?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Purpose
Previous studies have suggested that beta-alanine supplementation may benefit exercise performance, but current evidence regarding its effects on body composition remains unclear. This systematic review and meta-analysis aimed to investigate the effects of beta-alanine supplementation on body composition indices.
Methods
Online databases, including PubMed/Medline, Scopus, Web of Science, and Embase, were searched up to April 2021 to retrieve randomized controlled trials (RCTs), which examined the effect of beta-alanine supplementation on body composition indices. Meta-analyses were carried out using a random-effects model. The I2 index was used to assess the heterogeneity of RCTs.
Results
Among the initial 1413 studies that were identified from electronic databases search, 20 studies involving 492 participants were eligible. Pooled effect size from 20 studies indicated that beta-alanine supplementation has no effect on body mass (WMD: −0.15 kg; 95% CI: −0.78 to 0.47; p = 0.631, I2 = 0.0%, p = 0.998), fat mass (FM) (WMD: −0.24 kg; 95% CI: −1.16 to 0.68; p = 0.612, I2 = 0.0%, p = 0.969), body fat percentage (BFP) (WMD: −0.06%; 95% CI: −0.53 to 0.40; p = 0.782, I2 = 0.0%, p = 0.936), and fat-free mass (FFM) (WMD: 0.05 kg; 95% CI: −0.71 to 0.82; p = 0.889, I2 = 0.0%, p = 0.912). Subgroup analyses based on exercise type (resistance training [RT], endurance training [ET], and combined training [CT]), study duration (<8 and ≥8 weeks), and beta-alanine dosage (<6 and ≥6 g/d) demonstrated similar results. Certainty of evidence across outcomes ranged from low to moderate.
Conclusions
This meta-analysis study suggests that beta-alanine supplementation is unlikely to improve body composition indices regardless of supplementation dosage and its combination with exercise training. No studies have examined the effect of beta-alanine combined with both diet and exercise on body composition changes as the primary variable. Therefore, future studies examining the effect of the combination of beta-alanine supplementation with a hypocaloric diet and exercise programs are warranted.
1. Introduction
Various nutritional strategies are recommended to improve body composition by decreasing body fatness (both fat mass [FM] and body fat percentage [BFP]) and/or enhancing lean mass [Citation1–3]. The use of protein sources, often combined with exercise training to improve body composition, is prevalent among both athletes and the general population [Citation1]. Indeed, the beneficial effects of protein-rich foods, such as egg [Citation1], milk [Citation3], soy [Citation2], and meat [Citation4] on FM loss and lean mass gains are well established. Non-protein compounds are also used to improve body composition as evidence suggests they play important physiological roles, such as metabolic intermediates, biomolecular components, and post-translational modifiers [Citation5].
Beta-alanine, in particular, has gained considerable interest for this purpose and provides the focus of this investigation. Beta-alanine, a non-proteogenic amino acid, has become an increasingly popular dietary supplement as it boosts intramuscular carnosine (beta-alanyl-L-histidine) concentrations, which augments the fatigue threshold and improves high-intensity exercise performance [Citation6]. This beneficial advantage of beta-alanine has increased its utilization among athletes. In this regard, a systematic review of 19 randomized controlled trials (RCTs) showed that beta-alanine supplementation increases athletic performance [Citation7]. In another review study, its beneficial effects on exercise homeostasis and excitation-contraction coupling have also been indicated [Citation6]. Taken together, most of the literature has focused on beta-alanine’s effects on exercise performance [Citation6–11]. However, its effects on body composition are less studied. It has been hypothesized that beta-alanine supplementation could lead to improvements in lean mass by increasing the volume of training, although evidence is equivocal. For instance, beta-alanine supplementation increased lean mass after 3 weeks of high-intensity interval training (HIIT) in recreationally active college-aged men [Citation11]. On the other hand, Kern et al. did not report changes in body composition or lean mass after beta-alanine supplementation for 8 weeks in previously trained athletes [Citation12]. Additionally, 28 days of beta-alanine supplementation failed to affect body composition in female master athletes [Citation13]. Likewise, no significant effects of 10 weeks of resistance training combined with beta-alanine supplementation were observed on BFP [Citation14]. These conflicting outcomes indicate a need to conduct a systematic review and meta-analysis to assess the effects of beta-alanine supplementation on this topic. Therefore, we conducted a systematic review and meta-analysis to investigate beta-alanine’s effects on body composition indices (body mass, BFP, FM, and fat-free mass [FFM]).
2. Methods
This study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol to conduct and disseminate systematic reviews and meta-analyses [Citation15].
3. Search strategy
To find interrelated studies on beta-alanine supplementation in adults, we performed a comprehensive literature search in online databases including PubMed/Medline, Scopus, Web of Science, and Embase for the time period up to April 2021. The following terminology was utilized in the search: (“β-alanine’ OR ‘beta-alanine’ OR ‘b-alanine supplementation’ OR ‘beta-alanine supplementation’ OR ‘beta alanine’ OR ‘carnosine’ OR ‘βalanine’ and ‘beta-alanine’) AND (‘Intervention Study’ OR ‘Intervention Studies’ OR ‘controlled trial’ OR randomized OR randomized OR random OR randomly OR placebo OR ‘clinical trial’ OR ‘randomized controlled trial’ OR ‘randomized clinical trial’ OR RCT OR blinded OR ‘double blind’ OR ‘double blinded’ OR ‘clinical trial’ OR trials OR ‘Pragmatic Clinical Trial’ OR ‘Cross-Over Studies’ OR ‘Cross-Over’ OR ‘Cross-Over Study’ OR parallel OR ‘parallel study’ OR ‘parallel trial’). Search parameters were not restricted to publication date or original printed language. References from all relevant peer-reviewed investigations were consulted and cross-referenced against database searches to avoid omitting publications. All citations were subsequently included in the Endnote screening software, and duplicates were later removed from consideration in this study.
4. Inclusion criteria
In the present study, consideration was given to studies meeting all of the PICO criteria: (Participants) Adults (subjects older than 18 years), (Intervention) used a beta-alanine supplementation intervention/regimen, (Comparison) included a placebo or control group, (outcomes) body composition variables as an outcome (body mass, BFP, FM, and FFM). In the event of multiple cohort data publications from a single larger dataset, the more comprehensive article, whenever possible, was utilized in the present study. Studies containing more than one intervention group meeting the above criteria were considered independent datasets to determine the overall effect size.
5. Exclusion criteria
Investigation excluded from consideration comprised [Citation1]: cross-sectional or case-control design [Citation2], non-RCTs and literature reviews [Citation3], ecological studies [Citation4], control group manipulation of any sort [Citation5], lack of a placebo or control group [Citation6], performed on participants not meeting the minimum age criteria (<16 years), and [Citation7] the combination of beta-alanine with other supplements when compared with a placebo group.
6. Data extraction
Two independent investigators (DAL and OA) completed screening studies and data extraction from each qualified study. Extracted data contained the name of the primary investigator, year of publication, country of origin, study design, participant group size (placebo/control and intervention), participant demographics [(mean ± standard deviation [SD], age, body mass index (BMI), and sex)], beta-alanine dosage, duration of intervention, mean ± SD of body composition changes for both intervention and control groups, and any confounding variables utilized or accounted for in the randomized controlled trial (RCT). Dataset values were converted to the most common units of expression, whenever possible, for data analysis purposes.
7. Quality assessment
Study quality was measured by two independent reviewers (DAL and OA) using the Cochrane Collaboration modified risk of bias tool, which determines study bias in seven domains, including random sequence generation, allocation concealment, reporting bias, performance bias, detection bias, attrition bias, and other potential sources of bias [Citation16]. Consequently, terms including ‘Low’, ‘High’, or ‘Unclear’ were used to classify each domain of study bias. Dissimilarities between independent reviewers on the level of study bias in each domain were evaluated and resolved by the corresponding author.
8. Statistical analysis
Weighted mean differences (WMD) and SDs of body composition (body mass, FM, BFP, and FFM) from both intervention and control groups were extracted and used to generate overall effect sizes as determined by the random-effects model approach of DerSimonian and Laird [Citation17]. Additionally, when mean changes were not reported following beta-alanine supplementation (i.e. only mean value at baseline and again at post-intervention were noted in the study), the following formula was used to derive such changes: mean change = final post-intervention body composition indices value − baseline value for the same; and subsequently, changes in SDs of mean change scores were calculated by the following formula [Citation18]:
The correlation coefficient (R) was considered as 0.8 (between 0 and 1), which is in accordance with prior meta-analytic work [Citation18–20]. Moreover, reported standard errors (SEs), 95% confidence intervals (CIs), and interquartile ranges (IQRs) were converted to SDs using the method of Hozo et al. [Citation21]. Subsequently, a random-effects model, which incorporates between-study variations, was utilized to determine the overall body composition effect size. Heterogeneity between studies was performed using Cochran’s Q test and analyzed by an I-square (I2) statistic [Citation22] where I2 > 40% or p < 0.01 was considered as having high between-study heterogeneity [Citation23]. Sensitivity analysis was undertaken to determine the individual study effect on the overall estimation of effect [Citation24]. The possibility of publication bias was further verified through Begg’s test and funnel plots [Citation25]. STATA, version 11.2 (Stata Corp, College Station, TX), was used to perform statistical analysis. P-values <0.05 were considered statistically significant for all analyses.
9. Certainty assessment
The overall certainty of evidence across studies was assessed based on GRADE (Grading of Recommendations Assessment, Development, and Evaluation) guidelines working group (gradeworkinggroup.org) [Citation26]. The quality of evidence was subsequently classified into four categories according to the corresponding evaluation criteria, including high, moderate, low, and very low [Citation27].
10. Results
Study selection
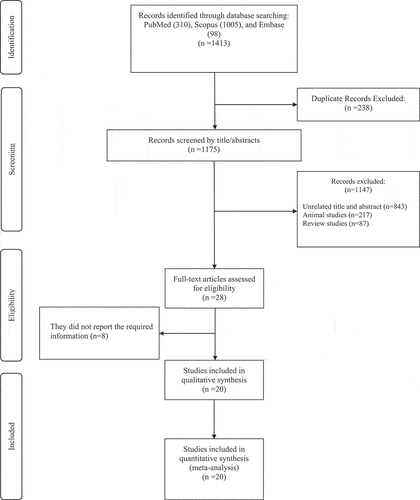
The initial databases search yielded 1413 studies, 238 of which were removed due to duplication. Another 1147 studies were excluded for the following reasoning: unrelated title and abstract not warranting full-text review (n = 843), animal (n = 217) and review studies (n = 87). Consequently, 28 relevant studies remained for full-text review and meta-analysis consideration. Eight studies were excluded because of a lack of necessary data reporting or other required information as outlined in the inclusion/exclusion criteria. Finally, 20 studies achieving all necessary criteria were included for meta-analysis in the present study ().
Study characteristics
The 20 included studies [Citation11–14,Citation28–43] contained a total of 25 intervention arms, which are shown in . These studies were published between 2008 and 2021, and in total, 492 participants were included. The study design of 19 studies was parallel (case = 242 participants and control = 242 participants), and one study had a crossover design (8 participants). Study duration varied from 3 to 10 weeks, while sample sizes ranged from 8 to 36 participants. Participants’ ages ranged from 17.4 to 53.5 years and baseline BFP from 7.8% to 35.7%. Beta-alanine dosage range was between 1.6 and 6.4 g/d. Except for two studies [Citation31,Citation39], others used beta-alanine supplementation combined with exercise training. Furthermore, most investigations (13 studies) were performed on men, whereas four studies utilized women and three included participants of both sexes. Quality assessment characteristics of studies are provided in .
Table 1. Characteristics of included studies in the meta-analysis
Table 2. Quality assessment
11. Meta-analysis
The effects of beta-alanine supplementation on body mass
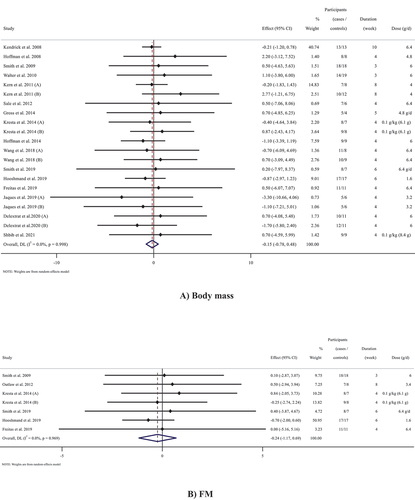
Outcomes analysis of the 16 studies (21 arms in total) [Citation11,Citation12,Citation14,Citation28–33,Citation36–38,Citation40–43] (n = 387) that measured body mass following beta-alanine supplementation did not show an overall effect of a significant change in body mass (WMD: −0.15 kg; 95% CI: −0.78 to 0.47; p = 0.631, I2 = 0.0%, p = 0.998) ()). In addition, all subgroup analyses did not indicate any changes in body mass following beta-alanine supplementation ().
Table 3. Subgroup analyses of beta-alanine supplementation on body composition
The effects of beta-alanine supplementation on FM
Based on the results of six studies [Citation11,Citation30–32,Citation39,Citation43] containing 7 total effect sizes (n = 154), beta-alanine supplementation failed to change FM (WMD: −0.24 kg; 95% CI: −1.16 to 0.68; p = 0.612, I2 = 0.0%, p = 0.969) ()) regardless of exercise type, study duration, and the dose of supplementation ().
The effects of beta-alanine supplementation on BFP
Overall result from 16 studies [Citation11,Citation13,Citation28–39,Citation43] containing 21 total effect sizes (n = 427) did not reveal significant alterations in BFP (WMD: −0.06%; 95% CI: −0.53 to 0.40; p = 0.782, I2 = 0.0%, p = 0.936) ()). Insignificant changes were shown in all subgroups ().
The effects of beta-alanine supplementation on FFM
Pooled effect sizes from 10 studies [Citation11,Citation30–33,Citation35,Citation37–39,Citation43] containing 13 arms (n = 276) did not reveal a significant change in FFM following beta-alanine supplementation (WMD: 0.05 kg; 95% CI: −0.71 to 0.82; p = 0.889, I2 = 0.0%, p = 0.912). Subgroup analyses demonstrated similar results () and ).
Publication bias
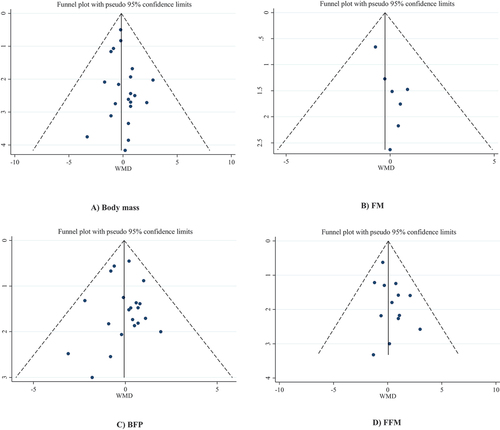
According to Begg’s regression test, there was no evidence of publication bias for studies examining the effect of beta-alanine supplementation on body mass (p = 0.786), FM (p = 0.548), BFP (p = 0.349), and FFM (p = 0.760). In addition, Egger’s regression test showed no significant publication bias for body mass (p = 0.285), BFP (p = 0.881), and FFM (p = 0.110), but there was evidence of publication bias found for FM (p = 0.031). The trim and fill analysis for FM demonstrated that, with the addition of 11 unpublished articles, the test for publication bias was no longer significant; however, the overall effect did not change significantly (WMD: −0.575, 95%CI: −1.382 to 0.232; p = 0.162). Funnel plots indicated no evidence of asymmetry in the effects of beta-alanine supplementation on all body composition indices except for FM ()).
Sensitivity analysis
Upon removing individual study effects for sensitivity analysis, the overall results did not significantly change for body mass, FM, BFP, and FFM.
Grading of evidence
The GRADE protocol was used to evaluate the certainty of the evidence (). The quality of evidence related to body mass, FM, and BFP was downgraded to moderate due to serious limitations in risk of bias. Moreover, the GRADE assessment for FFM was low due to concerns about both risk and publication bias.
Table 4. GRADE profile of beta-alanine supplementation on body composition
12. Discussion
The purpose of this study was to determine if beta-alanine supplementation in doses of 1.6-8.4 g/d improves body composition indices. Overall, beta-alanine supplementation exerted no significant impact on body mass, FM, BFP, and FFM. Subgroup analysis based on dosage (≥6 g or <6 g/d), study duration (8 ≥ or 8 < weeks), and exercise type (resistance, endurance, and combined training) indicated no significant changes following beta-alanine supplementation on body composition.
To our knowledge, this is the first systematic review and meta-analysis investigating the longitudinal effects of beta-alanine supplementation on body composition indices. Although its role as a precursor to the dipeptide carnosine has led recent researchers to consider beta-alanine as an ergogenic aid to improve exercise performance, some investigations have failed to show any significant improvements in exercise performance variables such as strength, endurance, and power following beta-alanine supplementation [Citation44]. The contribution of carnosine to intracellular buffering during intense exercise can attenuate intracellular acidosis as a possible factor contributing to reduced exercise performance [Citation45]. Other putative physiological effects of carnosine, such as increased calcium sensitivity [Citation46] and antioxidant capabilities [Citation47], may also have a positive impact on exercise performance, but the data is equivocal [Citation48]. However, most studies did not show any improvements in body composition indices following beta-alanine supplementation, indicating that the beneficial effects of intramuscular carnosine accumulation did not translate into body composition changes [Citation14,Citation39,Citation42,Citation49].
Pooled analysis of the studies included in this meta-analysis found no significant changes in body mass or FFM following beta-alanine supplementation. The results from our study were in line with previous RCTs, which did not observe any positive effects of beta-alanine supplementation on FFM [Citation11,Citation14,Citation35,Citation37,Citation40]. In this regard, in college-aged women, Outlaw et al. showed that beta-alanine supplementation (3.4 g/day) for 8 weeks combined with resistance training increased lower-body muscular endurance but had no effect on maximal strength, FFM, FM, or BFP [Citation39]. In addition, Kresta et al. assessed the influences of beta-alanine and creatine supplementation on muscle carnosine, body composition, and exercise performance in recreationally active females over 28 days and reported no FFM improvements [Citation43].
Although beta-alanine supplementation appears to be a valuable ergogenic aid in HIIT requiring a high degree of strength endurance, its capacity to boost hypertrophic responses during resistance training remains unknown. The observed beneficial effects of beta-alanine supplementation on lean mass in prior research can be attributable to beta-alanine’s ability to promote fluid shifts into muscle and subsequent increases in intramuscular water, which have been claimed to account for part of the gains in FFM [Citation12,Citation50]. However, Freitas et al. showed that 28 days of beta-alanine supplementation did not increase total or intracellular water content during resistance training after HIIT [Citation32]. In addition, recent studies were unable to measure intramuscular carnosine concentrations or myofibrillar protein content in the exercised muscles to prove their claims toward beneficial effects of beta-alanine supplementation on lean mass through muscle hypertrophy [Citation32,Citation40,Citation41]. As can be seen from the subgroup analysis, differences between studies in terms of study duration, dosages of beta-alanine, and exercise type did not change the overall impacts of beta-alanine supplementation on body mass or lean mass. It should be mentioned that only two of the 13 included studies on the effects of beta-alanine supplementation on FFM lasted 8 weeks [Citation12,Citation39]. The findings of a previous review implied that the supplementation time might be a modifying factor for the ergogenic effect of beta-alanine [Citation51]. It is possible that shorter supplementation procedures (e.g. 3 weeks) are insufficient to meet the threshold of muscle carnosine level increases required to improve Yo-Yo test performance [Citation51]. In this regard, one study found that beta-alanine supplementation and placebo treatment were equally effective at improving VO2peak, time to fatigue, and total work performed over 3 weeks of HIIT in young men [Citation11]; however, only the group that supplemented with beta-alanine showed an increase in total work performed and lean mass after 6 weeks of training [Citation11], supporting this theory. The usage of beta-alanine has been suggested by scientists as a method to improve training adaptation by enhancing the ability to train at a higher intensity with less muscle fatigue [Citation52,Citation53]. Future studies are needed with longer-term beta-alanine supplementation duration with HIIT or resistance training on alterations in FFM.
We also did not find significant alterations following beta-alanine supplementation on FM or BFP. Previous RCTs showed that both acute and chronic supplementation with beta-alanine in different practical settings resulted in small and non-significant effects on FM or BFP [Citation13,Citation33,Citation43,Citation54]. For example, Smith et al. found that 6 weeks of beta-alanine supplementation combined with HIIT was failed to significantly change FM in recreationally active men [Citation11]. Moreover, a recent study examined 6 weeks of beta-alanine supplementation in overweight women and reported increased time to exhaustion on a treadmill test compared to the control group, while FM remained unchanged [Citation31]. In a study conducted by Hoffman et al., a significant decrease in BFP was reported in the beta-alanine plus creatine supplementation group compared to the control but not different from the creatine supplementation group only [Citation42]. The potential benefit of adding beta-alanine with creatine for the 10-week duration may increase the fatigue resistance, allowing for greater training volume [Citation55]. Hoffman et al. reported no significant difference in total kcals between groups; thus, greater weekly training volume in the beta-alanine and creatine group vs. the placebo group would result in more significant kcal expenditure, thus potentially having a secondary effect on FM.
What remains to be seen is why the potential effects of beta-alanine supplementation on intramuscular carnosine concentrations and its contribution to intracellular buffering have yet to be translated into the improvements in body composition consistently. It should be noted that this lack of improvement could be due to some methodological and participant-related criteria in the previous studies. First of all, the primary aim of the majority of these studies was to increase exercise performance, and consequently, their training interventions were not specifically designed for achieving and maximizing body composition alterations. Second, it is important to note that most previous studies employed well-trained men with a lower baseline BFP; thus, the further fat loss was unexpected. Finally, the positive effects of beta-alanine supplementation on improvements in exercise performance are thought to be due to increases in intramuscular concentrations of carnosine, although the majority of RCTs included in our investigation did not measure carnosine concentrations [Citation11,Citation31,Citation32,Citation35–38,Citation40,Citation41,Citation54]. Therefore, the results of these studies should be interpreted with caution. It is worth mentioning that the increases in intramuscular concentrations of carnosine appear to be influenced by baseline levels and habitual dietary intake of carnosine-containing foods, which can potentially affect the impacts of beta-alanine supplementation on exercise performance and thereby body composition changes [Citation56,Citation57].
The current meta-analysis has some limitations. As mentioned above, in the majority of included RCTs, baseline intracellular carnosine concentrations, its changes during the study period, or dietary intake of carnosine and total protein using a validated methodology, such as 24-hour food recalls, were not investigated. Another significant limitation of this meta-analysis was the scarcity of research that used body composition indices as a primary outcome. However, low heterogeneity across the included studies’ results must be considered a strength of this study. Moreover, according to the GRADE profile, the quality of evidence for FFM is low. In other words, our confidence in the FFM-enhancing properties of beta-alanine supplementation is limited. However, in terms of quality of evidence of the effects of beta-alanine supplementation on body mass, BFP and FM, we are moderately confident in the effect estimate.
13. Conclusions
Beta-alanine supplementation does not improve body composition, and subgroup analysis based on study duration, beta-alanine dosage, and different training types did not alter the observed results. Because all studies included in the present systematic review and meta-analysis lasted less than 3 months, additional longer-term RCTs are necessary to expand our findings.
Availability of supporting data
Data sharing is applicable.
Authors’ contributions
DAL and RB conceived and designed the research. DAL and conducted experiments. DAL and RB contributed new reagents or analytical tools. OA analyzed data. DAL, RB, and MG wrote the manuscript. AW, RB, KS, and JRS revised the manuscript. All authors read and approved the manuscript.
Authors’ information
This was provided in the first page.
Consent for publication
We agree with publications after acceptance in JISSN.
Ethical Approval and Consent to participate
This is a review study, and there was no consent to participate.
Disclosure statement
Jeffrey R. Stout has conducted industry-sponsored research on creatine and other nutraceuticals over the past 25 years. Further, Jeffrey R. Stout has also received financial support for presenting the science of various nutraceuticals, like beta-alanine, at industry-sponsored scientific conferences
Additional information
Funding
References
- Bagheri R, Moghadam BH, Candow DG, et al. Effects of Icelandic yogurt consumption and resistance training in healthy untrained older males. Br J Nutr. 2021;1–26. DOI:10.1017/S000711452100310X
- Deibert P, Solleder F, König D, et al. Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males. Aging Male. 2011;14(4):273–279.
- Pourabbas M, Bagheri R, Hooshmand Moghadam B, et al. Strategic ingestion of high-protein dairy milk during a resistance training program increases lean mass, strength, and power in trained young males. Nutrients. 2021;13(3):948.
- Daly RM, O’Connell SL, Mundell NL, et al. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am J Clin Nutr. 2014;99(4):899–910.
- Haidari F, Asadi M, Mohammadi-Asl J, et al. Effect of weight-loss diet combined with taurine supplementation on body composition and some biochemical markers in obese women: a randomized clinical trial. Amino Acids. 2020;52(8):1115–1124.
- Blancquaert L, Everaert I, Derave W. Beta-alanine supplementation, muscle carnosine and exercise performance. Current Opin Clin Nutr Metab Care. 2015;18(1):63–70.
- Quesnele JJ, Laframboise MA, Wong JJ, et al. The effects of beta-alanine supplementation on performance: a systematic review of the literature. Int J Sport Nutr Exerc Metab. 2014;24(1):14–27.
- Artioli GG, Gualano B, Smith A, et al. Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med Sci Sports Exerc. 2010;42(6):1162–1173.
- Stout JR, Graves BS, Smith AE, et al. The effect of beta-alanine supplementation on neuromuscular fatigue in elderly (55–92 years): a double-blind randomized study. J Int Soc Sports Nutr. 2008;5(1):1–6.
- Ducker KJ, Dawson B, Wallman KE. Effect of beta-alanine supplementation on 800-m running performance. Int J Sport Nutr Exerc Metab. 2013;23(6):554–561.
- Smith AE, Walter AA, Graef JL, et al. Effects of β-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J Int Soc Sports Nutr. 2009;6:6.
- Kern BD, Robinson TL. Effects of β-alanine supplementation on performance and body composition in collegiate wrestlers and football players. J Strength Cond Res. 2011;25(7):1804–1815.
- Glenn JM, Gray M, Stewart RW Jr., et al. Effects of 28-day beta-alanine supplementation on isokinetic exercise performance and body composition in female masters athletes. J Strength Cond Res. 2016;30(1):200–207.
- Kendrick IP, Harris RC, Kim HJ, et al. The effects of 10 weeks of resistance training combined with β-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids. 2008;34(4):547–554.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177–188.
- Borenstein M, Hedges LV, Higgins JP, et al. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons; 2011.
- Asbaghi O, Khosroshahi MZ, Kashkooli S, et al. Effect of calcium‑vitamin d co‑supplementation on insulin, insulin sensitivity, and glycemia: a systematic review and meta-analysis of randomized clinical trials. Horm Metab Res. 2019;51(5):288–295.
- Asbaghi O, Sadeghian M, Rahmani S, et al. The effect of green coffee extract supplementation on anthropometric measures in adults: a comprehensive systematic review and dose-response meta-analysis of randomized clinical trials. Complement Ther Med. 2020;51:102424.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Tobias A. Assessing the influence of a single study in the meta-analysis estimate. STATA Tech Bull. 1999;8(47).
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. 2011;64(4):383–394.
- Gordon H, Oxman A, Vist G, et al. Rating quality of evidence and strength of recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926.
- Shbib S, Rashidlamir A, Hakak Dokht E. The effects of plyometric training and β-alanine supplementation on anaerobic power and serum level of carnosine in handball players. 2021;17(3):569–576.
- Impson-Davey GJK. Effects of supplementation with creatine monohydrate and beta-alanine, alone or combined, on repeated sprint performance and physiological parameters in amateur team and racket sport players. Kinesiology. 2020;52(1):115–123.
- Smith CR, Harty PS, Stecker RA, et al. A pilot study to examine the impact of beta-alanine supplementation on anaerobic exercise performance in collegiate rugby athletes. Sports. 2019;7(11):231.
- Hooshmand S, Halabchi F, Hashempour A, et al., Improving physical activity tolerance in sedentary overweight women under beta-alanine supplementation. Science & Sports. 2019;34(3):e217–e23.
- Freitas MC, Cholewa J, Panissa V, et al. Short-time β-alanine supplementation on the acute strength performance after high-intensity intermittent exercise in recreationally trained men. Sports. 2019;7(5):108.
- Jaques M, Glick D, Greco-Henderson D, et al. The effects of short term β-alanine supplementation on performance in division iii male and female rowers. Journal of Exercise and Nutrition. 2(3).
- Askari F, Rahmaninia F. The effect of 8 weeks beta-alanine supplementation and resistance training on maximal-intensity exercise performance adaptations in young males. Physical education of students. 2019;23(1):4–8.
- Jaffe D, Hewit J, Cholewa J, et al. Influence of sustained beta-alanine supplementation on body composition and physical performance in college-aged males seeking military commission. International Journal of Human Movement and Sports Sciences. 2018;6(1):1–9.
- Wang R, Fukuda DH, Hoffman JR, et al. Distinct Effects of Repeated-Sprint Training in Normobaric Hypoxia and β-Alanine Supplementation. Journal of the American College of Nutrition. 2019;38(2):149–161.
- Walter AA, Smith AE, Kendall KL, et al. Six weeks of high-intensity interval training with and without beta-alanine supplementation for improving cardiovascular fitness in women. Journal of Strength and Conditioning Research. 2010;24(5):1199–1207.
- Gross M, Bieri K, Hoppeler H, et al. Beta-alanine supplementation improves jumping power and affects severe-intensity performance in professional alpine skiers. Int J Sport Nutr Exerc Metab. 2014;24(6):665–673.
- Outlaw JJ, Smith-Ryan AE, Buckley AL, et al. Effects of β-alanine on body composition and performance measures in collegiate women. J Strength Cond Res. 2016;30(9):2627–2637.
- Sale C, Hill CA, Ponte J, et al. β-alanine supplementation improves isometric endurance of the knee extensor muscles. J Int Soc Sports Nutr. 2012;9(1):26.
- Hoffman JR, Landau G, Stout JR, et al. β-Alanine ingestion increases muscle carnosine content and combat specific performance in soldiers. Amino Acids. 2015;47(3):627–636.
- Hoffman JR, Ratamess NA, Faigenbaum AD, et al. Short-duration β-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr Res. 2008;28(1):31–35.
- Kresta JY, Oliver JM, Jagim AR, et al. Effects of 28 days of beta-alanine and creatine supplementation on muscle carnosine, body composition and exercise performance in recreationally active females. J Int Soc Sports Nutr. 2014;11(1):55.
- Huerta Ojeda Á, Tapia Cerda C, Poblete Salvatierra MF, et al. Effects of beta-alanine supplementation on physical performance in aerobic-anaerobic transition zones: a systematic review and meta-analysis. Nutrients. 2020;12(9):2490.
- Sale C, Saunders B, Harris RC. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids. 2010;39(2):321–333.
- Dutka TL, Lamboley CR, McKenna MJ, et al. Effects of carnosine on contractile apparatus Ca2⁺ sensitivity and sarcoplasmic reticulum Ca2⁺ release in human skeletal muscle fibers. J Appl Physiol. 2012;112(5):728–736. Bethesda, Md: 1985.
- Hipkiss AR, Michaelis J, Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995;371(1):81–85.
- Smith AE, Stout JR, Kendall KL, et al. Exercise-induced oxidative stress: the effects of β-alanine supplementation in women. Amino Acids. 2012;43(1):77–90.
- Kim KJ, Song HS, Yoon DH, et al. The effects of 10 weeks of β-alanine supplementation on peak power, power drop, and lactate response in Korean national team boxers. J Exerc Rehabil. 2018;14(6):985–992.
- Deldicque L, Theisen D, Bertrand L, et al. Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am J Physiol Cell Physiol. 2007;293(4):C1263–C71.
- Grgic J. Effects of beta-alanine supplementation on Yo–Yo test performance: a meta-analysis. Clin Nutr ESPEN. 2021;43:158–162.
- Stout JR, Cramer JT, Zoeller RF, et al. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids. 2007;32(3):381–386.
- Harris RC, Edge J, Kendrick IP, et al. The effect of very high interval training on the carnosine content and buffering capacity of V lateralis from humans. FASEB J. 2007;21(6):A944–A.
- Delextrat A, Targen N, Impson-Davey G, et al. Effects of supplementation with creatine monohydrate and beta-alanine, alone or combined, on repeated sprint performance and physiological parameters in amateur team and racket sport players. Kinesiology. 2020;52(1):115–123.
- Hobson RM, Saunders B, Ball G, et al. Effects of β-alanine supplementation on exercise performance: a meta-analysis. Amino Acids. 2012;43(1):25–37.
- Trexler ET, Smith-Ryan AE, Stout JR, et al. International society of sports nutrition position stand: beta-Alanine. J Int Soc Sports Nutr. 2015;12(1):30.
- Harris RC, Jones G, Hill CA, et al. The carnosine content of v lateralis in vegetarians and omnivores. FASEB J. 2007;21(6):A944–A.