018-0706-x.
- Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, Lockley SW, Cain SW. 2019. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci USA. 116(24):12019–12024.
- Pilorz V, Helfrich-Forster C, Oster H. 2018. The role of the circadian clock system in physiology. Pflugers Arch. 470(2):227–239. doi:https://doi.org/10.1007/s00424-017-2103-y.
- Pittendrigh C, Elliott J, Takamura T. 1984. The circadian component in photoperiodic induction. In: Porter R, Collins GM, editors. Ciba foundation symposium 104 ‐ photoperiodic regulation of insect and molluscan hormones. Vol. 104. p. 26–47.
- Portnov BA, Stevens RG, Samociuk H, Wakefield D, Gregorio DI. 2016. Light at night and breast cancer incidence in Connecticut: an ecological study of age group effects. Sci Total Environ. 572:1020–1024. doi:https://doi.org/10.1016/j.scitotenv.2016.08.006.
- Prayag AS, Münch M, Aeschbach D, Chellappa SL, Gronfier C. 2019. Light modulation of human clocks, wake and sleep. Clocks Sleep. 1(1):193–208. doi:https://doi.org/10.3390/clockssleep1010017.
- Prayag AS, Najjar RP, Gronfier C. 2019b. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J Pineal Res. 66(4):e12562. doi:https://doi.org/10.1111/jpi.12562.
- Price LLA, Lyachev A, Khazova M. 2017. Optical performance characterization of light-logging actigraphy dosimeters. J Opt Soc Am. 34(4):545–557. doi:https://doi.org/10.1364/JOSAA.34.000545.
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. 1998. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 95(1):340–345. doi:https://doi.org/10.1073/pnas.95.1.340.
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. 2000. A novel human opsin in the inner retina. J Neurosci. 20(2):600–605. doi:https://doi.org/10.1523/JNEUROSCI.20-02-00600.2000.
- Provencio I, Rollag MD, Castrucci AM. 2002. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 415(6871):493. doi:https://doi.org/10.1038/415493a.
- Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. 2014. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 37(2):271–281. doi:https://doi.org/10.5665/sleep.3396.
- Rahman SA, St Hilaire MA, Gronfier C, Chang AM, Santhi N, Czeisler CA, Klerman EB, Lockley SW. 2018. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol. 596(11):2147–2157. doi:https://doi.org/10.1113/JP275501.
- Rahman SA, St. Hilaire MA, Lockley SW. 2017. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol Behav. 177:221–229. doi:https://doi.org/10.1016/j.physbeh.2017.05.002.
- Razavi P, Devore EE, Bajaj A, Lockley SW, Figueiro MG, Ricchiuti V, Gauderman WJ, Hankinson SE, Willett WC, Schernhammer E. 2019. Shift work, chronotype, and melatonin rhythm in nurses. Cancer Epidemiol Biomark Prev. 28(7):1177–1186. doi:https://doi.org/10.1158/1055-9965.EPI-18-1018.
- Rea MS, Bierman A, Figueiro MG, Bullough JD. 2008. A new approach to understanding the impact of circadian disruption on human health. J Circadian Rhythms. 6(1):7. doi:https://doi.org/10.1186/1740-3391-6-7.
- Refinetti R. 2003. Effects of prolonged exposure to darkness on circadian photic responsiveness in the mouse. Chronobiol Int. 20(3):417–440. doi:https://doi.org/10.1081/CBI-120021443.
- Revell VL, Arendt J, Fogg LF, Skene DJ. 2006. Alerting effects of light are sensitive to very short wavelengths. Neurosci Lett. 399(1):96–100. doi:https://doi.org/10.1016/j.neulet.2006.01.032.
- Revell VL, Barrett DC, Schlangen LJ, Skene DJ. 2010. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiol Int. 27(9–10):1762–1777. doi:https://doi.org/10.3109/07420528.2010.516048.
- Revell VL, Skene DJ. 2007. Light‐induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 24(6):1125–1137. doi:https://doi.org/10.1080/07420520701800652.
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WG, Van Someren EW. 2008. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 299(22):2642–2655. doi:https://doi.org/10.1001/jama.299.22.2642.
- Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. 2009. A missense variant (p10l) of the melanopsin (opn4) gene in seasonal affective disorder. J Affect Disord. 114(1):279–285. doi:https://doi.org/10.1016/j.jad.2008.08.005.
- Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. 2013. Light and the human circadian clock. Handb Exp Pharmacol. 217:311–331.
- Roenneberg T, Merrow M. 2016. The circadian clock and human health. Curr Biol. 26(10):R432–R443. doi:https://doi.org/10.1016/j.cub.2016.04.011.
- Ross JK, Arendt J, Horne J, Haston W. 1995. Night-shift work in Antarctica: sleep characteristics and bright light treatment. Physiol Behav. 57(6):1169–1174. doi:https://doi.org/10.1016/0031-9384(95)00018-E.
- Royer M, Ballentine NH, Eslinger PJ, Houser K, Mistrick R, Behr R, Rakos K. 2012. Light therapy for seniors in long term care. J Am Med Dir Assoc. 13(2):100–102. doi:https://doi.org/10.1016/j.jamda.2011.05.006.
- Royer MP, Wilkerson A, Wei M, Houser KW, Davis RG. 2017b. Human perceptions of color rendition vary with average fidelity, average gamut, and gamut shape. Lighting Res. Technol. 49(8):966–991. doi:https://doi.org/10.1177/1477153516663615.
- Rüger M, St Hilaire MA, Brainard GC, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. 2013. Human phase response curve to a single 6.5 h pulse of short‐wavelength light. J Physiol. 591(1):353–363. doi:https://doi.org/10.1113/jphysiol.2012.239046.
- Russart KLG, Nelson RJ. 2018. Light at night as an environmental endocrine disruptor. Physiol Behav. 190:82–89. doi:https://doi.org/10.1016/j.physbeh.2017.08.029.
- Sadeghniiat-Haghighi K, Yazdi Z, Jahanihashemi H, Aminian O. 2011. The effect of bright light on sleepiness among rapid-rotating 12-hour shift workers. Scand J Work Environ Health. 37(1):77–79. doi:https://doi.org/10.5271/sjweh.3086.
- Sahin L, Figueiro MG. 2013. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol Behav. 116–117:1–7. doi:https://doi.org/10.1016/j.physbeh.2013.03.014.
- Schmidt TM, Chen S-K HS. 2011. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34(11):572–580. doi:https://doi.org/10.1016/j.tins.2011.07.001.
- Segal AY, Sletten TL, Flynn-Evans EE, Lockley SW, Rajaratnam SMW. 2016. Daytime exposure to short- and medium-wavelength light did not improve alertness and neurobehavioral performance. J Biol Rhythms. 31(5):470–482. doi:https://doi.org/10.1177/0748730416659953.
- Shimomura K, Menaker M. 1994. Light-induced phase shifts in tau mutant hamsters. J Biol Rhythms. 9(2):97–110. doi:https://doi.org/10.1177/074873049400900201.
- Sletten TL, Ftouni S, Nicholas CL, Magee M, Grunstein RR, Ferguson S, Kennaway DJ, O’Brien D, Lockley SW, Rajaratnam SM. 2017. Randomised controlled trial of the efficacy of a blue-enriched light intervention to improve alertness and performance in night shift workers. Occup Environ Med. 74(11):792–801. doi:https://doi.org/10.1136/oemed-2016-103818.
- Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. 2009. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 24(1):73–84. doi:https://doi.org/10.1177/0748730408328973.
- Smith KA, Schoen MW, Czeisler CA. 2004. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 89(7):3610–3614. doi:https://doi.org/10.1210/jc.2003-032100.
- Smith MR, Eastman CI. 2009. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 26(4):709–725. doi:https://doi.org/10.1080/07420520902927742.
- Smith MR, Eastman CI. 2012. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep. 4:111–132. doi:https://doi.org/10.2147/NSS.S10372.
- Smith MR, Fogg LF, Eastman CI. 2009a. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 32(11):1481–1489. doi:https://doi.org/10.1093/sleep/32.11.1481.
- Smith MR, Revell VL, Eastman CI. 2009b. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 10(3):287–294. doi:https://doi.org/10.1016/j.sleep.2008.05.005.
- Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L, Portaluppi F. 2016. Circadian disruption: new clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int. 1–19.
- Souman JL, Borra T, de Goijer I, Schlangen LJM, Vlaskamp BNS, Lucassen MP. 2018b. Spectral tuning of white light allows for strong reduction in melatonin suppression without changing illumination level or color temperature. J Biol Rhythms. 33(4):420–431. doi:https://doi.org/10.1177/0748730418784041.
- Souman JL, Tinga AM, Te Pas SF, van Ee R, Vlaskamp BNS. 2018. Acute alerting effects of light: a systematic literature review. Behav Brain Res. 337:228–239. doi:https://doi.org/10.1016/j.bbr.2017.09.016.
- Spitschan M. 2019. Photoreceptor inputs to pupil control. J Vis. 19(9):5. doi:https://doi.org/10.1167/19.9.5.
- Spitschan M, Lazar R, Yetik E, Cajochen C. 2019b. No evidence for s cone contribution to acute neuroendocrine and alerting responses to light. Curr Biol. 29(24):R1297–R1298. doi:https://doi.org/10.1016/j.cub.2019.11.031.
- Spitschan M, Stefani O, Blattner P, Gronfier C, Lockley SW, Lucas RJ. 2019a. How to report light exposure in human chronobiology and sleep research experiments. Clocks Sleep. 1(3):280–289. doi:https://doi.org/10.3390/clockssleep1030024.
- Stebelová K, Molčan L, Okuliarová M, Hanuliak P, Hartmann P, Hraska J, Zeman M. 2014. The influence of indoor lighting with low blue light dose on urine 6-sulphatoxymelatonin concentrations and sleep efficiency of healthy volunteers. Biol Rhythm Res. 46(1):137–145. doi:https://doi.org/10.1080/09291016.2014.963949.
- Stenvers DJ, van Dorp R, Foppen E, Mendoza J, Opperhuizen AL, Fliers E, Bisschop PH, Meijer JH, Kalsbeek A, Deboer T. 2016. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci Rep. 6:35662. doi:https://doi.org/10.1038/srep35662.
- Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS, Reinlib L. 2007. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 115(9):1357–1362. doi:https://doi.org/10.1289/ehp.10200.
- Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. 2014. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 64(3):207–218. doi:https://doi.org/10.3322/caac.21218.
- Stevens RG, Rea MS. 2001. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 12(3):279–287. doi:https://doi.org/10.1023/A:1011237000609.
- Stockman A, Sharpe LT. 1999. Cone spectral sensitivities and color matching. In: Gegenfurtner KR, Sharpe LT, editors. Color vision: from genes to perception. Cambridge (UK): Cambridge UP. p. 53–87.
- Stothard ER, McHill AW, Depner CM, Birks BR, Moehlman TM, Ritchie HK, Guzzetti JR, Chinoy ED, LeBourgeois MK, Axelsson J, et al. 2017. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr Biol. 27(4):508–513. doi:https://doi.org/10.1016/j.cub.2016.12.041.
- Sulli G, Manoogian ENC, Taub PR, Panda S. 2018. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 39(9):812–827. doi:https://doi.org/10.1016/j.tips.2018.07.003.
- Terman M, Terman JS. 1999. Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry. 60(11):799–808.
- Thapan K, Arendt J, Skene DJ. 2001. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 535(1):261–267. doi:https://doi.org/10.1111/j.1469-7793.2001.t01-1-00261.x.
- Torii M, Kojima D, Okano T, Nakamura A, Terakita A, Shichida Y, Wada A, Fukada Y. 2007. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 581(27):5327–5331. doi:https://doi.org/10.1016/j.febslet.2007.10.019.
- van de Werken M, Giménez MC, de Vries B, Beersma DGM, Gordijn MCM. 2013. Short-wavelength attenuated polychromatic white light during work at night: limited melatonin suppression without substantial decline of alertness. Chronobiol Int. 30(7):843–854. doi:https://doi.org/10.3109/07420528.2013.773440.
- van der Lely S, Frey S, Garbazza C, Wirz-Justice A, Jenni OG, Steiner R, Wolf S, Cajochen C, Bromundt V, Schmidt C. 2015. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. J Adolesc Health. 56(1):113–119. doi:https://doi.org/10.1016/j.jadohealth.2014.08.002.
- Van Dycke Kirsten CG, Rodenburg W, van Oostrom Conny TM, van Kerkhof Linda WM, Pennings Jeroen LA, Roenneberg T, van Steeg H, van der Horst Gijsbertus TJ. 2015. Chronically alternating light cycles increase breast cancer risk in mice. Curr Biol. 25(14):1932–1937. doi:https://doi.org/10.1016/j.cub.2015.06.012.
- Veitch JA, Fotios SA, Houser KW. 2019. Judging the scientific quality of applied lighting research. Leukos. 15(2–3):97–114. doi:https://doi.org/10.1080/15502724.2018.1550365.
- Vetter C. 2020. Circadian disruption: what do we actually mean? Eur J Neurosci. 51:531–550. doi:https://doi.org/10.1111/ejn.14255.
- Vetter C, Juda M, Lang D, Wojtysiak A, Roenneberg T. 2011. Blue-enriched office light competes with natural light as a zeitgeber. Scand J Work Environ Health. 37(5):437–445. doi:https://doi.org/10.5271/sjweh.3144.
- Vetter C, Phillips AJK, Silva A, Lockley SW, Glickman G. 2019. Light me up? Why, when, and how much light we need. J Biol Rhythms. 34(6):573–575. doi:https://doi.org/10.1177/0748730419892111.
- Vetter C, Scheer FAJL. 2019. A healthy lifestyle-reducing T2DM risk in shift workers? Nat Rev Endocrinol. 15(4):194–196. doi:https://doi.org/10.1038/s41574-019-0164-z.
- Viola AU, James LM, Schlangen LJM, Dijk DJ. 2008. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 34(4):297–306. doi:https://doi.org/10.5271/sjweh.1268.
- Wams EJ, Woelders T, Marring I, van Rosmalen L, Beersma DGM, Gordijn MCM, Hut RA. 2017. Linking light exposure and subsequent sleep: a field polysomnography study in humans. Sleep. 1; 40(12):zsx165. doi:https://doi.org/10.1016/0006-2944(75)90147-7.
- Webler FS, Spitschan M, Foster RG, Andersen M, Peirson SN. 2019. What is the ‘spectral diet’ of humans? Current Oopinion in Bbehavioral Ssciences. 30:80-86. doi:https://doi.org/10.1016/j.cobeha.2019.06.006.
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. 1993. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993 Oct;265(4 Pt 2):R846-57. doi: https://doi.org/10.1152/ajpregu.1993.265.4.R846. PMID: 8238456
- Wehr TA. 1991. The Ddurations of Hhuman Mmelaton in Ssecretion and Ssleep Respond to Changes in Daylength (Photoperiod). J Clin Endocrinol Metab. 73(6):1276–1280. doi:https://doi.org/10.1210/jcem-73-6-1276.
- Wehr TA. 1997. Melatonin and Seasonal Rhythms. J Bio Rhyth. 12(6):518-527. doi:https://doi.org/10.1177/074873049701200605.
- Wei M, Houser KW. 2016. Systematic changes in gamut size affects color preference. LEUKOS. 13(1):23–32. doi:https://doi.org/10.1080/15502724.2016.1192402.
- Wei M, Houser KW, Orland B, Lang DH, Ram N, Sliwinski MJ, Bose M. 2014. Field study of office worker responses to fluorescent lighting of different CCT and lumen output. J Environ Psychol. 39:62–76. doi:https://doi.org/10.1016/j.jenvp.2014.04.009.
- West KE, Jablonski MR, Warfield B, Cecil KS, James M, Ayers MA, Maida J, Bowen C, Sliney DH, Rollag MD, et al. 2011. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol. 110(3):619–626. doi:https://doi.org/10.1152/japplphysiol.01413.2009.
- White AJ, Weinberg CR, Park Y-M, D’Aloisio AA, Vogtmann E, Nichols HB, Sandler DP. 2017. Sleep characteristics, light at night and breast cancer risk in a prospective cohort. Int J Cancer. 141(11):2204–2214. doi:https://doi.org/10.1002/ijc.30920.
- Woelders T, Beersma DGM, Gordijn MCM, Hut RA, Wams EJ. 2017. Daily light exposure patterns reveal phase and period of the human circadian clock. J Biol Rhythms. 32(3):274–286. doi:https://doi.org/10.1177/0748730417696787.
- Wright KP, Badia P, Myers BL, Plenzler SC, Hakel M. 1997. Caffeine and light effects on nighttime melatonin and temperature levels in sleep-deprived humans. Brain Res. 747(1):78–84. doi:https://doi.org/10.1016/S0006-8993(96)01268-1.
- [IWBI] International Well Building Institute. WELL Building Standard v2, Q3 2020 Version. Section L03: Circadian Lighting Design. 2020. Available online at: https://v2.wellcertified.com/v/en/light/feature/3 (accessed November 2, 2020).
- Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. 2013. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 23(16):1554–1558. doi:https://doi.org/10.1016/j.cub.2013.06.039.
- Ye M, Zheng S, Wang M, Luo MR. 2018. The effect of dynamic correlated colour temperature changes on alertness and performance. LEUKOS. 50(7):1070–1081. doi: https://doi.org/10.1177/1477153518755617
- Yoon IY, Jeong DU, Kwon KB, Kang SB, Song BG. 2002. Bright light exposure at night and light attenuation in the morning improve adaptation of night shift workers. Sleep. 25(3):351–356.
- Yoshimura T, Ebihara S. 1996. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/ J (rd/rd) and normal CBA/N (+/+)mice. J Comp Physiol A. 178(6):797–802. doi:https://doi.org/10.1007/BF00225828.
- Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. 2000. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 526(3):695–702. doi:https://doi.org/10.1111/j.1469-7793.2000.00695.x.
- Zeitzer JM, Friedman L, Yesavage JA. 2011. Effectiveness of evening phototherapy for insomnia is reduced by bright daytime light exposure. Sleep Med. 12(8):805–807. doi:https://doi.org/10.1016/j.sleep.2011.02.005.
