ABSTRACT
We introduce a lighting technology designed to produce photoreceptor-directed lights (PrD). This photoreceptor-enhanced light therapy (PELT) differs from conventional supplemental lighting by using multiple limited-bandwidth primaries to generate spectra that appear white, and that are tailored to produce circadian equivalent (CE) lights for selectively increasing or decreasing the relative activation levels of specific photoreceptor classes in the human eye. Rather than designing a device to match a spectrum’s shape, we optimize the available hardware, so it best matches the biological effects of that spectrum. It goes beyond three and four dimensions (three cones plus melanopsin) to consider the biological responses mediated via all five photoreceptor classes (including rhodopsin); the inclusion of a fifth photoreceptor class is non-trivial both in implementation and biological effect. Here, we describe the technical specifications of the PELT device and its calibration procedures. Photoreceptor-directed lights with variable melanopsin and rhodopsin excitations and equal photometric luminance are presented. Device application examples are provided that include personalized supplemental light spectra for patients with photoreceptor sensitivity loss, for healthy people exposed to extreme seasonal or work-related variation in their ambient lighting patterns, and as a stimulus generator to evaluate the effects of light on human health and behavior mediated via the melanopsin expressing intrinsically photosensitive retinal ganglion cells (ipRGCs). In integrative lighting practice, the PELT method extends to dynamic control of the biological potency of the melanopsin and rhodopsin excitations over a large range, independent of perceived changes in correlated color temperature (CCT).
1. Introduction
Light is the catalyst for vision but also directly impacts a person’s nonvisual circadian, neuroendocrine and behavioral responses which are essential for human health and physiology (Berson et al. Citation2002; Cajochen Citation2007; Czeisler et al. Citation1989; Dijk et al. Citation1992; Markwell et al. Citation2010; Marshall Citation2016; Ruger and Scheer Citation2009; Shekleton et al. Citation2014). With variation in the illumination, both visual and nonvisual sensitivity depend on the activation of all five photoreceptor classes in the eye (Altimus et al. Citation2010; Barrionuevo et al. Citation2014; Dumpala et al. Citation2019; Houser et al. Citation2021; Uprety et al. Citation2022). Light is also a recognized therapy – a photoceutical. When people engage in activities such as international travel, occupational shift work or are exposed to lower ambient light levels during the winter months, their sleep-wake rhythms can deviate from the natural solar day–night cycle and they may experience sleep, mood and circadian disruption (Feigl et al. Citation2018; Hirakawa et al. Citation2020; Roecklein et al. Citation2013a, Citation2013b). With retinal and neurodegenerative disease, patients can experience these same disruptions even when exposed to natural light patterns. If degeneration of one or more of the five photoreceptors and/or their pathways occurs due to retinal pathology, patients can have abnormal physiological responses due to erroneous signaling received in circadian centers (Dumpala et al. Citation2019; Feigl et al. Citation2020; Maynard et al. Citation2015, Citation2017). Although supplemental bright light therapies are intended to limit the clinical impact of sleep and circadian disorders (Arendt Citation2010; Figueiro et al. Citation2016; Gooley Citation2008; Munch et al. Citation2011; Terman and Terman Citation2005; Tuunainen et al. Citation2004), an inherent limitation is that current supplemental spectra provide nonspecific photoreceptor excitation ratios. In other words, they are not personalized for the manifest retinal or circadian deficit. This is akin to using a nonspecific, broad-spectrum drug for diseases with different pathomechanisms.
Here, we introduce a photoreceptor-enhanced light therapy (PELT) with spectra designed to selectively target or supplement the five retinal photoreceptor classes in people who experience altered light exposures and circadian disruption. A key design property of PELT is that the light always appears “white” when selectively targeting the five photoreceptors in different excitation ratios (Zele et al. Citation2022). A white appearance is important because of the practical need for the device to easily integrate into a user’s environment without being visually intrusive or disrupting to their routines and tasks, as can be the case with chromatic lights (Killgore et al. Citation2020; Lieverse et al. Citation2011), all the while providing photoreceptor-directed light stimulation. PELT therefore has applications in scientific and clinical studies of the effects of light on circadian and sleep behavior, performance and mood mediated via different afferent pathways including the melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs).
There are existing spectrally tunable light engine concepts incorporating controllers and primary choices not dissimilar to ours, but that use different light integration techniques (Burgos et al. Citation2014; Chew et al. Citation2016; Fryc et al. Citation2005; Kolberg et al. Citation2011), including waveguides and/or mixing chambers having diffuser elements (Farràs Citation2019; Llenas and Carreras Citation2019a, Citation2019b). Designing a device having multiple channels for portable light therapy requires light homogenization to be considered more thoroughly than simply using an integrating sphere; commercially available supplemental lighting devices typically use only broadband (white) LEDs and so do not deal with integration as is done with PELT. Critically, one goal of tunable light engines is to generate spectra that are metamers along three dimensions (i.e., for recreating CIE illuminants and spectral power distributions of common lamps) or with extensions to four dimensions (to alter melanopsin excitation) but not all five dimensions (for rhodopsin excitation) as with PELT to provide full control of the biological effects of light on visual and nonvisual processes. Our goal is not simply to match or create a metamer of a white appearing spectrum (i.e., matching the three cone photoreceptor excitations at a specified CCT), but to match the biological effect of the spectrum driven by all five photoreceptor classes, and further, to match the biological effect of all five photoreceptor responses to one spectrum while it is being perceived as another different spectrum (e.g., effect of a higher color temperature on all five photoreceptor classes while perceived as lower CCT, and vice versa).
In the following, we introduce the device design and describe its manufacture, discuss the computational procedures to optimize the spectral outputs to target the five photoreceptors in specific daylight photoreceptor activation ratios. A calibration method is then presented for application in clinical practice and scientific studies using either CIE Standard Observer functions or through application of observer corrections to control for individual differences. We conclude with a demonstration using clinical applications of photoreceptor-enhanced light therapy (PELT).
2. Materials and methods
2.1. PELT device design
The PELT device () is a portable wide-field light box with a uniformly illuminated viewing area (Illumination area – 190 mm wide × 160 mm high viewed at 500 mm; Visual Angle – 21.5° horizontal, 18.2° vertical) capable of emitting photoreceptor-directed (PrD) light spectra at photopic luminance >1000 cd.m−2. It was designed and constructed using resources available in our laboratory. Here, we produced a 3D printed (X3D Polylactic acid) rectangular external case (250 mm wide × 225 mm high × 56 mm deep) using a Lulzbot TAZ 5 3D printer. The front façade and back cover were laser cut (Universal Laser Systems Industrial Series) from 2 mm thick Cast Acrylic (Mulford Plastics). Inside the case were light emitting diodes (LEDs) (Lumileds Luxeon Z) mounted facing inwards around all four sides of a clear acrylic panel (6 mm) with a custom dot raster on the front side to aid homogenization. Microcontroller-based pulse-width modulation (PWM) signals input to constant-current drivers (LEDdynamics) to control LED intensity, with diffusion (Lee #216 diffusion paper) in front of the acrylic, and rear reflective layers (Kodak Premium Gloss Photo Paper) set behind to maximize forward-directed light output. The microcontroller (Mega 2560 Pro Arduino clone, RobotDyn) can contain the personalized PELT protocol for independent operation or a script that allows real-time control using serial commands via USB, to allow the unit to be calibrated. Fundamentally, the device is used in the same manner as a commercial light therapy device; the user plugs the pre-programmed unit into the mains power, then presses the start button to begin a timed light exposure protocol that includes alerting sounds for compliance.
Fig. 1. A portable photoreceptor enhanced light therapy (PELT) device with spectral components optimized to provide a gamut capable of generating circadian equivalent photoreceptor-directed light. The PELT stimulation protocol is specified with reference to the excitations of all five photoreceptor classes in the human eye and written to the microcontroller via a USB connection accessed through a port on the back of the device. Power is connected via the right-side panel. A protocol is started using the red button on the top right corner of the device. A notification speaker can provide alerting sounds for compliance.

The PELT device includes a set of six primary lights () of peak wavelengths (full width at half maximum; color) at 448 nm (17 nm; royal blue), 469 nm (23 nm; blue), 506 nm (28 nm; cyan), 520 nm (30 nm; green), 598 nm (15 nm; amber) and 654 nm (18 nm; deep red) chosen to provide a gamut capable of producing photoreceptor-directed lights (PrD) lights (). Depending on the application, a primary at 596 nm (79 nm; PC-amber) may provide better performance, as described below. Theoretically, five primaries are sufficient to create PrD lights for five photoreceptor classes (Zele and Cao Citation2015); however, the addition of a sixth primary allows for the larger range of error that comes with practical implementation. Color mixing was performed using individually controllable LED groups mounted on metal core printed circuit boards (MCPCB) to aid with heat dissipation which can affect spectral output stability. All LEDs were run at currents of at most 500 mA (as per their test currents) to optimize the output performance within the space constraints. Currents were determined per-color to make the best use of the 8-bit-per-color PWM resolution for this application.
Fig. 2. Circadian equivalent photoreceptor-directed light spectrums implemented in the PELT device. (a) Normalized component spectral outputs of the six primary lights (solid lines) and the replacement primary (dashed line). All lights are specified with reference to their excitation ratios of the three cones (L-, M, S-), the rhodopsin (R) and melanopsin (i) photopigments. (b) Spectra designed as daylight metamers across all five photoreceptors at correlated color temperatures (CCT) between 2500 and 7500 K (). The spectra can be programmed to change dynamically and match the photoreceptor excitation ratios with time of day or be set to a static spectrum with the five photoreceptor excitation ratios matching the specific time of day as designated by the CCT and preferred by the user. (c) Photoreceptor directed (PrD) spectra having the three cone excitations set to a reference CCT (e.g., 3500 K) and a circadian equivalent (CE) melanopsin and rhodopsin excitation set at a higher CCT (e.g., 4500–7500 K). The appearance of all higher circadian equivalent lights matches closely with the 3500 K reference white. The change in color appearance of the white light due to the higher melanopsin and rhodopsin excitations is circumvented by applying cone corrections (Δl, Δs at equiluminance), but which introduce minor deviations in the evaluated CCT (). (d) Personalized spectral outputs for a PELT protocol that selectively alters the melanopsin excitation between a depleted (dashed line) and enhanced state (solid line), with reference to a ~3500 K light. This circadian equivalent increase in melanopsin excitation approaches 5500 K (Δ24% melanopsin Weber contrast) (). (e) Personalized spectral outputs for a PELT protocol that selectively alters the rhodopsin excitation between a depleted (dashed line) and enhanced state (solid line), with reference to a ~3500 K light. This circadian equivalent increase in rhodopsin excitation approaches 5000 K (Δ15.5% rhodopsin Weber contrast). Although the maximum melanopsin or rhodopsin photoreceptor directed contrasts are achieved using nonwhite chromaticities with the implemented spectral components, the practical settings generate a white light with high PrD contrasts by allowing a small tolerance in the unmodulated photoreceptor contrast ().
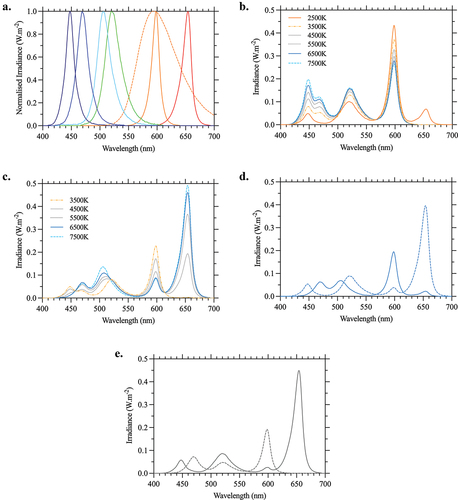
Table 1. The five photoreceptor excitation ratios for typical daylight, circadian equivalent photoreceptor-directed lights (PrD).
Table 2. Practical circadian equivalent stimulation settings for photoreceptor-enhanced light therapy (PELT).
To achieve uniformity for the chosen photoreceptor-directed contrasts, a four-side-address configuration with three different groups of MCPCB-mounted primaries were implemented using LED configurations having either (i) one royal blue, one green and two deep red primaries, (ii) one blue, one cyan and two amber or (iii) two amber and two deep red primaries. The first two configurations (i,ii) were grouped because each set of three primaries dominates a given state, (e.g., low or high melanopsin excitation) and the third group (iii) was required to increase each state’s overall output. The longer case side included three groups of LED banks, whereas the shorter case sides included two groups (total of 120 LEDs). The MCPCBs in each bank were attached to right-angle aluminum brackets with thermally adhesive tape, coupled to a finned aluminum heatsink, and aluminum billets that made a thermal connection between heatsinks. Within each LED bank, like-primaries were wired in series, and distributed to female socket headers running flush with the diffusion layers. Multiple primary groups were wired in parallel (where total LED group voltage drop exceeded 24 V) through PNP transistor-based current mirror circuits. Ten BuckPuck constant current LED drivers (LEDdynamics) of either 1000, 500 or 350 mA (depending on intensity requirements) were used to drive the six color groups, from which current was split as required to ensure individual LEDs were not driven above 500 mA.
The PELT protocol was written in Arduino script (Arduino IDE 1.8.10) and contained the calibrated PWM values corresponding to each photoreceptor-directed state, the protocol timing parameters and code to set the speed of the MOSFET-controlled fans (4x 12 V, for heat management), the 5 V notification speaker and 5 V-controlled 28VDC 10A relay (Songle), both powered from the Mega 2560 Pro. A 24 V 5A DC power supply (Powertran) was distributed to a 12 V regulator (to power the Mega 2560 Pro and the fans) and to the LED drivers via the relay. The relay was off by default to ensure the lights remain off until the protocol begins following the button press.
2.2. PELT calibration protocols
The system was pre-calibrated to set the personalized PELT protocol (). The spectral and luminance outputs of the primaries were measured to verify that mathematical solutions exist for the required PELT conditions which were then coded to the internal microcontroller via the USB connection. Spectral outputs were measured with a spectroradiometer (StellarNet, Tampa, FL, USA). Photometric and radiometric outputs were measured with an ILT1700 Research Radiometer (International Light Technologies, Inc., Peabody, MA, USA) and SpectraScan PR655 (Jadak, Syracuse, NY). Calibrations were performed using a custom MATLAB app (MATLAB R2018b, MathWorks) and microcontroller code (Arduino IDE 1.8.10) that allowed the LED intensities in the unit to be changed via real-time serial commands.
Photometric and radiometric calibrations were completed for a time equivalent to the duration of the light supplementation protocol (e.g., 30 min). The primary outputs were ramped-up from 90% to 100% over 5 minutes to minimize the initial and long-term thermal changes which affect the LED spectral output during a 30 min protocol thereby maximizing the spectral consistency throughout the protocol. Using the individually measured component spectra (), the theoretical LED intensity ratios for the PrD states were calculated and sent to the Mega 2560 Pro. The combined spectrum was measured and evaluated with reference to the original calibration values, including photoreceptor ratios and luminance. If necessary, recalibration or (more likely) luminance scaling was performed to ensure the output metrics of the measurement matched the reference metrics – this is entirely dependent on the circuit/driver/physical design’s ability to maintain independent component spectrum output linearity. Once verified, the PWM values of the primaries for each photoreceptor-directed state were stored on the PELT device. For a clinical trial, the device spectrum can be measured on both, allocation to and return from the patient to verify the unit’s spectral consistency while offsite.
The presence of minor deviations between an individual and the CIE 1964 10° Standard Observer due to differences in pre-receptoral filtering of the eye (e.g., lens) or L:M cone ratios are not critical because a participant typically only receives one PELT condition at a time; it is common industry practice to specify light using CIE standard functions. If a clinical or laboratory protocol requires participants to receive two PrD’s at different times, it is possible to correct for differences between the theoretical standard observer and participant by performing an individual observer calibration using heterochromatic flicker photometry (HFP) (Uprety et al. Citation2021) to equate the perceived brightness (Besenecker et al. Citation2016; Zele et al. Citation2018a, Citation2020) of the two PrD conditions (e.g., a depleted and enhanced melanopsin state). These measurements are restricted to a circular area of 10° visual angle within the center of the PELT device by application of an opaque black mask. To correct any perceived differences in color appearance due to the rhodopsin (Cao et al. Citation2008) and melanopsin contributions to human vision (Zele et al. Citation2018b), a perceptual match (the cone correction) between two PrD conditions is obtained by independently tuning the l- and s-cone excitations of the light at equiluminance (Zele et al. 2022). By correcting the visual contributions of melanopsin and rhodopsin, the circadian equivalent changes remain invisible, or difficult to perceive, while the PELT impacts the nonvisual biological responses (Zele et al. 2022).
The device can be programmed with any timing configuration including the duration of the light exposure protocol, the onset and offset parameters (e.g., temporal intensity profile ramps). Sounds can be programmed to alert the user (e.g., start, mid-point, 2-min to completion) and for timing and compliance measures wherein the purpose of the sound may be to alert a user to hold an actiwatch in front of the device to record the increase in ambient illumination and to verify the timing of the onset and offset of the supplemental light therapy. Device uniformity was measured in a grid pattern using a PR655 spot photometer (CIE 1931 2° field). The relative percent range of luminance was 15% over the central horizontal axis and 4% over the central vertical axis. The variation over the entire field was ~25%, similar to the homogeneity of commercial digital light processing (DLP) projectors which vary by ~29% across the image (Bayer et al. Citation2015). Any asymmetry that exists is a result of the LED configuration (in this case, the red/amber-only MCPCB of each LED bank), which could be improved with closer LED clusters.
Our PELT demonstration is presented as a simple, portable device; further practical improvements can be achieved depending on production capabilities and user requirements of the application, medical, commercial or otherwise. In addition to general production efficiency improvements, the application area can constrain the optimal choice of component spectra, including addition of broadband white primaries which, in turn, influences the output levels and LED driver circuitry to achieve the best intensity resolution. Future iterations could implement techniques to incorporate spectral feedback to accommodate changes in the same hardware over time (Llenas and Carreras Citation2019b). Another possibility is to include a broadband LED, acknowledging that the increased benefit in color rendering must be weighed against the likeliness of a reduced photoreceptor gamut. The design principles presented are intended to facilitate the initial translation of the PELT to the laboratory and clinical practice.
2.3. Generating photoreceptor-directed (PrD) lights
We specified the PrD lights with reference to the excitations of all five photoreceptor classes (Zele et al. Citation2019) using the CIE 1964 10° Standard Observer cone fundamentals (Smith and Pokorny Citation1975), the CIE 1951 scotopic luminosity function, and the melanopsin spectral sensitivity function (Adhikari et al. Citation2015; Enezi et al. Citation2011). Retinal illumination (photopic) was defined as the sum of L- and M-cone excitations with a 2:1 L:M cone ratio (MacLeod and Boynton Citation1979), such that for a white-appearing equal energy spectrum at 1 photopic Troland (Td), the photoreceptor excitation relative to photopic illumination is 0.6667 for L-cones (L), 0.3333 for M-cones (M), 1 for S-cones (S), 1 for rods (R), and 1 for melanopsin (i). We applied a luminance-normalization, wherein each photoreceptor excitation for a given light spectrum was divided by the photopic luminance (Vλ) of that spectrum. In the following analyses, the spectral distributions of natural daylights were referenced to the Illuminating Engineering Society (IES) TM-30 standard which uses a combination of CIE D-series illuminants and functions calculated using Planck’s radiation law. The spectral distributions of the natural daylights were correlated with the photoreceptor sensitivity functions to give five SMLRi values per natural daylight spectrum (). Each SMLRi group was then divided by the sum of its individual L and M components so that the L + M of each SMLRi equals 1. The SMLRi values are equivalent to the CIE S026/E:2018 α-opic efficacy of luminous radiation (ELR). Our data are based on scaling the photoreceptor spectral sensitivity functions relative to an equal energy spectrum before correlating with the luminance-normalized spectra, whereas CIE S026/E:2018 uses unit-normalized photoreceptor spectral sensitivity functions, correlates with a luminance-normalized spectra and then references to the D65 illuminant ELR. The Daylight Efficacy Ratio (DER) can be calculated from the SMLRi values () by dividing the test SMLRi by a reference SMLRi (e.g., at 6500 K, D65). Equivalent daylight illuminance (EDI) can be determined from DER and absolute illuminance measurements.
For CCTs within a desired range, for example from 3500 to 4000 K in intervals of 10 K, a CIE 1964 x,y chromaticity on the Planckian locus is chosen corresponding to the given CCT. This point is converted to XYZ (using Y = 1) and then to the L-, M- and S-cone excitations using the Smith and Pokorny (Citation1975) transform. The calculations can be performed using CIE S026/E:2018 to produce equivalent results. The SMLRi photoreceptor excitations of each of the LED primaries are computed (including a dark set [0 0 0 0 0]). The 5-dimensional convex hull made from these values describes the gamut of the group. For a desired SMLRi value (i.e., SML calculated from x,y) Grassman’s Laws are used to combine pairs of points on the hull to create new virtual-primary points which have the desired value for that photoreceptor class. A new hull is placed around those points and the next photoreceptor class’s iteration is performed. In this case, the result is a set of 5-dimensional points that share the same SML values but may have different Ri values. For every R value in the rhodopsin-melanopsin (Ri) hull, the maximum i range is computed using (almost always) a combination of two points on the hull with the closest R values below and above the reference point. The R value with the largest i range is chosen. Of the possible LED component intensity ratios that can achieve the maximum melanopsin contrast, the preferred solution can be chosen based on metrics such as color rendering or power efficiency.
3. Results
To support the full physiological effects of daylight, the biologically directed PrD spectra () were metameric to the spectral distribution of typical daylight (Judd et al. Citation1964) across a functional range of correlated color temperatures spanning sunrise to sunset (CCT = 2000–8000 K). gives the excitation ratios for different typical daylight PrD lights. A method for generating the daylight activation ratios of all five photoreceptors at each CCT can be found elsewhere (Zele et al. 2022). With six primaries (), these daylight photoreceptor activation ratios can be achieved to within 0.25% of the required theoretical melanopsin, rhodopsin and cone excitations. The biologically directed spectra () have a color rendering index that increases with CCT from 73 (3500 K) to 84 (7500 K). Replacing the amber primary with PC amber (, dashed line) increases the minimum CRI to 88 and achieves an average CRI of 92 across all CCTs, demonstrating that component choice can affect application suitability.
We applied Circadian equivalent PrD stimulation to selectively increase (enhance) or decrease (deplete) the quantal catch of one or more photoreceptor classes by setting a target group of SMLRi photoreceptor values. Because there is a non-linear relationship between photoreceptor classes over the range of daylight CCTs (), the PrD circadian equivalent lights were defined across five dimensions as given in . The three cone excitations were specified for a nominated CCT, and then the melanopsin and rhodopsin excitations were specified based on the user requirements. For example, with the L-, M- and S-cone excitations set to a desired CCT on the Planckian locus (e.g., 3500 K), the rhodopsin and melanopsin can be adjusted together in their respective circadian ratios over the equivalent range of 3500 to 7700 K (which equates to Weber contrasts of 50% and 69%, respectively, though not independently) (). This rhodopsin/melanopsin correlation is relevant when designing for a particular application. With the given cone excitations and rhodopsin held constant at the equivalent of 5350 K, melanopsin contrast can be up to 18%, whereas if rhodopsin is specified to the circadian equivalent of the cones, the available melanopsin contrast is reduced to 4.5%. In circumstances where additional limitations are imposed (such as CCT or CRI) a larger melanopsin contrast is possible if a small rhodopsin contrast can be tolerated. This is roughly a 1% to 1% ratio although it depends on the component spectra and CCT. In the latter two examples, a 3% contrast in rhodopsin would allow for 21% and 9.5% melanopsin contrasts, respectively. This is within the tolerable range of rhodopsin contrasts to limit interactions with the melanopsin signal (Uprety et al. Citation2022). Varying the cone excitations to shift the chromaticity away from the Planckian locus can also offer slightly increased contrasts, as shifting in one or more cone dimensions in a 5-dimensional photoreceptor space may allow for a longer vector in the dimension of interest (i.e., a larger PrD contrast). Changing cone excitations can also be used correct for the melanopsin (Zele et al. Citation2018b) and rod color contributions (Cao et al. Citation2008), to compensate for individual differences in color perception, or to adjust other metrics such as hue or chroma (Zele et al. 2022).
Similarly, at the same cone chromaticity (3500 K) and with melanopsin held constant at the equivalent of 4250 K, rhodopsin contrasts can be up to 13%. With melanopsin set to the 3500 K circadian equivalent, rhodopsin contrast drops to just under 4.5% but can be increased to 6.7% with a 3% melanopsin tolerance – less than a 1% to 1% ratio. Small variations in the cone excitations can be applied to correct for the color contributions of the rod pathway (Cao et al. Citation2008), or more likely, to shift the overall chromaticity toward a white that could increase the possible PrD contrast. Theoretically, similar-bandwidth component spectra with nearby peaks at 440, 477, 506, 520, 590 and 660 nm could reach a rod contrast of 20% (with melanopsin held constant at 4400 K), or alternatively higher contrasts can be achieved using narrower-bandwidth component spectra.
In one of our own applications of PELT lighting, the requirement was to generate a light between 3500 K and 4500 K that had a melanopsin contrast approaching 25% with minimal rod contrast using these commonly available component primaries (). Calculations showed that the maximum contrast possible was approximately 25% at x,y = 0.3591, 0.2591 (i.e., not close to the desired CCT range). An algorithmic approach was used to find the CIE 1964 10° chromaticity coordinates within the range of the desired CCTs, and iteratively search for the maximum melanopsin contrast, leaving the limited rhodopsin range to be determined as a by-product. A preferred (relative) enhanced or depleted option set was chosen and cone corrections were made to address perceived color variations (). A maximum melanopsin contrast of 24% was achieved at approximately 3500 K while limiting rhodopsin contrast to 3%. The excitation ratios for this and select other scenarios can be found in .
Using the same method to compute a white appearing, rhodopsin-directed light, the preferred set of ratios gave a 15.5% rod contrast with a 3% melanopsin tolerance and minimal cone corrections at 3500 K (, ). This differed considerably from the maximum possible nonwhite rod contrast of 27% () with this component spectra (), which can be explained by the rhodopsin spectral sensitivity’s higher correlation with the M-cone and relatively fewer component spectra that can accommodate an increased rhodopsin excitation in a white chromaticity (when compared with melanopsin). This lack of similarity in performance between melanopsin- and rod-directed stimulations when additional constraints were included (e.g., chromaticity), reinforces the need for application-specific spectral design consideration in PELT implementations.
3.1. Photoreceptor-enhanced light therapy (PELT) in clinical applications
A PELT device with six primary lights can create multiple different combinations of photoreceptor-enhanced lights (; ) to meet the requirements for study of the pathomechanisms and treatment of light dependent nonvisual functions such as circadian behavior and sleep in retinal or neurodegenerative disease, and in fundamental laboratory, educational and commercial settings in healthy people to study the effect of light on performance mediated via different afferent pathways. There is a high prevalence of sleep disruption in patients with eye diseases such as glaucoma (Wang et al. Citation2013), age-related macular degeneration (Khurana et al. Citation2016), retinitis pigmentosa (Gordo et al. Citation2001) and in diabetes with diabetic retinopathy (Skomro et al. Citation2001). All these conditions have varying degrees of melanopsin, rod and cone photoreceptor dysfunction in common that can be directly linked to circadian disruption (Adhikari et al. Citation2021, Citation2016b; Dumpala et al. Citation2019; Feigl et al. Citation2011; Gracitelli and Paranhos Citation2015; Maynard et al. Citation2017). For example, in the early stages of diabetes, only rods are dysfunctional and the ipRGCs are initially intact (Dumpala et al. Citation2019). With progression to diabetic retinopathy, melanopsin signaling becomes dysfunctional (Feigl et al. Citation2012b; Park et al. Citation2017), therein allowing a PELT protocol to transition from a rod-enhanced mode () to a rod and melanopsin enhanced mode of therapy with disease progression (). Likewise, sleep disorders are additional clinical symptoms in the neurodegenerative disorders such as Parkinson’s (Videnovic and Willis Citation2016) and Alzheimer’s disease (Uddin et al. Citation2020). Both diseases cause manifest melanopsin dysfunction (Feigl et al. Citation2020; Joyce et al. Citation2018; Romagnoli et al. Citation2020) and ipRGC loss has been confirmed by anatomical sections of postmortem human retina (La Morgia et al. Citation2016; Ortuno-Lizaran et al. Citation2018). A melanopsin-directed PELT () has the potential to improve sleep behavior in such populations, and the pathomechanisms determined in double-blind, randomized clinical trials designed to evaluate and optimize the application of light therapy and quantification of its treatment effects. This will determine the type, contrast and duration of the PELT. Photoreceptor dysfunction in disease has been reviewed elsewhere (Chougule et al. Citation2019; Feigl and Zele Citation2014; Kawasaki and Kardon Citation2007; La Morgia et al. Citation2018; Markwell et al. Citation2010; Zele and Gamlin Citation2020).
In personalizing the desired PELT protocol to re-align circadian rhythms to the 24-h day–night cycle, the rod, cone and melanopsin photoreceptor function can be quantified in practice using pupillometry (Adhikari et al. Citation2016a; Kelbsch et al. Citation2019). Alternatively, the literature can inform the typical manifest photoreceptor loss for the patient at their disease stage, or for the seasonal, work or travel-dependent desynchrony. The PrD stimuli are chosen depending on the user requirements in healthy people or those with disease and can be implemented as a room illuminant or portable device for bright light therapy (). Initially, it may be most practical to apply the maximum PrD contrast generated by a device to better utilize the available hardware to provide a physiological benefit. PELT can also be applied in laboratory studies to evaluate the effects of light mediated via all five photoreceptors on human physiology and performance, including the inadvertent negative impacts of artificial lighting such as headaches, fatigue, eye strain (Auffret et al. Citation2021; Joines et al. Citation2015) and photophobia (Zele et al. Citation2021). Such clinical and laboratory studies will be critical for providing the evidence-base for future light therapy protocols.
4. Discussion
Physicians do not treat different diseases with the same drug and any treatment with the same photoceutical can be expected to have lower clinical efficacy than with one that is personalized to target the neuroretinal pathways implicated in sleep and circadian disorders. Photoreceptor degeneration in disease can cause a progressive loss in visual acuity and contrast sensitivity (and irreversible blindness in later stages), attenuates photoreceptor electrical responses to light (Bearse et al. Citation2006; Feigl et al. Citation2005, Citation2012a; Moschos et al. Citation2011; Tanaka et al. Citation2021), delays visual adaptation (Dimitrov et al. Citation2008; Lamb and Pugh Citation2006), promotes the neural remodeling of interconnections within the retina and central nervous system (Marc et al. Citation2003), and these impact downstream processes including mood, sleep and circadian rhythms (Feigl et al. Citation2018; Hirakawa et al. Citation2020; Maynard et al. Citation2017; Roecklein et al. Citation2013a, Citation2013b). There arises an opportunity for evidence-based light therapy treatment protocols to supplement the photoreceptor sensitivity loss by increasing the stimulation level of the dysfunctional photoreceptors(s) in people affected by retinal and neurodegenerative disease. It can be also applied in healthy people who are exposed to irregular lighting schedules due to their profession (e.g., night shift, frequent travel through time zones) and in aging populations who spend increasing time indoors in artificial lighting.
To meet these requirements, a device needs to generate photoreceptor-directed light, especially given that in addition to the cone photoreceptors, both the melanopsin and rhodopsin signals contribute to circadian entrainment (Houser et al. Citation2021) and those current devices cannot independently modulate all five photoreceptors. Because the PELT spectrum appears white using different photoreceptor-directed stimulations matched to a user’s natural daily light rhythm or retinal pathology, it does not visually distract (as do currently available colored or very bright lights with photoreceptor excitation ratios that do not match the effects of natural daylight), it also can be used to test clinical hypotheses in controlled double-blind studies about the effects of photoreceptor-directed light on disease, as well as examine fundamental questions relating to the function of ipRGC projections to brain regions that modulate light-dependent mood, arousal, attention and decision-making, including in educational settings.
The perception of whiteness is subject to individual variations in optical and neural factors between and within observers, and over time (Bosten et al. Citation2015; Webster Citation2020). Because the relative outputs of the primary lights vary between the PrD conditions (), any manifest differences in chromatic adaptation between conditions will lead to color after-effects. A patient can however only view one PrD lighting condition at a time. With a maximum CRI of 92 for the evaluated primary combinations, the application of PELT in luminaires in the built environment can further increase color rendering by incorporating additional spectral primaries (e.g., broadband white LEDs). Such component changes must be considered in context of the purpose for they can lead to a reduction in maximum achievable PrD contrast. Similarly, it is yet to be determined how tolerant human vision is to integrative lighting, as advances in circadian spectral optimization may change our understanding of color rendering.
Daytime (healthy) exposure to PELT at bright levels should help consolidate sleep, increase nighttime sleep efficiency and daytime wakefulness. Similarly, PELT exposure in retinal and neurodegenerative disease should provide better health outcomes in patients. The implementation can be realized in luminaires for installation in homes, workplace and health-care settings. This is a positive step toward “healthy” lighting solutions that are purposeful for whom they are intended, considering both visual and nonvisual tasks as well as personalized for the settings, both night and day. The outcomes of controlled-studies using PELT will also provide the evidence-base to inform the spectral design of non-programmable solid-state luminaires for use in the built environment.
Personalizing light spectrums to deliberately boost photoreceptor function in healthy people exposed to abnormal diurnal patterns of light and darkness, and for patients with retinal disease might reduce the effects of biological variability by targeting exactly the site of operation within the visual system where phototransduction takes place. The photoreceptor-directed lights may help to unmask how the adaptation processes and neural networks controlling light-dependent visual and nonvisual processes, change in manifest disease. Personalized lighting may have an additional benefit for evidence-based studies by limiting the confounding effects of nonvisual factors such as mood, emotion, arousal that can influence health and well-being. Moreover, PELT should result in more efficient light treatment regimens; both in the timing, amount and duration of the supplemental exposure and ultimately lead to better health results for people, whether at home, in the workplace, in hospitals, schools or aged-care settings. The application of PELT affords the dynamic control of the biological potency of the melanopsin and rhodopsin excitations over a large range, independent of perceived changes in CCT, thereby allowing the effects of integrative lighting to be achieved with the consistency of a static color.
Disclosure statement
Authors (Zele, Feigl and Carter) are named inventors on international patent application WO 2022/094678 filed under the Patent Cooperation Treaty (PCT) that covers aspects of the device and method described here.
Data availability statement
Data underlying the results presented in this paper may be obtained from the authors upon reasonable request.
Additional information
Funding
References
- Adhikari P, Feigl B, Zele AJ. 2016a. Rhodopsin and melanopsin contributions to the early redilation phase of the Post-Illumination Pupil Response (PIPR). PLoS One. 11(8):e0161175.
- Adhikari P, Pearson CA, Anderson AM, Zele AJ, Feigl B. 2015. Effect of age and refractive error on the melanopsin mediated Post-Illumination Pupil Response (PIPR). Sci Rep. 5(1):17610.
- Adhikari P, Pradhan A, Zele AJ, Feigl B. 2021. Supplemental light exposure improves sleep architecture in people with type 2 diabetes. Acta Diabetol. 58(9):1201–1208.
- Adhikari P, Zele AJ, Thomas R, Feigl B. 2016b. Quadrant field pupillometry detects melanopsin dysfunction in glaucoma suspects and early glaucoma. Sci Rep. 6(1):33373.
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. 2010. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 13(9):1107–1112.
- Arendt J. 2010. Shift work: coping with the biological clock. Occup Med (London). 60(1):10–20.
- Auffret E, Gomart G, Bourcier T, Gaucher D, Speeg-Schatz C, Sauer A. 2021. Digital eye strain. Symptoms, prevalence, pathophysiology, and management. J Fr Ophtalmol. 44(10):1605–1610.
- Barrionuevo PA, Nicandro N, McAnany JJ, Zele AJ, Gamlin P, Cao D. 2014. Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Invest Ophthalmol Vis Sci. 55(2):719–727.
- Bayer FS, Paulun VC, Weiss D, Gegenfurtner KR. 2015. A tetrachromatic display for the spatiotemporal control of rod and cone stimulation. J Vis. 15(11):15.
- Bearse MA Jr., Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S. 2006. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 25(5):425–448.
- Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 295(5557):1070–1073.
- Besenecker UC, Bullough JD, Radetsky LC. 2016. Spectral sensitivity and scene brightness at low to moderate photopic light levels. Light Res Tech. 48(6):676–688.
- Bosten JM, Beer RD, MacLeod DI. 2015. What is white? J Vis. 15(16):5.
- Burgos FJ, Vilaseca M, Perales E, Herrera-Ramirez JA, Martinez-Verdu FM, Pujol J. 2014. Spectral LED-based tunable light source for the reconstruction of CIE standard illuminants. Image and Signal Processing ICISP 2014; Cherbourg, France: Springer International Publishing.
- Cajochen C. 2007. Alerting effects of light. Sleep Med Rev. 11(6):453–464.
- Cao D, Pokorny J, Smith VC, Zele AJ. 2008. Rod contributions to color perception: linear with rod contrast. Vision Res. 48(26):2586–2592.
- Chew I, Kalavally V, Tan CP, Parkkinen J. 2016. A spectrally tunable smart led lighting system with closed-loop control. IEEE Sens J. 16(11):4452–4459.
- Chougule PS, Najjar RP, Finkelstein MT, Kandiah N, Milea D. 2019. Light-induced pupillary responses in Alzheimer’s disease. Front Neurol. 10.
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. 1989. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 244(4910):1328–1333.
- Dijk DJ, Duffy JF, Czeisler CA. 1992. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1(2):112–117.
- Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. 2008. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 49(1):55–65.
- Dumpala S, Zele AJ, Feigl B. 2019. Outer retinal structure and function deficits contribute to circadian disruption in patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 60(6):1870–1878.
- Enezi J, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. 2011. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 26(4):314–323.
- Farràs AL. 2019. Spectral control methods and applications for multi-channel LED light engines [dissertation]. Barcelona (Spain): Universitat Politècnica de Catalunya.
- Feigl B, Brown B, Lovie-Kitchin J, Swann P. 2005. Adaptation responses in early age-related maculopathy. Invest Ophthalmol Vis Sci. 46(12):4722–4727.
- Feigl B, Dumpala S, Kerr GK, Zele AJ. 2020. Melanopsin cell dysfunction is involved in sleep disruption in Parkinson’s disease. J Parkinsons Dis. 10(4):1467–1476.
- Feigl B, Mattes D, Thomas R, Zele AJ. 2011. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 52(7):4362–4367.
- Feigl B, Morris CP, Brown B, Zele AJ. 2012a. Relationship among CFH and ARMS2 genotypes, macular pigment optical density, and neuroretinal function in persons without age-related macular degeneration. Arch Ophthalmol. 130(11):1402–1409.
- Feigl B, Ojha G, Hides L, Zele AJ. 2018. Melanopsin-driven pupil response and light exposure in non-seasonal major depressive disorder. Front Neurol. 9:764.
- Feigl B, Zele AJ. 2014. Melanopsin-expressing intrinsically photosensitive retinal ganglion cells in retinal disease. Optom Vis Sci. 91(8):894–903.
- Feigl B, Zele AJ, Fader SM, Howes AN, Hughes CE, Jones KA, Jones R. 2012b. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmol. 90(3):e230–234.
- Figueiro MG, Plitnick B, Rea MS. 2016. Research note: a self-luminous light table for persons with Alzheimer’s disease. Lighting Res Technol. 48(2):253–259.
- Fryc I, Brown D, Ohni Y. 2005. Spectral matching with an LED-based spectrally tunable light source. Proc SPIE. 59411:59411I.
- Gooley JJ. 2008. Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singap. 37(8):669–676. https://www.ncbi.nlm.nih.gov/pubmed/18797560
- Gordo MA, Recio J, Sanchez-Barcelo EJ. 2001. Decreased sleep quality in patients suffering from retinitis pigmentosa. J Sleep Res. 10(2):159–164.
- Gracitelli CPB, Paranhos Jr. A. 2015. Can glaucoma affect sleep quality? Arq Bras Oftalmol. 78(3):V–Vi.
- Hirakawa H, Terao T, Muronaga M, Ishii N. 2020. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 10(12):e01876.
- Houser KW, Boyce PR, Zeitzer JM, Herf M. 2021. Human-centric lighting: myth, magic or metaphor? Lighting Res Technol. 53(2):97–118.
- Joines S, James T, Liu SW, Wang WJ, Dunn R, Cohen S. 2015. Adjustable task lighting: field study assesses the benefits in an office environment. Work. 51(3):471–481.
- Joyce DS, Feigl B, Kerr G, Roeder L, Zele AJ. 2018. Melanopsin-mediated pupil function is impaired in Parkinson’s disease. Sci Rep. 8(1):7796.
- Judd DB, Macadam DL, Wyszecki G. 1964. Spectral distribution of typical daylight as function of correlated color temperature. J Opt Soc Am. 54(8):1031–1040.
- Kawasaki A, Kardon RH. 2007. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 27(3):195–204.
- Kelbsch C, Strasser T, Chen Y, Feigl B, Gamlin PD, Kardon R, Peters T, Roecklein KA, Steinhauer SR, Szabadi E, et al. 2019. Standards in pupillography. Front Neurol. 10:129.
- Khurana RN, Porco TC, Claman DM, Boldrey EE, Palmer JD, Wieland MR. 2016. Increasing sleep duration is associated with geographic atrophy and age-related macular degeneration. Retina. 36(2):255–258.
- Killgore WDS, Vanuk JR, Shane BR, Weber M, Bajaj S. 2020. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis. 134:104679.
- Kolberg D, Schubert F, Lontke N, Zwigart A, Spinner DM. 2011. Development of tunable close match LED solar simulator with extended spectral range to UV and IR. Energy Procedia. 8:100–105.
- Lamb TD, Pugh EN Jr. 2006. Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 47(12):5137–5152.
- La Morgia C, Carelli V, Carbonelli M. 2018. Melanopsin retinal ganglion cells and pupil: clinical implications for neuro-ophthalmology. Front Neurol. 9:1047.
- La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, Sambati L, Pan BX, Tozer KR, Barboni P, et al. 2016. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 79(1):90–109.
- Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. 2011. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 68(1):61–70.
- Llenas A, Carreras J. 2019a. Arbitrary spectral matching using multi-LED lighting systems. Opt Eng. 58(3):1.
- Llenas A, Carreras J. 2019b. A simple yet counterintuitive optical feedback controller for spectrally tunable lighting systems. Opt Eng. 58(3):1.
- MacLeod DI, Boynton RM. 1979. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J Opt Soc Am. 69(8):1183–1186.
- Marc RE, Jones BW, Watt CB, Strettoi E. 2003. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 22(5):607–655.
- Markwell EL, Feigl B, Zele AJ. 2010. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optom. 93(3):137–149.
- Marshall J. 2016. Light in man’s environment. Eye (London). 30(2):211–214.
- Maynard ML, Zele AJ, Feigl B. 2015. Melanopsin-mediated post-illumination pupil response in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 56(11):6906–6913.
- Maynard ML, Zele AJ, Kwan AS, Feigl B. 2017. Intrinsically photosensitive retinal ganglion cell function, sleep efficiency and depression in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 58(2):990–996.
- Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, Koutsandrea C. 2011. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol. 21(1):24–29.
- Munch M, Scheuermaier KD, Zhang R, Dunne SP, Guzik AM, Silva EJ, Ronda JM, Duffy JF. 2011. Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behav Brain Res. 224(2):272–278.
- Ortuño-Lizarán I, Esquiva G, Beach TG, Serrano GE, Adler CH, Lax P, Cuenca N. 2018. Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson's disease. Acta Neuropathol Commun 10;6(1):90.
- Park JC, Chen YF, Blair NP, Chau FY, Lim JI, Leiderman YI, Shahidi M, McAnany JJ. 2017. Pupillary responses in non-proliferative diabetic retinopathy. Sci Rep. 7(1):44987.
- Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M, Wood-Vasey WM, Franzen P. 2013a. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 210(1):150–158.
- Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC. 2013b. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci Biobehav Rev. 37(3):229–239.
- Romagnoli M, Stanzani Maserati M, De Matteis M, Capellari S, Carbonelli M, Amore G, Cantalupo G, Zenesini C, Liguori R, Sadun AA, et al. 2020. Chromatic pupillometry findings in Alzheimer’s disease. Front Neurosci. 14:780.
- Ruger M, Scheer FA. 2009. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 10(4):245–260.
- Shekleton JA, Flynn-Evans EE, Miller B, Epstein LJ, Kirsch D, Brogna LA, Burke LM, Bremer E, Murray JM, Gehrman P, et al. 2014. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep. 37(1):107–U324.
- Skomro RP, Ludwig S, Salamon E, Kryger MH. 2001. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2(5):417–422.
- Smith VC, Pokorny J. 1975. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 15(2):161–171.
- Tanaka H, Ishida K, Ozawa K, Ishihara T, Sawada A, Mochizuki K, Yamamoto T. 2021. Relationship between structural and functional changes in glaucomatous eyes: a multifocal electroretinogram study. BMC Ophthalmol. 21(1):305.
- Terman M, Terman JS. 2005. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 10(8):647–663. quiz 672 doi:10.1017/s1092852900019611.
- Tuunainen A, Kripke DF, Endo T. 2004. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. (2):CD004050. doi:10.1002/14651858.CD004050.pub2.
- Uddin MS, Tewari D, Mamun AA, Kabir MT, Niaz K, Wahed MII, Barreto GE, Ashraf GM. 2020. Circadian and sleep dysfunction in Alzheimer’s disease. Ageing Res Rev. 60:101046.
- Uprety S, Adhikari P, Feigl B, Zele AJ. 2022. Melanopsin photoreception differentially modulates rod-mediated and cone-mediated human temporal vision. iScience. 25(7):104529.
- Uprety S, Zele AJ, Feigl B, Cao D, Adhikari P. 2021. Optimizing methods to isolate melanopsin-directed responses. J Opt Soc Am A Opt Image Sci Vis. 38(7):1051–1064.
- Videnovic A, Willis GL. 2016. Circadian system - a novel diagnostic and therapeutic target in Parkinson’s disease? Mov Disord. 31(3):260–269.
- Wang H, Zhang Y, Ding J, Wang N. 2013. Changes in the circadian rhythm in patients with primary glaucoma. PLoS One. 8(4):e62841.
- Webster MA. 2020. The verriest lecture: adventures in blue and yellow. J Opt Soc Am A. 37(4):V1–V14.
- Zele AJ, Adhikari P, Cao D, Feigl B. 2019. Melanopsin driven enhancement of cone-mediated visual processing. Vision Res. 160:72–81.
- Zele AJ, Adhikari P, Feigl B, Cao D. 2018a. Cone and melanopsin contributions to human brightness estimation. J Opt Soc Am A. 35(4):B19–B25.
- Zele AJ, Cao D. 2015. Vision under mesopic and scotopic illumination. Front Psychol. 5:1594.
- Zele AJ, Dey A, Adhikari P, Feigl B. 2020. Rhodopsin and melanopsin contributions to human brightness estimation. J Opt Soc Am A. 37(4):A145–A153.
- Zele AJ, Dey A, Adhikari P, Feigl B. 2021. Melanopsin hypersensitivity dominates interictal photophobia in migraine. Cephalalgia. 41(2):217–226.
- Zele AJ, Feigl B, Adhikari P, Maynard ML, Cao D. 2018b. Melanopsin photoreception contributes to human visual detection, temporal and colour processing. Sci Rep. 8(1):3842.
- Zele AJ, Feigl B, Carter DD, inventors; Queensland University of Technology (QUT). 2022May12. Device, method, and system for biologically balanced lighting. World Intellectual Property Organization. Patent Cooperation Treaty. WO2022/094678A1.
- Zele AJ, Gamlin PD. 2020. Editorial: The pupil: behavior, anatomy, physiology and clinical biomarkers. Front Neurol. 11:211.
