?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Intermittent bright light during the night has shown to be able to generate circadian phase-shifting effects, suppress melatonin and induce alertness, but little attention has been devoted to the effects of diurnal intermittent bright light. Following a night of sleep restriction, forty participants were exposed in a counterbalanced within-subject design to an intermittent (100 lux – 1000 lux), a continuous dim (100 lux) and a continuous bright light condition (1000 lux) each lasting 90 min. Repeated assessments of self-reported sleepiness, cognitive performance and physiological arousal as well as subjective visual comfort were taken during each light condition. Results showed that alertness-related parameters were not significantly affected by the light conditions: neither the intermittent nor the bright condition improved alertness compared to the dim condition. Visual comfort was highest in the dim condition, followed by the intermittent and bright conditions respectively, even though the visualizations showed marked decreases in visual comfort during the bright light phases in the intermittent condition. The results illustrate the diversity in mechanisms underlying these visual experiences and neurobehavioral responses.
1. Introduction
The light that we typically are exposed to in daily life is highly dynamic and varies substantially throughout the day (Espiritu et al. Citation1994; Hébert et al. Citation1998; Okudaira et al. Citation1983; Savides et al. Citation1986). These unpredictable variations in the luminous environment are largely due to situational and behavioral factors (Roenneberg and Foster Citation1997). Situational factors may include the time of day and the weather, for instance. Behavioral factors comprise, among others, one’s location or gaze direction (Peeters et al. Citation2020). In the highly dynamic luminous profile that results from this, the cumulative exposure to bright light (e.g., more than 1000 lux at the eye) across the day is relatively short and varies approximately between one half hour and two and a half hours (Crowley et al. Citation2015; Espiritu et al. Citation1994; Hébert et al. Citation1998; Hubalek et al. Citation2010; Peeters et al. Citation2020; Savides et al. Citation1986; Smolders et al. Citation2013). This brighter light exposure is typically not bundled in one consecutive period, but is randomly distributed in shorter pulses of minutes up to about half an hour spread across the day (Hébert et al. Citation1998; Okudaira et al. Citation1983; Savides et al. Citation1986). This results in a luminous profile containing intermittent exposure to brighter light, in which periods above a certain bright light threshold alternate with periods below this threshold.
1.1. Circadian effects of nocturnal intermittent bright light
The effects of intermittent light during the night on the circadian system have attracted quite some research attention (Gronfier et al. Citation2004; Najjar and Zeitzer Citation2016; Rahman et al. Citation2021; Rimmer et al. Citation2000; Zeitzer et al. Citation2011b). Bright light pulses (~9500 lux at the eye) – ranging from 5 to 46 min, alternated with 20 to 60 min of darkness in between pulses – timed around the core body temperature minimum have been demonstrated to effectively generate phase-shifts (Gronfier et al. Citation2004; Rimmer et al. Citation2000). For example, intermittent light in which the effective duration of the bright light exposure was reduced by 37% resulted in a decrease of only 10% of the phase-shifting response compared to a continuous bright light episode of the same intensity (Rimmer et al. Citation2000). At certain interstimulus intervals, micro-second flashes of light appeared to even induce larger phase delays than continuous equiluminous light (Najjar and Zeitzer Citation2016). In intermittent light, the photic drive induced by the initial pulse is retained while the photoreceptors – bleached by this initial pulse – have the opportunity to recover and regain their sensitivity to light (at least partially) during the stimulus free/dark interval (Rimmer et al. Citation2000). The persistent and sustained firing of the intrinsically photosensitive retinal ganglion cells (ipRGC) might play a role in the retention of the photic drive in response to brief light pulses (Lucas et al. Citation2014). Furthermore, the intrinsic light sensitivity of ipRGCs can be influenced by their photic history, for instance, prior exposure to longer wavelengths can increase their intrinsic sensitivity whereas prior exposure to shorter wavelengths can decrease their sensitivity (Chellappa et al. Citation2014; Mure et al. Citation2009). Similarly, brighter preceding light exposure has been implicated to reduce the sensitivity to subsequent light exposure (Chang et al. Citation2011, Citation2013; Hébert et al. Citation2002; te Kulve et al. Citation2019; Zeitzer et al. Citation2011a). Prior studies have concluded that in many species the critical duration of a light pulse to achieve a phase-shifting response appears to be 5 to 10 min (Kronauer et al. Citation1999), though later studies have also demonstrated the effectiveness of millisecond flashes in phase-shifting responses under highly-controlled conditions (Najjar and Zeitzer Citation2016; Zeitzer et al. Citation2011b).
1.2. Acute effects of nocturnal intermittent bright light
Apart from these circadian effects, Yang et al. (Citation2018) and Yang et al. (Citation2019) have shown that three cycles of 30 min of bright light (1000 lux at the eye) alternated with 30 min of dim light (<5 lux at the eye) before bedtime can significantly induce continuous alertness improvements and momentary performance increments. Interestingly, hourly bright 10-min breaks (4700 lux at the eye) in the study by Lee et al. (Citation2020) decreased melatonin suppression compared to 10-min breaks in medium light intensity (430 lux at the eye) during a simulated night shift (1:00–6:00) with 550 lux at the eye. In contrast, a scenario with 10-min breaks in dim light (<1 lux at the eye) increased melatonin suppression compared to the condition with medium and bright light in the breaks. This would suggest that periods with lower light levels allow regeneration of photopigments in between bright light periods, and thereby might also benefit acute neurobehavioral responses. Yet, no statistically significant effects on subjective alertness or task performance were found in that study (Lee et al. Citation2020).
1.3. Acute effects of diurnal intermittent bright light
Although the acute and circadian effects of intermittent light in the evening or night are highly relevant, the effects of intermittent bright light during daytime are potentially even more important as the light we are exposed to during daytime is highly dynamic and results in frequent transitions between illuminance levels. However, most studies investigating acute alerting effects of diurnal light have focused on static light of one intensity during several minutes up to hours (e.g., Huiberts et al. Citation2016; Ru et al. Citation2019; Rüger et al. Citation2005; Smolders et al. Citation2012). To our knowledge, only one laboratory study has investigated to what extent intermittent bright light affects daytime behavior and experiences compared to static exposure to ordinary room light (Iskra-Golec and Smith Citation2008, Citation2011). Compared to 300 lux at the eye, six cycles of 15 min of bright light (4000 lux at the eye) alternated with 45 min of 300 lux at the eye between 11:00 and 17:00 improved global vigor (alertness, sleepiness, effort and weariness) and task performance during the late morning. Yet, intermittent bright light was found to be less comfortable as compared to ordinary room light (Iskra-Golec and Smith Citation2008), potentially due to the unusually high level of indoor bright lighting and/or by the abrupt transitions that were employed in the intermittent scenario (Kompier et al. Citation2021, Citation2020). More gradual transitions may ameliorate the visual experience of an intermittent bright light scenario (Chraibi et al. Citation2019). This is important as visual comfort may play a mediating role in the effect of light on alertness and mood (Veitch et al. Citation2011). Additionally, light scenarios designed for practical, real-life applications should strive to optimize both visual experience and acute alerting effects in integrative lighting solutions (CIE Citation2020). Therefore, in the current study we investigated the effects of daytime intermittent bright light on both alertness and visual comfort using fast, but gradual transitions.
1.4. Rationale and hypotheses
Prior research has shown that alerting effects of diurnal light on healthy participants are typically small and inconsistent (Lok et al. Citation2018; Souman et al. Citation2018). One potential explanation for this could be a ceiling level of alertness during daytime (Lok et al. Citation2019). Following partial sleep deprivation, the baseline alertness during the day is expected to decrease, leaving more room for improvements in alertness upon an environmental (light) intervention (Phipps-Nelson et al. Citation2003). The current study therefore employed sleep restriction (to 5 hr) for one night to study to what extent intermittent light can induce alertness in comparison to both constant bright (~1000 lux at the eye) and constant dim (~100 lux at the eye) light. The bright light pulse in the intermittent light condition lasted 10 min, in line with the duration of typical pulses in natural daylight exposure (Savides et al. Citation1986). The subsequent (relatively) dim light was chosen to represent a badly lit office environment and lasted 20 min to align the ratio bright to dim light with prior studies on the acute effects of intermittent light (Iskra-Golec and Smith Citation2008; Yang et al. Citation2019, Citation2018).
We expected that intermittent light would generate alerting effects comparable to those generated by continuous bright light. Furthermore, we examined to what extent intermittent light is perceived as visually comfortable in comparison to continuous bright and continuous dim light. By using fast, but gradual transitions in the intermittent light scenario, we expected to limit the visual discomfort that may result from abrupt transitions between bright and dim light. Last, we compared the temporal trajectories of both alertness and visual comfort during the static and intermittent light conditions to investigate the response dynamics of these variables.
2. Method
2.1. Design
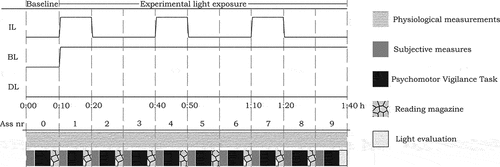
A balanced cross-over design was employed to test the effects of intermittent light (IL; 100–1000 lux at the eye) on alertness and comfort against a static dim (DL; 100 lux at the eye) and static bright (BL; 1000 lux at the eye) light condition. The intermittent light consisted of three cycles of ten minutes of bright light followed by twenty minutes of dim light (). Participants were exposed to each of the three conditions while repeatedly reporting on their alertness and visual comfort. Before each experimental light exposure, there was a 10-min baseline measurement in dim light (100 lux), resulting in 100-min sessions per condition.
Fig. 1. Visualization of the three experimental lighting conditions and measurement protocol of one session. IL = intermittent light, BL = bright light, DL = dim light, Ass nr = assessment number.
Participants experienced all light conditions on one day and the order was counterbalanced between participants. The first session started at, on average, 10:28 (median: 10:24, range: 10:14 to 10:45), the second one at 12:26 (median: 12:24, range: 12:10 to 12:47) and the third one at 14:22 (median: 14:30, range: 14:01 to 14:59). Between sessions, participants received a light snack and could visit the restroom.
The study was approved by the Institutional Ethical Review Board (HTI Ethical Review Board – experiment ID 1285). This study’s design and hypotheses were preregistered via the Open Science Framework (see: doi:10.17605/OSF.IO/UM29Q)
2.2. Participants
Power calculations were done using data from Kompier et al. (Citation2020), (Citation2021) with the Superpower package in R (Caldwell et al. Citation2021) and the spreadsheet belonging to Lakens (Citation2017). These analyses are described in the preregistration and yielded a required sample size of 41 participants. Forty-three participants (14 female) were recruited via a participant database and convenience sampling. They completed a screening questionnaire with self-report items before selection. Participants were generally healthy (SF-36 (van der Zee and Sanderman Citation2012); MGH = 80.7, SDGH = 13.7), reported no visual or auditory deficits, and passed the Ishihara Colorblindness test. All participants were between 18 and 30 years old (Mage = 22.7, SDage = 3.0), were free from medical and psychiatric disorders, and did not take medication (birth control not considered) structurally. Furthermore, participants were no extreme chronotypes (3.8 < midsleep < 6.8 based on Zavada et al. (Citation2005), assessed using the Munich Chronotype Questionnaire (Roenneberg et al. Citation2003)), reported no sleeping problems (Pittsburgh Sleep Quality Index < 6 (PSQI; (Buysse et al. Citation1989)), and slept habitually between 7 and 9 hours per night. Last, participants had not traveled between time zones or worked night shifts in the three months prior to the start of the study.
2.3. Sleep restriction protocol
Participants slept according to their regular schedule for three nights and then followed a sleep restriction protocol of 5 hr (between 1:00 and 6:00) on the night before the start of the study. Adherence was assessed using actigraphy (wrist-worn Axivity AX3 trackers) and the Core Consensus Sleep Diary (Carney et al. Citation2012). One participant did not adhere properly and was therefore excluded. Two others terminated the experimental session before completing all conditions. The forty included participants (13 female) had a mean sleep duration (Mrestriction) of 4.6 hours during the sleep restricted night (standard deviation: SDrestriction = 0.4, rangerestriction = 2.8–5.2), which was on average 3.1 hr less than on the three nights before (Mregular = 7.7 hours, SDregular = 1.3, rangeregular = 4.9–11.6). Furthermore, participants were instructed to not consume alcohol, drugs, caffeine or nicotine in the 24 hr before the start of the study. At the start of the experimental day, the mean KSS score was 5.1 (SDKSS-start = 1.8, range KSS-start = 2.0–8.0).
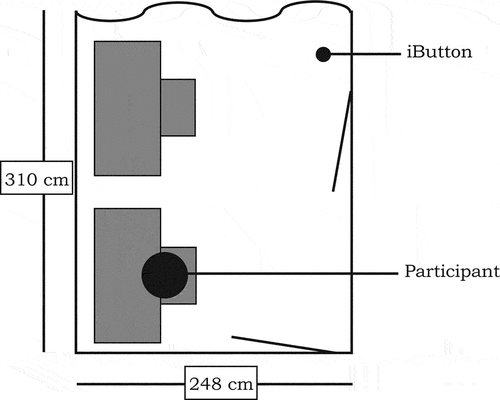
2.4. Setting
The experiment was conducted in rooms without daylight access measuring 2.5 × 3.6 m2 and fitted with a desk and a desktop computer (see ). The reflectance () of surfaces in the room was assessed by means of a calibrated JETI Specbos 1201 spectroradiometer, using
, where L is the luminance and Ev the illuminance. The front and back walls of the room were off-white (
), the curtain was light gray (
) and the left wall was gray (
). The doors were white (
, the desk was light gray (
) and the floor dark gray (
). Indoor climate was kept as constant as possible during all sessions, however due to use of fluorescent lamps considerable heat was produced. One iButton was placed 2.5 m from the participant at desk height (0.77 m) to measure the room temperature at a 10-min sampling interval (MTair-dim ± SD = 22.3 ± 2.0°C; MTair-intermittent = 22.5 ± 2.0°C; MTair-bright = 22.9 ± 2.0°C).
Each room was equipped with eight recessed Philips Savio (TBS770 3x54W/827/865HDFAC-MLOCVC) luminaires that covered the entire ceiling. Each luminaire contained three fluorescent tubes of 54 W, of which two were 6500 K and one was 2700 K, and was equipped with an acrylate micro-lens optic cover.
Illuminance at the eye (in the vertical plane) was measured using the calibrated spectroradiometer. shows the normalized spectral power distributions of the bright (1000 lux) and dim (100 lux) light settings, with equal CCT. displays the alpha-opic equivalent daylight illuminances (EDI), alpha-opic daylight efficacy ratios (DER), illuminance, CCT and color fidelity index (Rf) of the settings. Transitions between the settings spanned 2 s to create markedly visible, yet gradual light transitions.
Fig. 3. Normalized spectral power distribution of the bright (solid line) and dim (dotted line) light settings.
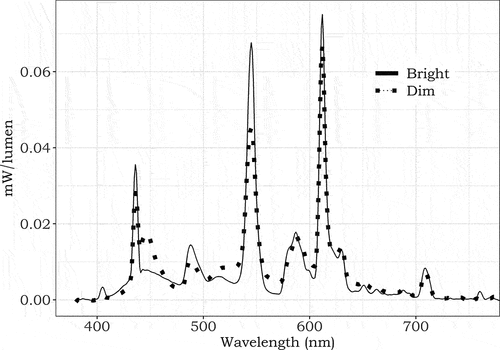
Table 1. Illuminance, CCT, Color fidelity index, α-opic EDI, and α-opic DER of the light settings (at the eye position).
2.5. Measures
2.5.1. Subjective experiences
Participants’ visual sensation was evaluated with two items probing experienced intensity and color (SensationVI and SensationVC) on 7-point rating scales ranging from Very Low (−3) to Very High (3) and Very Cool (−3) to Very Warm (3) respectively. Participants rated their acceptance of the lighting (AcceptanceV) on a binary scale (Acceptable/Unacceptable). Subjective visual comfort (ComfortV) was assessed on a 6-point rating scale ranging from Very Uncomfortable (−2) to Just Uncomfortable (0) and from Just Comfortable (1) to Very Comfortable (3), all as in Kompier et al. (Citation2020, Citation2021).
Mood was probed using eight items, of which six (Calm, Tense, Lively, Drowsy, Sleepy, Awake) from the Activation-Deactivation checklist (Thayer Citation1989) and two (Happy, Sad) from Smolders et al. (Citation2012). Response scales ranged from Definitely Not (1) to Definitely (5). As Lively, Awake, Sleepy (reverse coded) and Drowsy (reverse coded) had a Cronbach’s α of 0.89, these scores were averaged into one variable: Vitality. Calm and Tense had a Cronbach’s α of 0.74, and were also averaged after reverse coding Tense. Sad was excluded from analysis due to low variance. Subjective sleepiness was assessed using the KSS with a response scale ranging from Extremely Alert (1) to Extremely Sleepy (9) (Åkerstedt and Gillberg Citation1990).
2.5.2. Cognitive performance
In an auditory psychomotor vigilance task (PVT) of five minutes (Dinges and Powell Citation1985), participants responded as fast as possible to short auditory stimuli of 400 Hz that lasted 200 ms each, while keeping their dominant hand rested on the space bar and eyes focused on a fixation cross. Trials were terminated when participants pressed the spacebar or after 2 seconds without response. These trials without a response were counted as lapses. The inter-stimulus interval was randomly drawn from a range between 1 and 9 s. Mean reaction time (MRT), 10% fastest reaction times (MRT10%fast), 10% slowest reaction times (MRT10%slow), standard deviation of reaction time (SDRT), and the coefficient of variation in reaction time (CVRT = (SDRT/MRT) x 100) were used as indicators of vigilance. Three items from the Short Stress State Questionnaire (SSSQ; (Helton and Nöswall Citation2010)) assessed the motivation during the PVT on 5-point Likert scales ranging from Not at all (1) to Extremely (5) (see Supplementary Materials S1). PVT motivation was calculated as the mean of the three items (Cronbach’s α = 0.93).
2.5.3. Electrodermal activity
Electrodermal activity (EDA) was used as a proxy for physiological arousal that is related to alertness (Oken et al. Citation2006). EDA was measured using TMSi software with two electrodes placed on the first phalanx of the index and middle fingers. Processing and artifact detection was done using institutional software (Boschman Citation2015). Mean tonic skin conductance level (SCL) during the PVT, measured in micro Siemens, was taken as an indicator of physiological arousal.
2.5.4. Evaluation of the light per condition
Two self-formulated items probed Satisfaction and Pleasantness on 7-point Likert scales that ranged from Very Unsatisfied (1) to Very Satisfied (7), and Very Unpleasant (1) to Very Pleasant (7) respectively (see Supplementary Materials S1). The items were averaged into one Light appraisal variable (Cronbach’s α = 0.93). Headache and Eye-Strain Symptoms (HES) were evaluated on eight 4-point scales ranging from Absent (0) to Severe (3) (Giménez et al. Citation2017; Viola et al. Citation2008) and averaged into one HES-factor (Cronbach’s α = 0.85).
2.5.5. Start questionnaire
Upon arrival in the laboratory, participants completed a questionnaire probing the means and duration of transport to the study location and the amount of time spent outside in daylight prior to their visit (Kompier et al. Citation2021, Citation2020). Food and beverage intake were measured using multiple-choice questions; momentary sleepiness was assessed using the KSS (Åkerstedt and Gillberg Citation1990). These control variables were considered as potential covariates in the analyses.
2.5.6. Additional parameters
The pupil diameter was measured using a Tobii 4C Eye-tracker with a frequency of 90 Hz for an exploratory investigation to examine whether pupil variation could be used as a proxy of sleepiness in light exposure (Lüdtke et al. Citation1998; Wilhelm et al. Citation1998). Furthermore, two iButtons were attached to the underarm and fingertip of the left hand to provide an indication of vasoconstriction of the participants (Rubinstein and Sessler Citation1990). Thermal sensation (SensationT) was evaluated on a 7-point scale ranging from Cold (−3) to Hot (3) based on ASHRAE 55 (ASHRAE Citation2004); thermal acceptance (AcceptanceT) on a binary scale (Acceptable/Unacceptable), and thermal comfort (ComfortT) on a 6-point rating scale identical to the one for visual comfort and as used in Kompier et al. (Citation2020), (Citation2021). These items are out of scope for this article, and thus not presented in this article.
2.6. Procedure
The study was conducted between April 12th and June 3rd, 2021. Subjects completed all three conditions on one experimental day. Upon arrival at 10:00, participants were welcomed and completed the start questionnaire. Subsequently, they received further instructions, applied the sensors and practiced the PVT for three minutes. Participants briefly read a magazine until the baseline period of the first session started. In the 10-min baseline assessment, participants completed the subjective measures, performed the 5-min PVT and evaluated their motivation during the task. After the baseline assessment, identical assessments followed every 10 min in the 90-min experimental light exposure. In between assessments, participants read magazines provided by the experimenters. At the end of each session, participants evaluated the light conditions before they could visit the bathroom and consumed a calorie-controlled snack (~300 kcal). Subsequently, the baseline for the next experimental light exposure started (second ~12:30 and third ~14:20). The procedure of the experimental sessions is shown in . At the end of the day, participants were debriefed and received a monetary compensation for their participation.
2.7. Statistical analysis
All statistical analyses were done in Rstudio 1.3.1073 with the “lme4,” “lmer,” “emmeans” and “Hmisc”-packages. Visualizations were created using “ggplot2.” Observations that deviated more than three standard deviations from the mean were identified as outliers and coded as missing (142 observations for MRT,CVRT and SDRT, 106 for SCL, 95 for MRT10%fast and MRT10%slow from a total of 1080 observations). Non-normally distributed variables were transformed (log transformations for SCL) or recoded (Calm into three categories: 1–3.5 = 1, 4–4.5 = 2, 5 = 3). The binary variable (AcceptanceV) was not analyzed statistically, but only examined visually due to the skewed distribution. For all statistical tests, an α-criterion of 0.01 was used to account for the multitude of dependent variables.
Linear mixed models (LMMs) were used to test the differences in alertness and visual experience-related parameters between the light conditions. We tested the main effect Light condition across all experimental assessments. In addition to these preregistered analyses, exploratory LMM analyses were done on two subsets of the data to explore the differences between conditions for the bright and dim phases of the intermittent light separately. One set contained the data of assessments 1, 4 and 7 of all light conditions (referred to as intermittent-bright set) the other contained all data of assessments 2, 3, 5, 6, 8 and 9 (referred to as intermittent-dim set). Post-hoc contrasts with Tukey correction tested the differences between the three light conditions in the complete set, in the intermittent-bright set and in the intermittent-dim set respectively. Cohen’s was calculated for all fixed effects in the models using
, in which R2 is the marginal R2 (Selya et al. Citation2012). Cohen’s
reflects the unique portion of variance accounted for by parameter
. In the result section, we report the post-hoc contrasts as well as the other fixed effects of the models. The statistics of the main effect of Light condition are reported in Supplementary Materials S2.
Subjective vitality, sleepiness, PVT metrics, SCL and mood were analyzed using separate LMMs with Participant and Session (1,2,3; nested within Participant) included as random intercepts. Random slopes for the effect of condition were explored to test interindividual differences (see Supplementary Materials S3). To correct for the effect of time-in-session, a parabolic function was fitted to the data of the continuous dim condition, and subsequently subtracted from the actual scores during all conditions for each of the dependent variables. The fixed effects were Light condition (as factor), General health, Room temperature and the Baseline score. Covariates were included if they correlated sufficiently (i.e., a significant correlation > 0.4) with the dependent variable, and did not show multicollinearity with other predictors (based on VIF scores). The intermittent condition and the bright condition were expected to produce similar alertness levels, therefore we tested the equivalence of the scores of the alertness-related parameters averaged over all experimental assessments in these conditions using the two one-sided tests procedure for dependent samples (Lakens Citation2017). shows the employed upper and lower equivalence bounds in these analyses, which were set to 51.3% of the standard deviation of the respective variable as described in the preregistration.
Table 2. Upper (ΔU) and lower (ΔL) equivalence bounds that were used in the equivalence test for the alertness-related parameters.
In the models for the comfort-related variables, Participant and Session (1,2,3; nested within Participant) were also included as random intercepts. Light condition was added as a fixed factor, and Room temperature and Baseline scores as covariates. For these variables (and for mood), we tested differences between the intermittent and bright condition rather than equivalence as we did not expect the outcomes to be equivalent. The power for the main effect of Light condition and the contrasts can be found in the Supplementary Materials S2.
For the end-of-condition variables Light appraisal and HES-factor, two-level models were used with only Participant as random intercept. Light condition was the only fixed effect in the model and marginal R2 was calculated as effect size measure of the main effect. Again, post-hoc analyses tested contrasts with Tukey correction. Power is reported in the Supplementary Materials S2.
The dataset as used for the analysis is available via the Open Science Framework (doi: 10.17605/OSF.IO/BSA9N.
3. Results
3.1. Alertness
3.1.1. Subjective vitality and sleepiness
Overall, subjective vitality did not significantly differ between the dim (Estimated Marginal Mean (EMM) ± Standard Error (SE) = 3.39 ± 0.08), intermittent (3.40 ± 0.08) and bright light conditions (3.48 ± 0.08; ). This is in line with the equivalence test between the intermittent and bright condition (t(39) = 2.34, p = .01), yet in contrast with what we expected regarding differences with the dim condition. The pooled vitality scores for assessments 1, 4 and 7 (i.e., the intermittent-bright set) also showed no significant differences between conditions (all p’s > 0.25; dim: 3.40 ± 0.08, intermittent: 3.56 ± 0.08, and bright: 3.51 ± 0.08). Likewise, no significant differences (all p’s > 0.41) existed between the dim (3.39 ± 0.09), intermittent (3.31 ± 0.09), and bright light (3.47 ± 0.09) conditions for assessments 2, 3, 5, 6, 8 and 9 (i.e., the intermittent-dim set). Baseline vitality (F1,116 = 125.69, p < .001, B ± SE = 0.64 ± 0.06, = 0.61) and Room temperature (F1,115 = 8.86, p = .003, B ± SE = −0.06 ± 0.02,
< 0.01) both significantly predicted self-reported vitality; a higher vitality score at baseline and a lower room temperature during assessments predicted higher vitality. General health was not significantly associated with vitality (F1,32 = 1.25, p = .27,
= 0.01).
Fig. 4. Temporal trajectory of a) vitality, b) sleepiness, c) mean reaction time for PVT, d) standard deviation for the PVT, e) mean reaction time for the 10% slowest responses of the PVT, f) coefficient of variation for the PVT, g) mean reaction time for the 10% fastest responses of the PVT, and h) PVT motivation. The graphs are based on the models without the correction for time in session. The error bars represent SE.
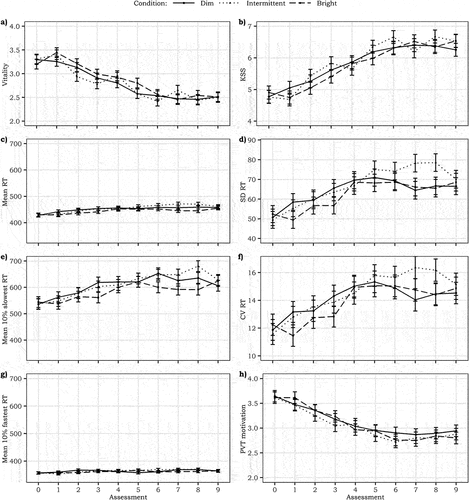
Similarly, there was no statistically significant difference in sleepiness between the intermittent (4.75 ± 0.17) and bright conditions (4.60 ± 0.17) compared to the dim condition (4.69 ± 0.17; ). The equivalence test for the intermittent and bright condition also indicated equivalence of these two conditions (t(39) = −2.47, p = .009). Sleepiness scores in the intermittent-bright set again showed no statistically significant differences between the dim (4.70 ± 0.15), intermittent (4.44 ± 0.15) or bright (4.60 ± 0.15) conditions (all p’s > 0.45). Similarly, in the intermittent-dim set no statistically significant differences (all p’s > 0.47) occurred between the dim (4.68 ± 0.19), intermittent (4.91 ± 0.18) and bright conditions (4.62 ± 0.18). Baseline KSS (F1,77 = 148.80, p < .001, B ± SE = 0.74 ± 0.05, = 0.74) and Room temperature (F1,106 = 7.90, p = .002, B ± SE = 0.12 ± 0.04,
< 0.01) both had a significant positive relationship with self-reported sleepiness. General health did not significantly predict sleepiness (F1,35 = 0.50, p = .48,
< 0.01).
3.1.2. Cognitive performance
There were no significant differences between the dim and intermittent or bright light conditions for the MRT, MRT10%fast, MRT10%slow, SDRT, CVRT or Motivation in the PVT (all p’s > 0.05; ). The equivalence tests indicated equivalence between the intermittent and bright light conditions for all PVT metrics (t(39) = 2.53–4.90, all p’s < 0.01). Moreover, the models testing the effect of Light condition in intermittent-bright and intermittent-dim sets separately showed no significant differences between the conditions for any of the performance metrics (all p’s > 0.08). Baseline PVT scores (MRT, MRT10%fast or MRT10%slow, SDRT, CVRT and motivation) did significantly and positively predict the respective PVT scores (all F’s > 16.80, B ± SE = 0.34–0.92 ± 0.04–0.10, all p’s ≤ 0.001, ’s = 0.06–4.14). None of the PVT metrics were significantly associated with the Room temperature (all F’s < 4.69, all p’s > 0.03, all
’s ≤ 0.01) or General health (all F’s < 4.99, all p’s > 0.03, all
’s ≤ 0.09), except that the latter was associated with the motivation during the PVT (F1,23 = 8.13, p = .009, B ± SE = 0.02 ± 0.01,
= 0.18).
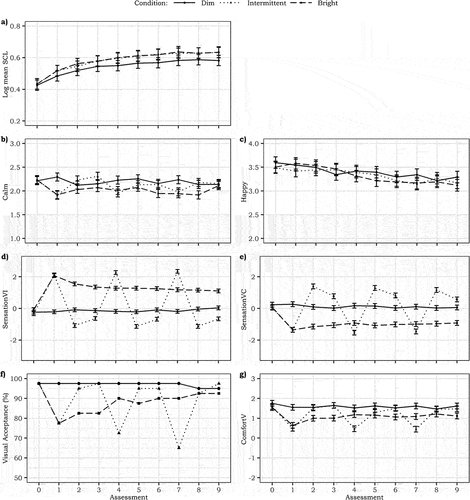
3.1.3. Skin conductance level
There were no significant differences in SCL in the intermittent (0.48 ± 0.03) or bright (0.48 ± 0.03) light conditions compared to the dim light condition (0.44 ± 0.03; see ). Equivalence between the intermittent and bright condition was accepted (t(39) = 4.02, p < .001). The separate models for intermittent-bright set and intermittent-dim set also showed no significant differences between the light conditions (all p’s > 0.24). Baseline SCL did positively predict SCL in the experimental assessments (F1,80 = 145.44, p < .001, B ± SE = 0.44 ± 0.03, = 0.60). Room temperature nor perceived General health predicted SCL significantly (F1,830 = 1.04, p = .31,
= 0.04 and F1,30 = 1.54, p = .22,
= 0.04, respectively).
Fig. 5. Temporal trajectory of a) mean SCL, b) calm, c) happy, d) sensation of light intensity, e) sensation of color temperature, f) visual acceptance (in % – no statistical testing), and g) visual comfort. The graphs are based on the models without the correction for time in session. The error bars represent SE.
3.2. Mood
Overall, there was no statistically significant difference in calmness between the dim (2.24 ± 0.07), intermittent (2.15 ± 0.07) and bright conditions (2.03 ± 0.07; all p > .02; ). However, in the separate analysis of the intermittent-bright set, calmness was higher in the dim condition (2.28 ± 0.07) compared to both the intermittent (1.98 ± 0.07; p = .003) and the bright conditions (1.99 ± 0.07; p = .005). Between the latter two, there was no significant difference (p = .99). In contrast, there were no statistically significant differences between the three conditions when looking only at the intermittent-dim set (all p’s > 0.11). Calmness at baseline and Room temperature both significantly and positively predicted calmness (F1,62 = 60.19, p < .001, B ± SE = 0.51 ± 0.07, = 0.40 and F1,69 = 9.90, p = .002, B ± SE = 0.06 ± 0.02,
= 0.08, respectively). Perceived General health was not significantly related to calmness (F1,17 = 6.53, p = .02,
< 0.01).
Happiness did not significantly differ between conditions (dim: 3.59 ± 0.11, intermittent: 3.53 ± 0.11, bright: 3.54 ± 0.11; all p’s > 0.71; ). This pattern also occurred when analyzing the assessment sets separately for the intermittent-dim and intermittent-bright phases (all p’s > 0.44). Baseline happiness and perceived General health were both significantly and positively associated with happiness (F1,106 = 11.58, p < .001, B ± SE = 0.24 ± 0.07, = 0.08 and F1,28 = 8.28, p = .008, B ± SE = 0.02 ± 0.01,
= 0.16, respectively). Room temperature showed no significant relationship with happiness (F1,565 = 3.28, p = .07,
< 0.01).
3.3. Visual comfort
shows that, across all assessments, the dim condition (−0.19 ± 0.09) was not perceived significantly different from the intermittent one (0.08 ± 0.09; p = .05), but both were experienced dimmer than the bright light condition (1.32 ± 0.09; p < .001). Yet, the close-up analyses of the intermittent-bright set showed that SensationVI in the intermittent condition (2.20 ± 0.11) was significantly higher than in the bright condition (1.49 ± 0.11; p < .001). Both were perceived also significantly brighter than the dim light condition in these phases (−0.25 ± 0.11; both p’s < 0.001).In contrast, the analysis of the intermittent-dim set showed that the intermittent-dim condition (−0.98 ± 0.10) was perceived significantly dimmer than the dim light condition in these assessments (−0.18 ± 0.10; p < .001). The bright condition (1.25 ± 0.10) was perceived significantly brighter than the dim and intermittent-dim conditions in this set (both p’s < 0.001). Baseline SensationVI significantly predicted SensationVI (F1,113 = 18.28, p < .001, B ± SE = 0.28 ± 0.07, =0.04), whereas Room temperature did not (F1,57 = 1.12, p = .30,
< 0.01). Even though the CCT of the light did not change, the bright condition (−0.91 ± 0.12) was perceived significantly cooler than the dim (0.27 ± 0.12; p < .001) and the intermittent light condition (0.33 ± 0.12; p < .001), while no significant differences existed between the dim and intermittent condition (p = .93; ). The close-up analysis showed that in the intermittent-bright set, the dim condition (0.33 ± 0.14) was perceived significantly warmer than the intermittent (−1.33 ± 0.14; p < .001) and bright light condition (−0.98 ± 0.14; p < .001). No significant differences existed between the SensationVC of the intermittent and bright conditions in these assessments (p = .15). In the intermittent-dim set, all conditions differed significantly from each other (all p’s < 0.001): the bright condition (−0.88 ± 0.12) was perceived significantly cooler than the dim condition (0.24 ± 0.12), and both the bright and the dim condition were perceived significantly cooler than the intermittent condition (1.15 ± 0.12). Neither Baseline SensationVC nor Room temperature predicted SensationVC during assessments (F1,116 = 3.85, p = .05,
< 0.01 and F1,70 = 2.73, p = .10,
< 0.01, respectively).
A visual inspection of the visual acceptance votes suggested highest acceptance in the dim light condition (). During the second and third intermittent-bright phases (assessment 4 and 7), acceptance of the bright setting was lower than in the continuous bright condition, whereas in the intermittent-dim phases the acceptance of the dim setting was very close to the acceptance of the static dim condition.
Across all assessments, the dim light condition (1.69 ± 0.10) was significantly more comfortable than the intermittent (1.24 ± 0.10; p = .001) and bright light conditions (1.13 ± 0.10; p < .001; ), but the latter two did not significantly differ (p = .59). Yet, when only focusing on the intermittent-bright set, the intermittent condition (0.56 ± 0.14) was perceived as less comfortable than both the dim (1.68 ± 0.14; p < .001) and the bright light condition (1.04 ± 0.14; p = .01). The dim condition was also perceived significantly more comfortable than the bright condition (p < .001). In the intermittent-dim set, the intermittent condition (1.58 ± 0.11) did not differ with respect to the experienced visual comfort from the dim (1.68 ± 0.11; p = .78) or bright conditions (1.19 ± 0.11; p = .02), but the visual comfort in the bright condition was significantly lower than in the dim condition (p = .003). Baseline ComfortV significantly predicted visual comfort in the assessments (F1,114 = 25.34, p < .001, B ± SE = 0.32 ± 0.06, = 0.09), whereas Room temperature did not (F1,104 = 0.62, p = .43,
< 0.01).
In the dim light condition (5.69 ± 0.20), significantly more pleasantness and satisfaction with the lighting were reported compared to the intermittent (4.84 ± 0.20; p = .001) and bright conditions (4.62 ± 0.20; p < .001). No significant differences existed between the bright and intermittent condition (p = .60). For the HES symptoms, light condition did not significantly add explained variance to the model (χ2 (2) = 5.28, p = 0.07). There were no statistically significant differences between the dim (1.10 ± 0.10), intermittent (1.14 ± 0.10) and bright condition (1.30 ± 0.10; all p’s > 0.08).
4. Discussion
Bright light pulses during the night have shown to effectively modulate circadian rhythms (Gronfier et al. Citation2004; Rahman et al. Citation2021; Rimmer et al. Citation2000). Short bursts of light during the night can also suppress melatonin, although disproportionately less so than phase shifting (Rahman et al. Citation2021), and it can mitigate declines in alertness (Yang et al. Citation2019, Citation2018). However, whether intermittent light during daytime can elicit acute alerting effects remains to be established (Iskra-Golec and Smith Citation2008). We compared diurnal intermittent bright light to continuous dim and continuous bright light for subjective, behavioral and physiological indicators of alertness. In addition, visual comfort experienced with each of the three light conditions was investigated. Results showed that the highest visual comfort was experienced in the dim light condition. No significant alerting effects of the bright or the intermittent light condition were found, as well as no statistically significant effects for happiness, skin conductance or performance metrics. Furthermore, the dim-bright pattern of the intermittent light condition was clearly visible in the temporal trajectories of the visual experience.
4.1. Alerting effects of light
Despite conditions with markedly different illuminances (100 vs. 1000 lux; ED65mel = 67 vs. 624 lux), an exposure duration of 90 min, a relatively large, partially sleep-deprived sample and repeated measurements, no statistically significant differences were found between the intermittent, bright and dim light conditions with respect to subjective alertness, task performance or skin conductance levels. This contrasts findings for subjective alertness in various studies (e.g., Huiberts et al. Citation2015; Kaida et al. Citation2006; Leichtfried et al. Citation2015; Phipps-Nelson et al. Citation2003; Smolders et al. Citation2012; Vandewalle et al. Citation2006). However, this is also not the first study in which no significant alerting effects of bright light during the day were found (Lok et al. Citation2018; Souman et al. Citation2018) and the publication bias for positive findings should be considered too. Yet, the reason for these inconsistent results of daytime light exposure across studies has not been established to date. The data showed a large standard deviation for the alertness-related variables, indicating high variance in the responses that could not be attributed to the different light conditions. Participants may have responded differently to the light manipulation due to interindividual differences in light sensitivity (Phillips et al. Citation2019) and/or vulnerability to sleep loss (Vandewalle et al. Citation2009; van Dongen et al. Citation2004). However, random slope analyses (reported in Supplementary Materials S3) indicated that interindividual differences only affected the results significantly for the 10% fastest response times and the color sensation of the light. Furthermore, intraindividual variability in sleepiness may have occurred at the start of each condition due to variations in sleepiness across the day. Although potential differences in the baseline scores of each condition were accounted for in the models of the alertness-related parameters, the effect of bright or intermittent light may have varied with participants’ momentary alertness or homeostatic sleep pressure. The high variation could possibly be explained by the employed design, in which participants were exposed to the experimental light conditions at different times of day, and thus different circadian phases and homeostatic sleep pressure levels. Yet, when exposing participants to experimental light conditions at the same circadian phases and/or homeostatic sleep pressure, sessions need to be planned at different days and day-to-day variability may occur. With one night of sleep restriction before the experimental day, we reduced participants’ baseline level of alertness to avoid a potential ceiling effect. Although the sample was on average more sleepy at the start of the experiment compared to prior studies, alertness levels were still high at the start of some sessions for some participants (see Supplementary Materials S4) leaving little room for the bright and intermittent conditions to improve alertness (Lok et al. Citation2019; Smolders and de Kort Citation2014). For other individuals, sleep pressure may have been too high for alerting effects in response to bright and intermittent light to emerge (Gabel et al. Citation2015; St Hilaire et al. Citation2017).
The lack of differences in subjective sleepiness or vitality between the dim and intermittent condition contradicted the expectations based on the findings of Iskra-Golec and Smith (Citation2008), who investigated the effect of 6 hrs of exposure to intermittent light. They reported increased global vigor and performance in the late morning as a result of 15-min bright light pulses of 4000 lux alternated with 45 min of 300 lux. Iskra-Golec and Smith (Citation2008) found the alerting effects in the late morning only, yet in the current study responses were averaged over the entire day and time-dependent variations were not investigated due to power limitations. Although the current analyses yielded no significant main effects on the alertness-related parameters, a subtle reflection of the intermittent light pattern could be observed in the graphical visualizations of the vitality, sleepiness and calmness data. The visualizations showed systematic, albeit subtle, variations in line with the bright light periods in the intermittent light condition. The direction of these delicate variations in the responses is in line with the acute and momentary changes in subjective experiences as a result of light transitions that have been reported before (Kompier et al. Citation2021, Citation2020). To further explore the differences between the three conditions, we subdivided the data for assessments 1, 4 and 7 (intermittent-bright set) and assessments 2, 3, 5, 6, 8 and 9 (intermittent-dim set). In the intermittent-bright set, only calmness was significantly lower in the intermittent and bright condition compared to the dim condition, which could be indicative of cognitive associations between light settings and arousal/activity related concepts (e.g., Schietecat et al. Citation2018).
Similar to the subjective experiences related to alertness, no significant effects of intermittent or bright light on task performance were found. Despite the high power of the current study, the results reported by Phipps-Nelson et al. (Citation2003) could not be replicated. They reported improved task performance in static bright light amongst sixteen sleep restricted individuals. The bright light levels in both studies were similar, yet the dim light was <5 lux on the eye in the study of Phipps-Nelson et al. (Citation2003) whereas we employed 100 lux (ED65mel = 67 lux) on the eye. The dim condition in this study can be considered as only relatively dim compared to earlier studies who employed dim conditions below 5 or even 1 lux (e.g., Phipps-Nelson et al. Citation2003; Rüger et al. Citation2005; Sahin et al. Citation2014; te Kulve et al. Citation2017; Vandewalle et al. Citation2006), but was chosen in view of ecological validity. This suggests that 100 lux (corresponding to ED65mel = 67 lux in the current study) may already suffice to improve performance on the PVT as compared to darkness or very dim light settings. Yet, this would also imply that increases in luminous exposure in real office environments are not likely to be an effective strategy to boost performance, especially since nowadays most tasks in office environments are screen-based and thus self-illuminated. The visualizations of the PVT metrics seemed to suggest a worsening of performance in intermittent light in the second half of the session. Potentially, the alterations in light were mentally exhausting and compromised PVT performance (Langner et al. Citation2010). Although the motivation for the task seemed to decrease over time in every condition, there were no differences in motivation between the light conditions. Thus, a compensatory change in motivation is not a likely explanation for the null effects of the light condition with respect to performance metrics. It remains important to further study in which exact conditions bright light can improve task performance.
4.2. Visual experience of the light conditions
Both the repeated assessments of visual comfort and the one-time end-of-session measures of satisfaction and pleasantness demonstrated marked differences between the three conditions with respect to visual comfort. Overall, the dim light condition was perceived as most comfortable, and on average the intermittent condition was evaluated as more comfortable than the bright condition. These differences could not be explained by the self-reported headache or eye-strain symptoms for which no significant differences were found between the light conditions. The most reported complaints were “difficulty concentrating,” “difficulty focusing” and “eye fatigue,” in line with the evaluation of the dynamic lighting in Giménez et al. (Citation2017). However, in the current study the severeness was not significantly different in all three conditions. Based on Altomonte et al. (Citation2016), we hypothesize that these negative experiences regarding the lighting may be explained by the general fatigue that was induced by the sleep restriction. Clearly, the sensation of each new light setting was influenced by the prior light setting in such a way that the difference between the light conditions was amplified and an “overshoot” in sensation occurred. This emphasizes the relative nature of our perceptual system as was already described in Kompier et al. (Citation2020). The temporal trajectories of both the visual color and the intensity sensation in comparison to visual acceptance and visual comfort further demonstrated the role of adaptation as was also discussed in Kompier et al. (Citation2020, Citation2021). The visualization of the temporal trajectory of visual comfort demonstrated that in the bright light condition visual comfort increased after an initial drop and then stabilized rapidly. However, still the bright condition was consistently evaluated as less comfortable than the dim light condition, which contrasts the review by Fotios (Citation2017) who reported that illuminances below 300 lux were perceived at unpleasant. The graphical visualization of the repeatedly assessed visual experience highlights the added value of recurrent measurements during a light condition in contrast with one-time retrospective measures.
When implementing recurrent measurements, the measurement interval should be carefully chosen. The interval should be short enough to get detailed information about the temporal trajectory, yet long enough to overcome potential temporal response set bias. Previous studies have used measurement intervals of 13 to 20 min (Huiberts et al. Citation2015; Kompier et al. Citation2020, Citation2021; Smolders et al. Citation2012, Citation2016, Citation2018; Smolders and de Kort Citation2014, Citation2017). This study demonstrated that with a measurement interval of 10 min, the data still contains variance within experimental sessions. Repeated measurements are highly important for constant and fluctuating light conditions to gain detailed insights in the response dynamics. The decrease in acceptance for every subsequent bright light pulse in the intermittent condition was intriguing and underlines the care that is required when implementing dynamic light scenarios as these may compromise visual comfort (Aries et al., Citation2020; de Kort and Smolders Citation2010; Iskra-Golec and Smith Citation2008). When solely looking at the main effects one may conclude that the intermittent condition was perceived more comfortable than the bright condition, but instead the repeated measures indicated that the bright light in the intermittent condition was evaluated as more uncomfortable than the static bright light. This might be (partially) attributed to the transition speed to the bright phase of the intermittent light. Even though the employed transition in this study spanned 2 s, slower transitions may be required for such a large change in illuminance to diminish a drop in experienced visual comfort (Chraibi et al. Citation2019). Extreme care should be taken when using electric light fluctuations, as the bright light phases in a fluctuating light scenario may result in reduced visual comfort.
4.3. Reflections and implications
With intermittent bright light, we expected to be able to generate acute alerting effects, as preceding light exposure can alter the sensitivity to subsequent light (Chang et al. Citation2011, Citation2013; te Kulve et al. Citation2019). In this study, intermittent light could not improve momentary or overall alertness, which may be because of the dim light being too bright for the photoreceptors to recover and (partially) regain their sensitivity to light (Rimmer et al. Citation2000), though Iskra-Golec and Smith (Citation2008) found some alerting effects with pulses of 4000 lux at the eye alternated with 300 lux at the eye in between pulses. Whereas the main aim of this study was to examine whether intermittent bright light would be able to exert alerting effects similar to the effects bright light can have (Huiberts et al. Citation2015; Kaida et al. Citation2006; Kompier et al. Citation2021, Citation2020; Leichtfried et al. Citation2015; Phipps-Nelson et al. Citation2003; Smolders et al. Citation2012; Vandewalle et al. Citation2006), our results emphasized that bright light does not always generate alerting effects. While the research community is aware of the delicacy when it comes to these effects and still exerts great efforts to unravel the mechanism behind potential alerting effects of daytime light exposure (Lok et al. Citation2019), applications and lighting solutions have already been developed and are being advertised as solutions for dips in alertness.
Furthermore, an increasing amount of attention is being paid to the importance of integrative lighting, in which both visual experience, acute alerting and circadian effects of light settings are combined to optimally benefit human users. This study demonstrated that bright light conditions, and especially transitions toward bright light, are perceived as less comfortable compared to continuous dim light. Rapid transitions to or from higher illuminances can even compromise user acceptance and comfort, and thus it is a risky undertaking to carelessly apply bright light at every opportunity to align the circadian rhythm or possibly increase alertness.
Third, comparisons of the trajectories of response parameters demonstrate the marked variability in response dynamics for different outcome measures. For instance, graphical visualizations of trajectories for subjective sleepiness, vitality and calmness were quite similar, but clearly distinct from the response patterns related to task performance and skin conductance. All of these also differed markedly from the response trajectories of visual sensation and visual comfort. These temporal dynamics can help in understanding processes, or at the very least they illustrate the diversity in underlying pathways. Similar to studies suggesting decoupling of the processes and pathways responsible for circadian phase shifting vs. melatonin suppression (Rahman et al. Citation2018) and for learning vs. mood (Fernandez et al. Citation2018), we argue that there must be multiple pathways underlying the range of responses tracked in the current study (sensation vs. comfort vs. subjective alertness/vitality vs. mood vs. cognitive performance vs. physiological arousal). This warrants a word of caution against the overgeneralization of pathways as either being visual/image-forming or nonvisual/ non-image forming/ipRGC-influenced as this may result in overly simple solutions or rules of thumb for “integrative light” (CIE Citation2020).
The variance and dynamic nature of temporal responses also emphasize the importance of explicitly considering the timing of measurements in research. Especially the comparison of the repeated measurements with the one-time, retrospective measure of visual experience illustrated that, when using a one-time measure, detailed insights in the development of the variable of interest might be missed. Furthermore, a multi-measure approach with repeated subjective, behavioral and physiological assessments identifies the (diversity in) response dynamics of light-induced responses.
4.4. Limitations and future research
A limitation to the current study is that all sessions were completed on one experimental day, and thus the effects were tested at different circadian phases and sleep pressure levels. Despite complete counterbalancing of the order of the conditions between participants, this may have induced additional variability in the responses. However, also in real-life conditions circadian phase and sleep pressure at a given clock time will vary across individuals. Moreover, even when testing the different conditions on different days at the same internal (i.e. circadian) or external (i.e. clock) time, factors such as time spent outdoors, prior light exposure, or sleep quality of the night before the session might result in increased variability in the responses. Furthermore, the variable room temperature due to the use of fluorescent tubes to create the desired light settings may have resulted in additional variability in the data. Although we attempted to compensate the generated additional heat in the bright light settings with the use of air-conditioning, the temperature was not entirely stable within and between experimental sessions and conditions. The temperature in the room was continuously monitored and the variability in the temperature was accounted for statistically in the analyses by including the room temperature of every assessment as a covariate in the models, yet still room temperature may have masked effects of the light conditions.
5. Conclusion
In this study, intermittent light did not generate alerting effects compared to continuous dim light, yet neither did continuous bright light. Even amongst partially sleep deprived participants, exposure to 1000 lux for one-and-a-half hours (ED65mel = 624 lux) was not enough to decrease self-reported sleepiness or increase task performance or physiological arousal. Whereas the circadian benefits of daytime light exposure are well established (Boubekri et al. Citation2014; Brown et al. Citation2022; Hébert et al. Citation2002; Papatsimpa et al. Citation2021; Schlangen and Price Citation2021; White et al. Citation2013), further research is needed to identify under exactly which circumstances light is able to acutely improve daytime alertness. Moreover, the ambition to strive for integrative lighting dictates that the visual experience of light should be explicitly considered as well. The graphical visualizations emphasized the importance of repeated assessments timed deliberately in the measurement protocol, not only throughout one light setting but also during the transition into a new light setting, as the only way to gain insight in the comprehensive experience of a light condition. A detailed exploration of such dynamics will help to further characterize and understand the marked variability in the neural mechanisms underlying light-induced responses. Furthermore, when employing bright light in daytime contexts with the aim to increase alertness, visual quality and comfort of the lighting conditions (and their transitions) should be sufficiently secured.
Supplemental Material
Download PDF (105.3 KB)Supplemental Material
Download PDF (128.6 KB)Supplemental Material
Download PDF (136.3 KB)Supplemental Material
Download PDF (99.6 KB)Acknowledgments
We would like to thank Meike Heldoorn, Robin Galama, Mitchell Schijen, Timon Doornhein and Tom van Hoesel for their help in gathering the data. Additionally, we express our gratitude to Martin Boschman and Joost van Gennip for their technical assistance of the materials and the laboratory.
Disclosure statement
LJMS’s full time position at Eindhoven University of Technology has been partially funded by Signify, he is also active in various unpaid roles within the International Commission on Illumination (CIE). MEK, KCHJS and YAWK declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The coded collected data is available to the general public in an online data repository (10.17605/OSF.IO/BSA9N).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502724.2022.2068573
Additional information
Funding
References
- Åkerstedt T, Gillberg M. 1990. Subjective and objective sleepiness in the active individual. Int. J Neurosci. 52(1–2):29–37.
- Altomonte S, Kent MG, Tregenza PR, Wilson R. 2016. Visual task difficulty and temporal influences in glare response. Build Environ. 95:209–226.
- Aries MBC, Beute F, Fischl G. 2020. Assessment protocol and effects of two dynamic light patterns on human well-being and performance in a simulated and operational office environment. J Environ Psychol. June 2019; 69. 101409. doi:10.1016/j.jenvp.2020.101409.
- ASHRAE. 2004. Standard-55, thermal environment conditions for human occupancy. American society of heating, refrigerating and air-conditioning engineering. Atlanta.
- Boschman MC. 2015. GSRMonitor - user manual (Version 1.0). Eindhoven: Eindhoven University of Technology, Human Technology Interaction group.
- Boubekri M, Cheung IN, Reid KJ, Wang CH, Zee PC. 2014. Impact of windows and daylight exposure on overall health and sleep quality of office workers. J Clin Sleep Med. 10(6):603–611.
- Brown TM, Brainard GC, Cajochen C, Czeisler CA, Hanifin JP, Lockley SW, Lucas RJ, Munch M, O’Hagan JB, Peirson SN, et al. 2022 December. Recommendations for healthy daytime, evening, and night-time indoor light exposure. PLoS Biol. 20(3):e3001571. doi:10.20944/preprints202012.0037.v1.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. 1989. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28(2):193–213.
- Caldwell AR, Lakens D, Parlett-Pelleriti CM 2021. Power analysis with superpower.
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. 2012. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 35(2):287–302.
- Chang AM, Scheer FAJL, Czeisler CA. 2011. The human circadian system adapts to prior photic history. J Physiol. 589(5):1095–1102.
- Chang AM, Scheer FAJL, Czeisler CA, Aeschbach D. 2013. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep. 36(8):1239–1246.
- Chellappa SL, Ly JQM, Meyer C, Balteau E, Degueldre C, Luxen A, Phillips C, Cooper HM, Vandewalle G. 2014. Photic memory for executive brain responses. Proc Natl Acad Sci U S A. 111(16):6087–6091.
- Chraibi S, Creemers P, Rosenkötter C, van Loenen EJ, Aries MBC, Rosemann ALP. 2019. Dimming strategies for open office lighting: user experience and acceptance. Light Res Technol. 51(4):513–529.
- CIE. 2020. CIE international lighting vocabulary. International Standard CIE 017/E. doi:10.25039/S017.2020.
- Crowley SJ, Molina TA, Burgess HJ. 2015. A week in the life of full-time office workers: work day and weekend light exposure in summer and winter. Appl Ergon. 46(Part A):193–200.
- de Kort YAW, Smolders KCHJ. 2010. Effects of dynamic lighting on office workers: first results of a field study with monthly alternating settings. Light Res Technol. 42(3):345–360.
- Dinges DF, Powell JW. 1985. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 17(6):652–655.
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR, Kaplan OJ. 1994. Low illumination experienced by San Diego adults: association with atypical depressive symptoms. Biol Psychiatry. 35(6):403–407.
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, et al. 2018. Light affects mood and learning through Distinct Retina-Brain Pathways. Cell. 175(1):71–84.e18.
- Fotios SA. 2017. A revised kruithof graph based on empirical data. LEUKOS - J Illum Eng Soc N Am. 13(1):3–17.
- Gabel V, Maire M, Reichert CF, Chellappa SL, Schmidt C, Hommes V, Cajochen C, Viola AU. 2015. Dawn simulation light impacts on different cognitive domains under sleep restriction. Behav Brain Res. 281:258–266.
- Giménez MC, Geerdinck LM, Versteylen M, Leffers P, Meekes GJBM, Herremans H, de Ruyter B, Bikker JW, Kuijpers PMJC, Schlangen LJM. 2017. Patient room lighting influences on sleep, appraisal and mood in hospitalized people. J Sleep Res. 26(2):236–246.
- Gronfier C, Wright KP, Kronauer RE, Jewett ME, Czeisler CA. 2004. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol - Endocrinol Metab. 287(1 50–1):174–181.
- Hébert M, Dumont M, Paquet J. 1998. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 15(1):59–70.
- Hébert M, Martin SK, Lee C, Eastman CI. 2002. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 33(4):198–203.
- Helton WS, Nöswall K. 2010. Short stress state questionnaire: factor structure and state change assessment. Eur J Psychol Assess. 31(1):20–30.
- Hubalek S, Brink M, Schierz C. 2010. Office workers’ daily exposure to light and its influence on sleep quality and mood. Light Res Technol. 42(1):33–50.
- Huiberts LM, Smolders KCHJ, de Kort YAW. 2015. Shining light on memory: effects of bright light on working memory performance. Behav Brain Res. 294:234–245.
- Huiberts LM, Smolders KCHJ, de Kort YAW. 2016. Non-image forming effects of illuminance level: exploring parallel effects on physiological arousal and task performance. Physiol Behav. 164:129–139.
- Iskra-Golec IM, Smith L. 2008. Daytime intermittent bright light effects on processing of laterally exposed stimuli, mood, and light perception. Chronobiol Int. 25(2–3):471–479.
- Iskra-Golec IM, Smith L. 2011. Bright light effects on ultradian rhythms in performance on hemisphere-specific tasks. Appl Ergon. 42(2):256–260.
- Kaida K, Takahashi M, Haratani T, Otsuka Y, Fukasawa K, Nakata A. 2006. Indoor exposure to natural bright light prevents afternoon sleepiness. Sleep. 29(4):462–469.
- Kompier ME, Smolders KCHJ, de Kort YAW. 2021. Abrupt light transitions in illuminance and correlated colour temperature result in different temporal dynamics and interindividual variability for sensation, comfort and alertness. PLoS ONE. 16(3):1–24.
- Kompier ME, Smolders KCHJ, van Marken Lichtenbelt WD, de Kort YAW. 2020. Effects of light transitions on measures of alertness, arousal and comfort. Physiol Behav. 223. doi:10.1016/j.physbeh.2020.112999
- Kronauer RE, Forger DB, Jewett ME. 1999. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J Biol Rhythms. 14(6):501–516.
- Lakens D. 2017. Equivalence tests: a practical primer for t tests, correlations, and meta-analyses. Soc Psychol Personal Sci. 8(4):355–362.
- Langner R, Steinborn MB, Chatterjee A, Sturm W, Willmes K. 2010. Mental fatigue and temporal preparation in simple reaction-time performance. Acta Psychologica. 133(1):64–72.
- Lee SI, Kinoshita S, Noguchi A, Eto T, Ohashi M, Nishimura Y, Maeda K, Motomura Y, Awata Y, Higuchi S. 2020. Melatonin suppression during a simulated night shift in medium intensity light is increased by 10-minute breaks in dim light and decreased by 10-minute breaks in bright light. Chronobiol Int. 37(6):897–909.
- Leichtfried V, Mair-Raggautz M, Schaeffer V, Hammerer-Lercher A, Mair G, Bartenbach C, Canazei M, Schobersberger W. 2015. Intense illumination in the morning hours improved mood and alertness but not mental performance. Appl Ergon. 46(Part A):54–59.
- Lok R, Smolders KCHJ, Beersma DGM, de Kort YAW. 2018. Light, alertness, and alerting effects of white light: a literature overview. J Biol Rhythms. 33(6):589–601.
- Lok R, van Koningsveld MJ, Gordijn MCM, Beersma DGM, Hut RA. 2019. Daytime melatonin and light independently affect human alertness and body temperature. J Pineal Res. 67(1):1–10.
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PDR, Lockley SW, O’Hagan JB, et al. 2014. Measuring and using light in the melanopsin age. Trends Neurosci. 37(1):1–9.
- Lüdtke H, Wilhelm B, Adler M, Schaeffel F, Wilhelm H. 1998. Mathematical procedures in data recording and processing of pupillary fatigue waves. Vision Res. 38(19):2889–2896.
- Mure LS, Comut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM. 2009. Melanopsin bistability: a fly’s eye technology in the human retina. PLoS ONE. 4(6). doi:10.1371/journal.pone.0005991
- Najjar RP, Zeitzer JM. 2016. Temporal integration of light flashes by the human circadian system. J Clin Invest. 126(3):938–947.
- Oken BS, Salinsky MC, Elsas SM. 2006. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 117(9):1885–1901.
- Okudaira N, Kripke DF, Webster JB. 1983. Naturalistic studies of human light exposure. Am J Physiol - Regul. Integr. Comp. Physiol 14(4):19–21.
- Papatsimpa C, Schlangen LJM, Smolders KCHJ, Linnartz JPMG, de Kort YAW. 2021. The interindividual variability of sleep timing and circadian phase in humans is influenced by daytime and evening light conditions. Sci Rep. 11(1):1–14.
- Peeters ST, Smolders KCHJ, de Kort YAW. 2020. What you set is (not) what you get: how a light intervention in the field translates to personal light exposure. Build Environ. 185(September):107288.
- Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, Lockley SW, Cain SW. 2019. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci U S A. 116(24):12019–12024.
- Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SMW. 2003. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 26(6):695–700.
- Rahman SA, Brainard GC, Czeisler CA, Lockley SW. 2021. Spectral sensitivity of circadian phase resetting, melatonin suppression and acute alerting effects of intermittent light exposure. Biochem Pharmacol. 191(March):114504.
- Rahman SA, St Hilaire MA, Gronfier C, Chang AM, Santhi N, Czeisler CA, Klerman EB, Lockley SW. 2018. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol. 596(11):2147–2157.
- Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. 2000. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol - Regul. Integr. Comp. Physiol 279(5):R1574–R1579.
- Roenneberg T, Foster RG. 1997. Twilight times: light and the circadian system. Photochem Photobiol. 66(5):549–561.
- Roenneberg T, Wirz-Justice A, Merrow M. 2003. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 18(1):80–90.
- Ru T, de Kort YAW, Smolders KCHJ, Chen Q, Zhou G. 2019. Non-image forming effects of illuminance and correlated color temperature of office light on alertness, mood, and performance across cognitive domains. Build Environ. 149:253–263.
- Rubinstein EH, Sessler MD. 1990. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology. 73(3):541–545.
- Rüger M, Gordijn MCM, Beersma DGM, de Vries B, Daan S. 2005. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol - Regul. Integr. Comp. Physiol 290(5):R1413–R1420.
- Sahin L, Wood BM, Plitnick B, Figueiro MG. 2014. Daytime light exposure: effects on biomarkers, measures of alertness, and performance. Behav Brain Res. 274:176–185.
- Savides TJ, Messin S, Senger C, Kripke DF. 1986. Natural light exposure of young adults. Physiol Behav. 38(4):571–574.
- Schietecat AC, Lakens D, IJsselsteijn WA, de Kort YAW. 2018. Predicting context-dependent cross-modal associations with dimension-specific polarity attributions part 1 – brightness and aggression. Collabra: Psychol. 4(1):1–20.
- Schlangen LJM, Price LLA. 2021 March. The lighting environment, its metrology, and non-visual responses. Front Neurol. 12. doi:10.3389/fneur.2021.624861.
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. 2012. A practical guide to calculating Cohen’s f 2, a measure of local effect size, from PROC MIXED. Front Psychol. 3(APR):1–6.
- Smolders KCHJ, de Kort YAW. 2014. Bright light and mental fatigue: effects on alertness, vitality, performance and physiological arousal. J Environ Psychol. 39:77–91.
- Smolders KCHJ, de Kort YAW. 2017. Investigating daytime effects of correlated colour temperature on experiences, performance, and arousal. J Environ Psychol. 50:80–93.
- Smolders KCHJ, de Kort YAW, Cluitmans PJM. 2012. A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol Behav. 107(1):7–16.
- Smolders KCHJ, de Kort YAW, Cluitmans PJM. 2016. Higher light intensity induces modulations in brain activity even during regular daytime working hours. Lighting Res Technol. 48(4):433–448.
- Smolders KCHJ, de Kort YAW, van den Berg SM. 2013. Daytime light exposure and feelings of vitality: results of a field study during regular weekdays. J Environ Psychol. 36:270–279.
- Smolders KCHJ, Peeters ST, Vogels IM, de Kort YAW. 2018. Investigation of dose-response relationships for effects of white light exposure on correlates of alertness and executive control during regular daytime working hours. J Biol Rhythm. 33(6):649–661.
- Souman JL, Tinga AM, te Pas SF, van Ee R, Vlaskamp BNS. 2018. Acute alerting effects of light: a systematic literature review. Behav Brain Res. 337:228–239.
- St Hilaire MA, Rüger M, Fratelli F, Hull JT, Phillips AJK, Lockley SW. 2017. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 40(1). doi:10.1093/sleep/zsw009
- te Kulve M, Schlangen LJM, Schellen L, Frijns AJH, van Marken Lichtenbelt WD. 2017. The impact of morning light intensity and environmental temperature on body temperatures and alertness. Physiol Behav. 175(March):72–81.
- te Kulve M, Schlangen LJM, van Marken Lichtenbelt WD 2019. Early evening light mitigates sleep compromising physiological and alerting responses to subsequent late evening light. Sci Rep. 9(1). doi:10.1038/s41598-019-52352-w
- Thayer RE. 1989. The biopsychology of mood and arousal. Oxford: Oxford University Press.
- van der Zee K, Sanderman R. 2012. Het meten van de algemene gezondheidstoestand met de Rand-36. Groningen: Research Institute SHARE.
- van Dongen HPA, Baynard MD, Maislin G, Dinges DF. 2004. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 27(3):423–433.
- Vandewalle G, Archer SN, Wuillaume C, Balteau E, Degueldre C, Luxen A, Maquet P, Dijk DJ. 2009. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J Neurosci. 29(25):7948–7956.
- Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, Albouy G, Darsaud A, Desseilles M, Dang-Vu TT, et al. 2006. Daytime light exposure dynamically enhances brain responses. Curr Biol. 16(16):1616–1621.
- Veitch JA, Stokkermans MGM, Newsham GR. 2011. Linking lighting appraisals to work behaviors. Environ Behav. 45(February):198–214.
- Viola AU, James LM, Schlangen LJM, Dijk DJ. 2008. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 34(4):297–306.
- White M, Ancoli-Israel S, Wilson R. 2013. Senior living environments: evidence-based lighting design strategies [CEU]. Int J Environ Res Public Health. 7(1):60–78.
- Wilhelm B, Wilhelm H, Lüdtke H, Streicher P, Adler M. 1998. Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep. 21(3):258–265.
- Yang M, Chen Q, Zhu Y, Zhou Q, Geng YG, Lu CC, Wang GF, Yang C-M. 2019. The effects of intermittent light during the evening on sleepiness, sleep electroencephalographic spectral power and performance the next morning. Light Res Technol. 51(8):1159–1177.
- Yang M, Ma N, Zhu Y, Su Y-C, Chen Q, Hsiao F-C, Ji Y, Yang C-M, Zhou G. 2018. The acute effects of intermittent light exposure in the evening on alertness and subsequent sleep architecture. Int J Environ Res Public Health. 15(3):524.
- Zavada A, Gordijn MCM, Beersma DGM, Daan S, Roenneberg T. 2005. Comparison of the munich chronotype questionnaire with the horne-östberg’s morningness-eveningness score. Chronobiol Int. 22(2):267–278.
- Zeitzer JM, Friedman L, Yesavage JA. 2011a. Effectiveness of evening phototherapy for insomnia is reduced by bright daytime light exposure. Sleep Med. 12(8):805–807.
- Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. 2011b. Response of the human circadian system to millisecond flashes of light. PLoS ONE. 6(7):1–5.