ABSTRACT
Background
Exercise and diet have positive effects on hepatic fat reduction, and protein supplementation is known to lower hepatic fat accumulation. However, the effect of a combination of exercise and whey protein supplementation (WPS) on hepatic fat content (HFC) is unknown.
Methods
We investigated the effect of WPS on HFC during resistance exercise and diet control intervention for four weeks. A total of 34 sedentary males participated and were randomly assigned to two groups: a protein supplement group (PSG, n = 18) and a control group (CG, n = 16). The PSG took 60 g of WPS per day, and the CG took 60 g of an isocaloric placebo per day. All participants were fed a calorie-controlled diet throughout the study period, with their daily caloric intake determined by their resting metabolic rate and physical activity level. Both groups performed resistance exercises supervised by experts at 60–70% of their maximum efforts for 60 min/day, 6 days/week for 4 weeks. HFC was assessed using the controlled attenuation parameter (CAP) after an 8 h fast, at pre-, mid-, and post-intervention. Liver enzymes and lipid profile were also analyzed after an 8 h fast and pre- and post-intervention.
Results
The CAP was significantly reduced after 4 weeks of intervention in both groups (PSG, p < .001; CG, p = .002). However, there was no significant interaction between the group and changes in CAP. Interestingly, when comparing the pre- and mid-tests, both groups also had significantly reduced CAP (PSG, p = .027; CG, p = .028), but there was a significant difference in the amount of change in CAP between the two groups (PSG, -47.2 ± 25.4 dB/m; CG, -19.5 ± 15.1 dB/m; p = .042). For liver enzymes, there was a significant interaction between the two groups and a change in aspartate transaminase (AST) (p = .038). However, alanine aminotransferase (ALT) levels were significantly decreased only in the PSG group (p = .002). In lipids, both groups showed significantly decreased total cholesterol (p < .001) and low-density lipoprotein cholesterol (p < .001) after the intervention.
Conclusion
Our data showed that WPS may not enhance the overall effects of resistance exercise on HFC and lipid profiles. However, in part, WPS may have a beneficial effect on liver enzymatic changes and rapid response to resistance exercise-induced HFC reduction.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD), a condition in which triglycerides accumulate excessively in the liver [Citation1], has a worldwide prevalence of approximately 25% [Citation2]. The hepatic fat content (HFC) indicates the accumulation of triglycerides in the liver and is widely used as a diagnostic criterion for NAFLD [Citation3]. Therefore, there is growing interest in the reduction and management of HFC.
The European Association for the Study of the Liver and European Association for the Study of Diabetes recommend exercise as an effective way to reduce HFC in patients [Citation4]. Indeed, numerous studies have reported that both aerobic exercise and resistance exercise reduced HFC in individuals with various metabolic disorders [Citation5,Citation6]. Recent studies have shown that resistance exercise has the advantage of reducing HFC without much difference from aerobic exercise, even though it consumes less energy [Citation7].
Protein supplementation is known to enhance the effects of resistance exercise [Citation8]. Based on the fact that they are generally composed of amino acids, such as casein, branched chain amino acids, and carnitine, they consequently promote the synthesis of proteins necessary for muscle growth [Citation9]. Therefore, the number of people taking protein supplements during resistance exercise continues to increase.
High-protein diets also reduce hepatic fat accumulation by promoting increased hepatic fat oxidation [Citation10]. In fact, Bortolotti et al. found that whey protein supplementation (WPS) reduced intra-hepatocellular lipids by 20% in females with obesity [Citation11]. Thus, protein supplementation may increase HFC reduction during resistance exercise. However, it is difficult to determine whether this effect is actually caused by WPS or diet because there is no dietary control for daily food intake. Therefore, it is necessary to investigate the effect of WPS on HFC during resistance exercise under controlled conditions.
In this study, we designed a double-blind, randomized, placebo-controlled trial to investigate the effects of protein supplementation on resistance exercise-induced HFC reduction in individuals with sedentary lifestyle under a normal diet.
2. Materials and methods
2.1. Recruitment
Thirty-six male participants in their 20s participated in the study. The study was conducted as a double-blind, randomized, placebo-controlled trial, with a protein supplement group (PSG) and a control group (CG).
The recruitment was conducted by posting recruitment notices for study participants on bulletin boards within the university and on online communities. Individuals who wished to participate underwent 1:1 interviews with the research team to confirm the inclusion and exclusion criteria. The inclusion criteria for the study were as follows: males with sedentary lifestyle between 20 and 30 years of age; standard physique for Korean males in their 20s (height: 174 ± 5 cm, weight: 74 ± 4 kg) [Citation12]. The exclusion criteria for the study were as follows: alcohol consumption within the last 3 months, regular exercise within the last 3 months, smoking within the last 3 months, liver disease, diabetes mellitus, or any congenital carbohydrate metabolism disorder such as allergy to dairy or lactose intolerance. The study was approved by the Institutional Review Board (Protocol #2020_136_HR; date of approval: 31/05/2021). All participants signed a consent form and were informed of the purpose of the study and the possible risks. A physical ability readiness questionnaire (PAR-Q+) was administered to determine participants’ health status and risk before the experiment [Citation13].
2.2. Whey protein supplement or placebo intake and diet control
The PSG took a protein powder supplement, and the CG took a placebo with equal calories (99 kcal/pack) consisting of carbohydrates. Protein powder supplements (Selex PRO-FITTM) and placebo were manufactured by Maeil Health Nutrition Co. Ltd. (Gyeonggi-do, Korea). One pack of protein powder supplement contained 20 g of whey protein isolate, 8 g of carbohydrate, and 6.4 g of dietary fiber, whereas one pack of placebo contained 25 g of carbohydrate (0 g of dietary fiber) instead of protein. All participants took a protein powder supplement or a placebo with water, three times a day, after waking up, after exercise, and before bed. All powders were chocolate-flavored to mask the contents and provided in unlabeled packing so that neither the study participants nor the researcher could distinguish them.
All participants were provided with three meals per day equal to the calories obtained by multiplying their resting metabolic rate by the activity factor per day during the 4-week intervention period. Each meal was commissioned by a packed meal company (Salady Inc., Seoul, Korea) and prepared with a protein: fat: carbohydrate ratio of 10:30:60, according to the calculated individual daily calorie requirement. All meals were delivered daily and served directly to the participants. The nutritional components and calories of the meals provided to the participants were analyzed once a week basis. Information regarding the composition and quantity of the meals was obtained from the company, and actual calorie intake of the participants was calculated by referring to the nutritional information provided the company. To check whether the participants consumed all the provided meals correctly, we monitored the video sent by the participants recording what they had consumed at each meal.
2.3. Resistance exercise program
As shown in , all participants performed resistance exercise for 4 weeks. Each muscle group was trained 2–3 days a week with at least 48 h of rest [Citation14]. All participants received detailed education on resistance exercise in an interview before the study began and were encouraged to maintain their daily activities during the study period.
Table 1. Resistance exercise program.
2.4. Measurements
2.4.1. Body composition
Body weight, body mass index (BMI), body fat percentage, fat mass, and skeletal muscle mass were assessed using a bioelectrical impedance analyzer (BIA) (Inbody 620, Seoul, Korea). This equipment has an intraclass correlation coefficient greater than 0.99 for estimating all body composition parameters in men [Citation15]. According to the manufacturer’s instructions, a fast of at least 4 hours was required, and all participants maintained an 8-h fasting period on the same day as the resting metabolic rate (RMR) test. Participants were instructed to restrict food, beverages, alcohol, caffeine, and other substances that could affect body composition test results during the 8-h fasting period.
2.4.2. Resting metabolic rate
The RMR test was performed using a gas analyzer (Quark RMR, COSMED, Italy). The participants visited the laboratory after an 8-h overnight fast [Citation16]. After arriving at the laboratory, participants rested for 10–15 min, put their head in the canopy, and maintained a lying position on the bed. While the examiner set up the equipment, the participants rested in a lying position for 10 min. Inhalation and exhalation through the mouth and nose were measured using a breath-by-breath method. Except for the values collected during the first 5 min after the start of the measurement, the average value measured for the remaining 10 min was used to determine RMR. To collect accurate data, the participants were instructed not to fall asleep during the measurement.
2.4.3. Hepatic fat content
The controlled attenuation parameter (CAP) was measured using a FibroScan device (FibroScan® Mini + 430, Echosens, Paris, France) by a single technician who was blinded to the clinical data of the participants. The measurement was performed using a 3.5-MHz standard probe on the right hepatic lobe through the intercostal spaces with the participant lying supine [Citation17]. Measurements were considered valid if the following criteria were met: (1) at least 10 valid shots, (2) the success rate was at least 60%, and (3) the interquartile range was less than 30% of the median values of the CAP. The final CAP was recorded as the median value of all measurements and expressed as dB/m [Citation18]. CAP was measured pre- (week 0), mid- (week 2), and post-intervention (week 4). However, due to COVID-19, only 12 participants were able to complete mid-intervention test.
2.4.4. Blood biochemistry assay
The blood samples were collected once during the morning 1–2 days before the start of the exercise intervention (week 0) and once during the morning 1–2 days after the last day of exercise (week 4). Following a fasting period of 8 h, 10 mL of venous blood samples were obtained. Then, the blood samples were centrifuged at 3000 rpm for 20 min within 2 h after blood collection and stored at -70 ℃ until the assay. Blood samples were tested within 24 h of sample collection. Aspartate transaminase (AST), alanine aminotransferase (ALT), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) levels were analyzed and quantified using a clinical automatic chemistry analyzer, DXC 700 AU (Beckman Coulter, CA, USA). Low-density cholesterol (LDL-C) level was calculated using Friedewald’s equation [Citation19]. shows the study process, including the time-point measurements used in this study.
2.5. Data analysis
We calculated our sample size using G*power software (G*power 3.1; Kiel University, Kiel, Germany). We determined our sample size with a power of 0.8, an effect size (ES) of 0.27, and an alpha error of 0.05, including 20% dropout rate [Citation20]. Data were analyzed using SPSS version 27 for Windows (IBM Corporation, Armonk, NY, USA). All data were checked for a normal distribution using kurtosis and skewness [Citation21]. Repeated measures ANOVA with a mixed design was used to evaluate the group-by-time interaction effect. If no significant interaction was observed, a paired t-test was used to evaluate the difference in pre- and posttests within the group. An independent t-test was used to evaluate the homogeneity between the groups. To confirm the effect of the intervention, the ES was calculated. Cohen’s d was used as the ES for the paired t-test. For the analysis of non-parametric data, the Wilcoxon signed rank test was used to evaluate the difference between pre-, mid-, and postests within the group, and the Mann–Whitney U-test was used to evaluate the homogeneity between groups. Pearson’s correlation was performed to evaluate the associations between CAP and liver enzymes. The statistical significance level was set at p < .05.
3. Results
3.1. Participant demographics
Thirty-six participants participated in the study. However, two participants in the CG dropped out, mainly due to COVID-19 symptoms. Therefore, data from 34 participants were included in the statistical analyses. The age and BMI of the PSG (n = 18) and CG (n = 16) were 23.9 ± 2.5 vs. 24.8 ± 3.0 years, and 24.0 ± 1.2 vs. 24.0 ± 1.8 kg/m2, respectively. There were no statistical differences in age and BMI between the groups.
3.2. Calorie intake
The calculated calorie intake for PSG participants was 2343.2 ± 202.2 kcal/day, and actual calorie intake was 2242.7 ± 122.6 kcal/day. The difference was only 100.5 kcal/day, but the difference was statistically significant (p = .002). The calculated calorie intake for CG participants was 2414.3 ± 256.0 kcal/day, and actual calorie intake was 2320.8 ± 158.9 kcal/day. Similarly, the difference was also only 93.5 kcal/day, but the difference was statistically significant (p = .040). However, these differences were not statistically different between the two groups. The actual calorie intake of carbohydrates, protein, and fat for PSG participants was 1224.4 ± 73.6 kcal (54.6%), 434.6 ± 12.3 kcal (19.4%), 583.7 ± 36.8 kcal (26.0%), respectively. Those of CG participants were 1511.3 ± 95.3 kcal (65.1%), 202.4 ± 15.9 kcal (8.7%), 607.1 ± 47.7 kcal (26.1%), respectively.
3.3. Body composition and resting metabolic rate
As shown in , body weight, body fat mass, and body fat percentage significantly decreased in both groups after 4 weeks of resistance exercise (p < .001). The RMR was significantly increased by 128.9 kcal in PSG (p = .036), but not in CG. However, there were no significant interactions between the group and these changes. Skeletal muscle mass did not show significant differences in both groups after 4 weeks of resistance exercise, and there was no interaction between group and the change in skeletal muscle mass.
Table 2. Body composition and resting metabolism.
3.4. Hepatic fat content
3.4.1. Pre- vs. postest
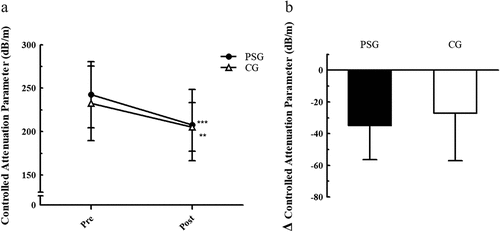
The CAP was significantly decreased in both groups after 4 weeks of resistance exercise (PSG: 242.4 ± 38.2 to 207.5 ± 41.2, p < .001; CG: 232.4 ± 42.9 to 205.3 ± 27.9, p = .002). The CAP reduction was about 29% larger in PSG (-34.9 dB/m) than in CG (-27.1 dB/m), but there was no significant interaction between the group and the CAP reduction ().
3.4.2. Pre- vs. mid-test
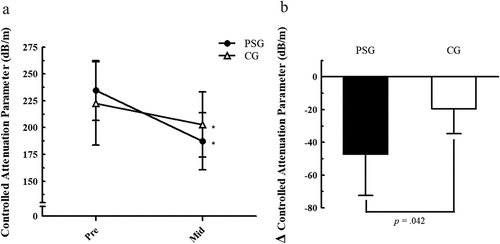
Due to COVID-19, the experimental conditions were very limited; therefore, only 12 participants (PSG, n = 6; CG, n = 6) could complete the mid-test. Since the data were not normally distributed and the number of participants was small, non-parametric statistics were used for the analysis. The CAP was significantly decreased in both groups after 2 weeks of resistance exercise (PSG: 234.5 ± 28.0 to 187.3 ± 26.6, p = .027; CG: 222.3 ± 38.8 to 202.8 ± 30.4, p = .028). In addition, CAP reduction was significantly different between the groups (p = .042). The CAP reduction was approximately 142% larger in PSG (-47.2 dB/m) than in CG (-19.5 dB/m) ().
3.4.3. Mid- vs. posttest
There was no significant change in CAP from week 2 to week 4 in either group (PSG: 187.3 ± 26.6 to 194.6 ± 14.2; CG: 202.8 ± 30.4 to 189.3 ± 32.8). There were no significant differences in the changes in CAP between the two groups.
3.5. Liver enzyme and lipid profile
The changes in liver enzymes are shown in . There was a significant interaction between group and AST change (p = .038). PSG, but not CG, showed significantly decreased AST (p < .001) and ALT levels (p=.002). However, there was no significant interaction between the groups and changes in ALT levels.
Table 3. Liver enzymes and lipid profile.
The lipid profiles are presented in . HDL-C did not change in the CG but significantly decreased by 6.2 mg/dL in the PSG group (p = .001). TC and LDL-C levels significantly decreased in both groups (p < .001). However, there were no significant changes in the TG levels in either group.
3.6. Correlations between hepatic fat content and liver enzymes
The CG showed a significant positive correlation between CAP and AST (r = 0.635, p < .01), and ALT (r = 0.770, p < .01) in the pretest, whereas PSG did not. However, there was no significant correlation between CAP and liver enzymes in the posttest for both groups. Additionally, there was no significant correlation between the changes in CAP and the changes in liver enzymes for both groups.
4. Discussion
The main finding of this study was that WPS did not have an overall additional effect on resistance exercise-induced HFC reduction. WPS did not affect the pattern of CAP reduction or lipid profile changes after a 4-week resistance exercise. Interestingly, however, WPS had an additional benefit, in part, on the rapid HFC reduction response. As a result of the mid-test, the reduction of CAP was approximately 142% greater in PSG than in CG, which means that WPS decreased HFC more rapidly over 2 weeks of resistance exercise. These results are similar to those of previous studies of high-protein diets. It has been reported that a high-protein diet in humans without exercise has a positive effect on HFC reduction [Citation11,Citation22,Citation23]. For instance, Xu et al. (2020) reported a 42.5% reduction in intrahepatic lipids in patients with obesity who consumed a high-protein diet for 3 weeks [Citation22]. Meanwhile, Markova et al. (2017) reported that a diet rich in animal and plant protein (both diets accounted for 28.8–30.0% of total energy intake) reduced intrahepatic fat by 48.0% and 35.7%, respectively, within 6 weeks in individuals with type 2 diabetes and NAFLD [Citation23]. Moreover, Bortolotti et al. (2011) found that WPS reduced intra-hepatocellular lipid levels by 20% in females with obesity [Citation11].
Several mechanisms have been proposed to explain the benefits of dietary protein supplementation. Increased protein intake can lead to an increase in hepatic lipid oxidation due to the energy-demanding process of amino acid catabolism [Citation10]. Amino acids can also affect liver metabolism by altering the expression of numerous genes [Citation24] through regulation of transcription factors such as sterol regulatory element binding protein-1 (SREBP-1), bile acid-activated farnesoid X receptor (FXR), adenosine monophosphate-activated protein kinase (AMPK), and peroxisome proliferator-activated receptor gamma co-activator 1α (PGC1α) [Citation10,Citation25]. It is known that in hepatocytes, FXR, AMPK, and SREBP-1 inhibit de novo lipogenesis, while PGC1α promotes fat oxidation [Citation26–29]. Based on these mechanisms, it is speculated that the faster response of PSG to the reduction in HFC over the 2-week period in this study may be due to the additional intake of amino acids through WPS. In addition, genes such as SREBP-1, AMPK, and PGC1α are known to increase in response to exercise [Citation30,Citation31]. Therefore, protein supplementation and exercise can enhance the effect of each other through an interaction effect by increasing the expression of these genes, promoting hepatic fat metabolism, and inhibiting de novo lipogenesis. On the other hand, the fact that the effect of WPS on HFC reduction was attenuated after 2 weeks might be related to the adaptation of protein supplementation in hepatic metabolism. Given the “diminishing returns” commonly observed in the benefits of exercise training [Citation32] and protein supplementation [Citation8], it can be speculated that the benefits of protein supplementation on hepatic metabolism diminish as supplementation continues. However, further research is needed to clarify the exact mechanisms underlying the synergistic effects of resistance exercise and protein supplementation, as well as the adaptive response of hepatic metabolism to protein supplementation.
In humans, AST is found in decreasing order of concentration in the liver, cardiac muscle, skeletal muscle, kidneys, brain, pancreas, lungs, leukocytes, and erythrocytes, making it less specific to the liver than ALT [Citation33]. ALT is a more specific enzyme for the liver as it is found in both the liver and muscle, but in much lower concentrations in muscle [Citation33,Citation34]. These two aminotransferase levels are therefore used as sensitive indicators of liver cell injury and are helpful in recognizing liver diseases [Citation33]. Our study showed that WPS had a positive effect against liver damage by significantly reducing AST and ALT levels. PSG significantly decreased AST by 5.2 U/L (23.2%) and ALT by 11.1 U/L (41.0%), whereas CG did not. These results are consistent with a recent study showing that WPS reduces the increased serum AST and ALT levels induced by marathon running [Citation35]. However, since AST and ALT levels in this study were within the normal range in all participants before and after the intervention and resistance exercise also influenced the decrease in AST and ALT levels, further studies are needed to elucidate the clinical effects of WPS on liver damage, particularly in patients with NAFLD. Additionally, since muscle tissue, which is larger than the liver tissue, can contain more AST and ALT even if their concentrations are lower than those in the liver, intensive acute muscular exercise may temporarily increase AST and ALT levels [Citation36]. Therefore, the elevation of these enzymes may not necessarily indicate liver damage [Citation37]. Nevertheless, the finding that 4 weeks of WPS and resistance exercise significantly reduced AST and ALT is still interesting and warrants further investigation. Finally, we recommend assessing γ-glutamyl transferase (GGT) along with AST and ALT to more accurately evaluate liver function as GGT is also a sensitive marker for the presence or absence of hepatobiliary disease [Citation33].
Interestingly, WPS did not have any significant additional effects on lipid profiles in this study. Despite a previous study showing that WPS reduced the de novo synthesis of cholesterol in the liver [Citation38], we did not find any interaction between the group and changes in TC and LDL-C. This discrepancy may be due to the different amounts and durations of protein supplementation between studies. Another possible reason is that TC and LDL-C were decreased in both groups in this study, similar to a previous study [Citation39]; therefore, the effect of resistance exercise may mask the effect of WPS on the reduction of TC and LDL-C. There was also no interaction between the groups and change in TG levels. This indicates that WPS had no effect on TG change due to resistance exercise, which is consistent with a previous study reporting that WPS did not improve TG [Citation40]. Surprisingly, HDL-C decreased in the PSG group, which may be considered an adverse effect of WPS. However, a meta-analysis revealed similar results [Citation41], and it has been suggested that HDL-C can be decreased due to WPS downregulating the expression of genes involved in cholesterol metabolism and lipogenesis [Citation42]. In addition, even though HDL-C decreased in this study, it remained above the normal range of 40 mg/dL. Thus, more studies on the effect of WPS and resistance exercise on changes in lipid profiles are needed.
We controlled daily food intake in this study to investigate the effect of resistance exercise on HFC, independent of dietary influence. We found a significant CAP reduction in CG by 27.1 dB/min (11.7%) and 19.5 dB/min (8.8%) after 4 weeks and 2 weeks of resistance exercise, respectively. Our results are similar to those of previous studies, in which there was no dietary control. Hallsworth et al. (2011) reported that moderate-to-high-intensity resistance exercise three times a week for 8 weeks significantly reduced hepatic fat by 13% [Citation43]. Shamsoddini et al. (2015) also found that resistance exercise three times a week for 8 weeks significantly decreased hepatic fat grade by 0.7 [Citation44]. Thus, resistance exercise seems to reduce HFC independent of diet. The potential mechanisms by which resistance exercise improves HFC are not yet fully understood. Previous studies [Citation7,Citation43] have suggested several possibilities, including energy balance, circulatory lipids, and insulin sensitivity. One possibility is that it induces changes in muscle fiber type (hypertrophy of type II fibers) leading to increased glycolysis and improved insulin resistance [Citation7]. This improvement in insulin resistance may inhibit de novo lipogenesis by upregulating SREBP-1c and ChREBP expression in the liver [Citation45]. Another possibility is through the myokine Irisin, which facilitates muscle-liver crosstalk and inhibits lipogenesis in hepatocytes [Citation7]. To the best of our knowledge, this study is the first to report that a reduction in HFC can occur after 4 weeks of resistance exercise and even after 2 weeks. Since the liver is an organ that occupies a central position in the metabolic homeostasis of the entire body, it responds sensitively to direct or indirect influences from other organs [Citation46]. Thus, it is possible that even 2 weeks of resistance exercise would have increased the catabolism of hepatic fat to provide energy for fatty acid oxidation [Citation47]. In addition, considering that there is a dose–response relationship between the improvement of HFC and the frequency of exercise [Citation48], the high frequency (six times a week) of exercise in this study may have had a positive effect on reducing HFC, even if the duration of exercise was relatively short in this study.
A meta-analysis showed that exercise had a positive effect on improving liver enzyme levels by reducing liver damage in patients with NAFLD under weight-loss conditions [Citation49]. However, in this study, there was no significant change in AST and ALT levels in the CG after resistance exercise, despite a significant reduction in body weight. This discrepancy may be because the participants in this study were not patients with NAFLD; in fact, their AST and ALT levels before and after the intervention were mostly within the normal range. Considering that previous studies [Citation50,Citation51] reported changes in liver enzyme levels after 8 or more weeks of resistance exercise, it is possible that the 4-week resistance exercise period in this study was insufficient to induce significant changes in liver enzyme levels, and that changes may become apparent over time due to the reduction in HFC.
The correlation between serum liver enzymes and HFC has been an area of interest among many researchers. In this study, only CG showed a significant correlation between HFC and liver enzymes before exercise training (AST: r = .635, p < .01; ALT: r = .770, p < .01). It is not clear why PSG did not show this correlation, but considering that the correlation between HFC and liver enzymes was still significant when the pretest values of CG and PSG were combined (AST: r = .394, p < .05; ALT: r = .456, p < .01, data not shown), this could be a statistical power issue. Indeed, previous research [Citation52] found that there was a mild positive correlation between degree of steatosis and ALT (ρ = 0.259, p < .05) in apparently healthy individuals, although no correlation was found with AST. Interestingly, there were no correlation between HFC and liver enzymes after exercise training in the present study. There was also no correlation between the changes in these levels with exercise. These results might suggest that HFC and liver enzymes are differently responsive to resistance exercise in relatively healthy individuals. However, further research is needed to clarify this ambiguity.
In this study, TC and LDL-C levels were significantly decreased in the CG after resistance exercise. These results are similar to those of Kelley and Kelley (2009) [Citation39]. In contrast, Goldberg et al. (2011) reported that resistance exercise for 12 weeks or less may not be sufficient to induce changes in blood lipids and lipoproteins [Citation53]. Braith and Stewart (2006) also reported that there was no evidence that resistance exercise improved blood lipids and lipoproteins [Citation54]. Thus, the effects of resistance exercise on lipid profiles are not yet clear.
We tried to better control the diet to minimize its effect on our findings. In this study, compliance with dietary intake was high, and attendance of exercise intervention was 100%, providing reliable results. On the other hand, the actual calories intake was approximately 100 kcal/day less than the calculated calories in both groups, indicating that all study participants would have consumed less calories than needed. However, this difference did not affect the results as there were no statistically significant differences between the groups. Nevertheless, it is possible that there may have been a calorie restriction effect in this study.
This study has some limitations that should be mentioned. First, we used a noninvasive method, CAP, without liver biopsy, which is considered the gold standard for measuring HFC. However, the sensitivity of CAP for discriminating mild hepatic steatosis is 91.9% and the specificity is 85.7%; therefore, it has adequate performance and is also correlated with the histopathological grade of hepatic steatosis [Citation55,Citation56]. It can be used as an effective noninvasive tool for screening and quantifying liver steatosis in clinical practice because it examines an area that is approximately 100 times larger than that of a liver biopsy and has less sampling error [Citation57]. Therefore, considering that the participants of this study were not patients with NAFLD, the use of CAP was safer and more valid. Second, we used BIA instead of the gold standard dual-energy X-ray absorptiometry to evaluate body composition. The use of BIA may lead to an overestimation of skeletal muscle mass depending on an individual’s hydration status [Citation58]. Therefore, if the hydration status of the study participants had changed during the study, it may affect their body composition. Third, in this study, we did not evaluate the participants’ sleep quality and health status such as the Pittsburgh sleep quality index and the general health questionnaire-28, and could not control for hydration status. This may have affected the study results. However, since all participants were encouraged not to change their lifestyle during the study period, it is estimated that the variations on sleep patterns, health status, and hydration status were minimized. Nevertheless, it cannot be ruled out that the improvement in the quality of sleep and health status due to exercise had a positive effect on the reduction of HFC. Finally, we did not evaluate GGT, which is frequently used as a biochemical marker of liver diseases, along with AST and ALT. Since this study targeted Korean males with average BMI who were not patients, we focused mainly on AST and ALT, which represent liver function. If GGT is additionally evaluated in future studies, more definitive results may be obtained.
Despite the limitations, this is the first clinical study to examine the effects of WPS during resistance exercise under a controlled diet. In addition, objective results were obtained in a double-blind, randomized, placebo-controlled trial. For dietary control, individual caloric intake was determined, and three meals per day were provided for 4 weeks to monitor each meal. All the exercise sessions were directly controlled by experts. Therefore, it can be said that the results of this study are reliable, although the number of participants is relatively small. Since the participants were not patients with NAFLD, there were no significant differences observed in most hepatic metabolism parameters between the groups. However, it is important to note that the effects of WPS may be more pronounced in patients with NAFLD.
In conclusion, this study indicates that WPS may not enhance the overall effects of resistance exercise on HFC and lipid profile; in part, WPS may have a beneficial effect on liver enzymatic changes and a rapid response to resistance exercise induced HFC reduction. This suggests that WPS during resistance exercise may help improve the HFC and liver enzyme levels.
Acknowledgments
We thank Gi-jae Yang Jong-hoon Park, Jong-hwa Won, and Tae-hoon Kim for their assistance with this study.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Cohen, JC, Horton, JD, Hobbs, HH. Human fatty liver disease: old questions and new insights. Sci. 2011;332(6037):1519–482.
- Younossi, ZM, Koenig, AB, Abdelatif, D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatol. 2016;64(1):73–84.
- Hashimoto, E, Taniai, M, Tokushige, K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28:64–70.
- European Association for the Study of The Liver, & European Association for the Study of Diabetes (EASD). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9(2):65–90. DOI:10.1159/000443344
- Berzigotti, A, Saran, U, Dufour, JF. Physical activity and liver diseases. Hepatol. 2016;63(3):1026–1040.
- Keating, SE, Hackett, DA, George, J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157–166.
- Hashida, R, Kawaguchi, T, Bekki, M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142–152. DOI:10.1016/j.jhep.2016.08.023
- Stokes, T, Hector, AJ, Morton, RW, et al. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutr. 2018;10(2):180.
- Wolfe, RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72(2):551S–557S.
- de Wit, NJ, Afman, LA, Mensink, M, et al. Phenotyping the effect of diet on non-alcoholic fatty liver disease. J Hepatol. 2012;57(6):1370–1373.
- Bortolotti, M, Maiolo, E, Corazza, M, et al. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin Nutr. 2011;30(4):494–498.
- Korean Agency for Technology and Standards. Korean 7th Anthropometric Survey. Eumseong, Republic of Korea: Korean Agency for Technology and Standards; 2015. https://www.kats.go.kr/en/main.do
- Liguori, G, American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. New York: Lippincott Williams & Wilkins; 2020.
- American College of Sports Medicine. American college of sports medicine position stand. progression models in resistance training for healthy adults. Med & Sci In Sports & Ex. 2009;41(3):687–708.
- Anderson, LJ, Erceg, DN, Schroeder, ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nut Res. 2012;32(7):479–485.
- Fullmer, S, Benson-Davies, S, Earthman, CP, et al. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non–critically ill individuals. J Acad Nutr Diet. 2015;115(9):1417–1446.
- Castera, L, Forns, X, Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–847.
- Sandrin, L, Fourquet, B, Hasquenoph, J, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. DOI:10.1016/j.ultrasmedbio.2003.07.001
- Friedewald, WT, Levy, RI, Fredrickson, DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
- Winters-van Eekelen, E, Verkouter, I, Peters, HP, et al. Effects of dietary macronutrients on liver fat content in adults: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2021;75(4):588–601.
- West, SG, Finch, JF, Curran, PJ. 1995. Structural equation models with nonnormal variables: Problems and remedies. Structural Equation Modeling Concepts, issues, and applications. Thousand Oaks: Sage Publications, Inc.
- Xu, C, Markova, M, Seebeck, N, et al. High‐protein diet more effectively reduces hepatic fat than low‐protein diet despite lower autophagy and FGF21 levels. Liver Int. 2020;40(12):2982–2997.
- Markova, M, Pivovarova, O, Hornemann, S, et al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterol. 2017;152(3):571–585.e8.
- Song, S, Hooiveld, GJ, Li, M, et al. Dietary soy and meat proteins induce distinct physiological and gene expression changes in rats. Sci Rep. 2016;6(1):1–12.
- Hashidume, T, Sasaki, T, Inoue, J, et al. Consumption of soy protein isolate reduces hepatic SREBP-1c and lipogenic gene expression in wild-type mice, but not in FXR-deficient mice. Biosci Biotechnol Biochem. 2011;75(9):1702–1707.
- Jiao, Y, Lu, Y, Li, XY. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol Sin. 2015;36(1):44–50.
- Jeon, SM. Regulation and function of AMPK in physiology and diseases. Experiment Molecul Med. 2016;48(7): e245-e245.
- Ruiz, R, Jideonwo, V, Ahn, M, et al. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J Biol Chem. 2014;289(9):5510–5517.
- Morris, EM, Meers, GM, Booth, FW, et al. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303(8):G979–992.
- van der Windt, DJ, Sud, V, Zhang, H, et al. The effects of physical exercise on fatty liver disease. Gene Expr. 2018;18(2):89.
- Pilegaard, H, Saltin, B, Neufer, PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546(3):851–858.
- Morton, RW, Colenso-Semple, L, Phillips, SM. Training for strength and hypertrophy: an evidence-based approach. Curr Opin Physiol. 2019;10:90–95.
- Pratt, DS, Kaplan, MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–1271.
- Lim, AK. Abnormal liver function tests associated with severe rhabdomyolysis. World J Gastroenterol. 2020;26(10):1020.
- Huang, WC, Chang, YC, Chen, YM, et al. Whey protein improves marathon-induced injury and exercise performance in elite track runners. Int J Med Sci. 2017;14(7):648.
- Chalasani, N. Clinical meaning of elevated aminotransferase activity. In Hepatotoxicity Special Interest Group Meeting Silver Springs, MD, USA; 2008.
- Lee, TH, Kim, WR, Poterucha, JJ. Evaluation of elevated liver enzymes. Clin Liver Dis. 2012;16(2):183–198.
- Gouni-Berthold, I, Schulte, DM, Krone, W, et al. The whey fermentation product malleable protein matrix decreases TAG concentrations in patients with the metabolic syndrome: a randomised placebo-controlled trial. British Journal Of Nutrition. 2012;107(11):1694–1706.
- Kelley, GA, Kelley, KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Preventive Med. 2009;48(1):9–19.
- Wirunsawanya, K, Upala, S, Jaruvongvanich, V, et al. Whey protein supplementation improves body composition and cardiovascular risk factors in overweight and obese patients: a systematic review and meta-analysis. J Am Coll Nutr. 2018;37(1):60–70.
- Badely, M, Sepandi, M, Samadi, M, et al. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta-analysis. Diabetes Metabol Syndrome: Clin Res Rev. 2019;13(6):3121–3131.
- Chen, Q, Reimer, RA. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutr. 2009;25(3):340–349.
- Hallsworth, K, Fattakhova, G, Hollingsworth, KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278–1283. DOI:10.1136/gut.2011.242073
- Shamsoddini, A, Sobhani, V, Chehreh, MEG, et al. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepatitis Mon. 2015;15(10). DOI:10.5812/hepatmon.31434
- Xu, X, So, JS, Park, JG, et al. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis. 2013;33(4):301–311.
- Berghe, G. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J Inherit Metabol Disease. 1991;14(4):407–420.
- Thyfault, JP, Rector, RS. Exercise combats hepatic steatosis: potential mechanisms and clinical implications. Diabetes. 2020;69(4):517–524.
- Charatcharoenwitthaya, P, Kuljiratitikal, K, Aksornchanya, O, et al. Moderate-intensity aerobic vs resistance exercise and dietary modification in patients with nonalcoholic fatty liver disease: a randomized clinical trial. Clin Transl Gastroenterol. 2021;12(3):e00316.
- Katsagoni, CN, Georgoulis, M, Papatheodoridis, GV, et al. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabol. 2017;68:119–132.
- Eckard, C, Cole, R, Lockwood, J, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6(4):249–259.
- Hallsworth, K, Thoma, C, Hollingsworth, KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129(12):1097–1105.
- Ardigò, D, Numeroso, F, Valtueña, S, et al. Hyperinsulinemia predicts hepatic fat content in healthy individuals with normal transaminase concentrations. Metabol. 2005;54(12):1566–1570.
- Goldberg, AC, Hopkins, PN, Toth, PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the national lipid association expert panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5(3):133–140.
- Braith, RW, Stewart, KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulat. 2006;113(22):2642–2650.
- Jun, BG, Park, WY, Park, EJ, et al. A prospective comparative assessment of the accuracy of the fibroscan in evaluating liver steatosis. PLoS ONE. 2017;12(8):e0182784. DOI:10.1371/journal.pone.0182784
- Wong, GL. Transient elastography: kill two birds with one stone? World J Hepatol. 2013;5(5):264.
- Wang, Y, Fan, Q, Wang, T, et al. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8(10):17654.
- Composition of the ESPEN Working GroupKyle, UG, Bosaeus, I. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243.