ABSTRACT
Objective
We examined the effects of short-term KD on exercise efficiency and hormonal response during and after the graded exercise testing.
Methods
Fourteen untrained healthy adults (8 males, 6 females, age 26.4 ± 3.1 [SD] years; BMI 24.8 ± 4.6 kg/m2; peak VO2max 54.0 ± 5.8 ml/kg FFM/min) completed 3-days of a mixed diet (MD) followed by another 3-days of KD after 3-days of washout period. Upon completion of each diet arm, participants underwent graded exercise testing with low- (LIE; 40% of VO2max), moderate- (MIE; 55%), and high-intensity exercise (HIE; 70%). Exercise efficiency was calculated as work done (kcal/min)/energy expenditure (kcal/min).
Results
Fat oxidation during the recovery period was higher in KD vs. MD. Despite identical workload during HIE, participants after having KD vs. MD showed higher energy expenditure and lower exercise efficiency (10.1 ± 0.7 vs. 12.5 ± 0.3%, p < .01). After KD, free fatty acid (FFA) concentrations were higher during MIE and recovery vs. resting, and beta-hydroxybutylate (BOHB) was lower at HIE vs. resting. Cortisol concentrations after KD was higher during recovery vs. resting, with no significant changes during graded exercise testing after MD.
Conclusions
Our data suggest that short-term KD is favorable to fat metabolism leading increased circulating FFA and BOHB during LIE to MIE. However, it is notable that KD may cause 1) exercise inefficiency manifested by increased energy expenditure and 2) elevated exercise stress during HIE and recovery. Trial registration: KCT0005172, International Clinical Trials Registry Platform.
1. Background
Obesity is a global health concern [Citation1] as it can cause various metabolic health disorders including insulin resistance, metabolic syndrome, and type 2 diabetes [Citation2]. Although pharmacological treatment of those metabolic diseases is an efficient solution, lifestyle modifications including diet and exercise are considered the first-line preventive/therapeutic approach to tackle any metabolic disturbances with minimal adverse events compared to drug treatments [Citation3,Citation4]. Of them, a ketogenic diet (KD), which is comprised of very low carbohydrate contents (20–50 g/day) [Citation5], has been suggested to treat obesity [Citation6], metabolic syndrome [Citation3], and type 2 diabetes [Citation7] by improving their metabolic, inflammatory and dysglycemic biomarkers.
Despite potential merits of KD on metabolic health [Citation3,Citation6,Citation7], it is controversial whether KD has positive or negative impact on exercise performance [Citation8–10] and exercise efficiency [Citation11,Citation12], and exercise fatigue [Citation13,Citation14] during endurance exercise. A recent systematic review and meta-analysis of 10 studies reported that mid- (3–6 weeks) to long-term (>3–4 months) KD had no striking effects on aerobic capacity measured by maximal oxygen consumption (VO2max), and exercise performance measured by time to exhaustion, and fatigue measured by the rating of perceived exertion (RPE) during endurance exercise [Citation15]. In addition, two studies reported inconsistent findings with respect to the effect of KD on exercise efficiency [Citation11,Citation12]. Cole et al. [Citation11] observed no changes (within each group) and differences (between two groups) in exercise efficiency after 3 days of moderate amount of carbohydrate diet vs. after 3 days of low-carbohydrate diet in 15 healthy trained male cyclists. Conversely, Shaw et al. [Citation12] observed that a 31 days of KD reduced exercise efficiency during treadmill exercising at > 70% VO2max (exercise efficiency was preserved at < 60% VO2max) in eight trained male endurance athletes. Furthermore, previous findings [Citation16–19], including well-trained endurance athletes or competitive recreational athletes, showed that both short-term (≤7 days) and long-term KD (>3–4 months) alters metabolic response to exercise, resulting in changes in substrate utilization, particularly a shift toward increased fat oxidation and reduced reliance on carbohydrates compared to moderate and high carbohydrate diets. However, individual responses to the KD can vary depending on the duration of diet periods and training status.
As such, there is accumulating interest in KD research with respect to exercise performance and/or exercise efficiency and/or substrate utilization in well-trained endurance athletes [Citation15,Citation17,Citation20,Citation21]; however to date, limited evidence is available in terms of 1) very short-term (within 2 weeks) KD effects and 2) exercise-related metabolic response to the KD in non-trained healthy adults. Therefore, the purpose of this study was to investigate potential effects of a short-term (3 days) KD on exercise efficiency and metabolic/hormonal responses at rest and during graded exercise testing and recovery in untrained healthy adults.
2. Methods
2.1. Participants
A total of 14 adults (age 18–45 years, eight males and six females) participated in the present study, which was approved by the Yonsei University Institutional Review Board, Seoul, South Korea (Yonsei IRB no. 201701-HR-744-03). A signed consent form was collected from each participant. The exclusion criteria were: history of hypertension or cardiovascular disease or diabetes; use of any medications in the past 3 months, including any hormonal contraceptive use that could affect the responsiveness to aerobic endurance training; pregnant and/or menstruating individuals.
2.2. Experimental procedure
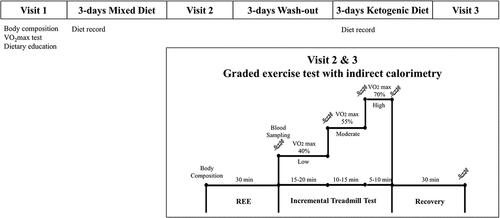
This study consisted of three visits before and after each intervention (i.e. two diet arms). All participants came to the Yonsei Exercise Physiology Laboratory at 7:30 am after a 10 h overnight fast, and they were asked to abstain from caffeine, smoking, drinking alcohol, and moderate to vigorous physical activity for at least 24 h prior to measurements. All participants completed the following study procedures (). At Visit 1, body composition was measured with a bioelectrical impedance analysis device (InBody 720, Biospace, Seoul, South Korea). To measure cardiorespiratory fitness level, VO2max test was determined by peak VO2 , and conducted on a treadmill employing a computerized cardiac stress testing system (Cardiac Science, Q-stress TM65, Waukesha, WI, USA). During the test, participants wore a non-rebreathing facemask (Hans Rudolph, Rudolph series 7910, Kansas, MO, USA), while their heart rate (HR) was continuously measured using radiotelemetry (Polar, Electro Oy, Finland). Oxygen consumption was continuously monitored breath-by-breath through utilization of a computerized metabolic measurement system (ParvoMedics, TrueOne 2400, Sandy, UT, USA). A well-trained researcher followed the Bruce protocol [Citation22]. Together with voluntary exhaustion, the following criteria were used to determine whether VO2max was reached: a plateau in VO2 despite an increase in workload and a respiratory exchange ratio (RER) ≥1.10, within 5 bpm of the HRmax. At Visit 2 (after the completion of Mixed Diet [MD]), body composition and resting energy expenditure (REE) were measured. During REE testing, all participants rested for 30 min, while wearing a non-rebreathing facemask in a quiet and private room within the exercise physiology lab, where the temperature and humidity were carefully controlled. The modified Weir equation was used to calculate REE [Citation23]. After the REE measurement, participants ran on a treadmill with a 5% incline until they expended 100 kcal at exercise intensities (a total of 300 kcal) of low (40% of VO2max), moderate (55% of VO2max), and high (70% of VO2max) [Citation24]. At the end of an exercise session, participants rested for 30 min in a supine position. During graded exercise testing, RPE was measured using the Borg scale 6–20 at the end of each exercise [Citation25]. After the second visit, participants completed a 3-day washout period, followed by 3 days of a KD. At Visit 3, the same protocol and identical measurements of the Visit 2 were applied to all participants who completed KD 3 days.
2.3. Dietary intervention: 3 days of a Mixed Diet (MD) or Ketogenic Diet (KD)
All participants were instructed to be on a MD for 3 days followed by a KD for 3 days after having 3-day washout periods. Participants were educated on dietary composition for the MD and the KD at the first visit, and they were educated about the benefits and negative effects of both dietary interventions. The recommended proportions of each nutrient for MD were 15% fat, 25% protein, and 60% carbohydrate, whereas the KD was composed of 75% fat, 20% protein, and 5% carbohydrate [Citation26]. Participants were asked to keep a dietary record during the 3 days of MD or KD. The dietary composition was analyzed using CAN Pro version 5.0 software (Computer-Aided Nutritional Analysis Program, 2016 Korean Nutrition Society).
2.4. Analyses of biomarkers
All participants fasted at least 10 h before blood sampling and did not consume any food during testing. The blood samples were collected at the following time points during the second and third visits: 1) baseline (after testing REE), 2) after each exercise (low, moderate, high), and 3) 30-min post-exercise recovery. Blood samples (10 mL each) were collected by standard venipuncture into a plain tube. The blood samples were centrifuged at 3,000 rpm for 10 min. The serum was separated and stored at −80°C until further analysis. Serum concentrations of fasting glucose, total cholesterol, high-density lipoprotein cholesterol, free fatty acids (FFAs), beta-hydroxybutylate (BOHB), and triglycerides were assayed using the ADVIA 1650 Chemistry system (Siemens, Tarrytown, NY, USA), with inter-assay coefficient of variation (CV) 0.5–1.6% and intra-assay CV 0.9–2.4%. Fasting insulin was assessed with an electrochemiluminescence immunoassay using the Elecsys 2010 (Roche, Indianapolis, IN, USA). Epinephrine, norepinephrine, triiodothyronine (T3), and tetraiodothyronine (T4) were measured with enzyme immunoassay kits (Mesdia, Seoul, Korea).
2.5. Exercise efficiency
The calculation of exercise efficiency was based on gross efficiency [Citation27], which divides the work accomplished per minute (Watts converted to kcal/min) by the total energy cost required to do the work:
Gross Efficiency (%) = Work Done (kcal/min)/Energy Expenditure (EE) (kcal/min) × 100
EE at rest and during steady-state exercise was calculated using the Weir equation [Citation23]: EE (kcal/min) = 3.9 × VO2 (l/min) + 1.1 × VCO2 (l/min)
2.6. Statistical analyses
Descriptive analysis was conducted for all variables. Mauchly’s W tests were used to verify the estimated sphericity, and the Greenhouse–Geisser correction was conducted when the assumption of sphericity was found to be violated. Before implementing parametric tests, the assumption of normality was confirmed using the Kolmogorov–Smirnov test. Within-group changes from the MD to the KD were examined using Student’s paired-tests for dependent variables. A two-way repeated-measures analysis of variance was used to examine the main effect of the diets, the main effect of exercise protocol time points, and interaction between diet and exercise protocol time points for each variables of interest. A post-hoc analysis was conducted when a significant effect was observed using Bonferroni adjustments for multiple comparisons. Data were analyzed using SAS 6.4 software (SAS Institute, Cary, NC, USA) and presented as mean ± SD, unless otherwise specified, with significance set at P < .05.
3. Results
Fourteen untrained healthy adults successfully completed all requirements associated with the current study protocols. At baseline, mean values (with SD) of the participants’ age, body mass index (BMI), and maximal oxygen consumption (VO2max) were 26.4 ± 3.1 years, 24.8 ± 4.6 kg/m2, and 54.0 ± 5.8 ml/kg FFM/min, respectively (). shows that all participants have “good” or “excellent” VO2max scores when compared to sex and age-appropriate reference values (men: >42.5 ml/kg/min, women: >33.0 ml/kg/min) [Citation24]. Male had higher height, weight, muscle mass, fat-free mass, body fat percent, and VO2max compared with female, with no differences in age, BMI, and fat mass. All participants complied with the recommended MD and KD compositions. Fat and protein intake was higher and carbohydrate intake was lower in the KD vs. the MD. However, no significant difference in total energy intake was observed between the two diet groups (Supplementary Table S1).
Table 1. Participants’ characteristics.
3.1. Body composition in response to ketogenic diet vs. mixed diet
Compared with MD group, KD group had lower in bodyweight (74.9 ± 16.2 vs. 70.7 ± 15.6 kg, P = .001), muscle mass (32.0 ± 8.1 vs. 30.0 ± 7.9 kg, p = .001), FFM (56.7 ± 13.3 vs. 54.0 ± 12.5 kg, P = .001), and total body water (40.4 ± 9.8 vs. 38.8 ± 9.4, P = .001), while BMI, body fat percentage, and fat mass did not change (data not shown).
3.2. Exercise efficiency at rest and during graded exercise
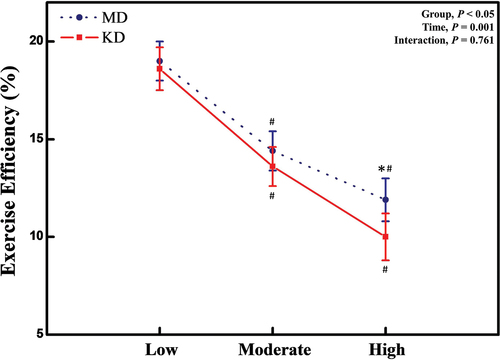
Exercise efficiency measured by gross efficiency decreased in the KD vs. MD (P = .045), and during exercise testing (P = .001), while there was no significant interaction between group (KD vs. MD) and time (LIE vs. MIE vs. HIE) (P = .761) (). Exercise efficiency gradually decreased as exercise intensity increased from low to mid to high in both MD and KD groups (P = .001). Exercise efficiency was lower during HIE after the KD compared with the MD (P < .05) (). There were no significant differences in exercise efficiency during LIE and MIE between the two diet arms.
3.3. Substrate utilization at rest and during graded exercise
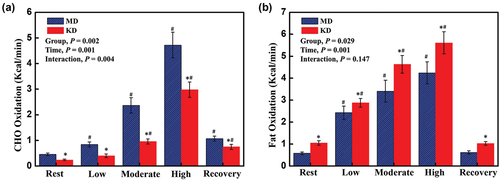
demonstrates statistically significant effects of diet intervention on carbohydrate oxidation (P = .002), and fat oxidation (P = .029), and significant effects of exercise in both substrate utilization during the graded exercise testing were observed in both groups (MD and KD, all P = .001). Moreover, a significant interaction in the carbohydrate oxidation was detected (P = .004). When comparing fuel oxidation rates between the two diet groups, carbohydrate oxidation was ~2.3-fold higher in the MD than in the KD at rest, during LIE, MIE, HIE, and recovery period (all P < .01) (), while fat oxidation was ~1.3-fold higher in participants consuming the KD than the MD at rest, during LIE, MIE, HIE, and recovery period (all P < .05) ().
Figure 3. Substrate utilization at mixed diet and after short-term ketogenic diet. CHO (a); fat (b). Abbreviation: MD; mixed diet, KD; ketogenic diet, CHO; carbohydrate. *: significant difference of P < .05, between MD and KD. #: significant difference of P < .05, between compared to baseline value.
indicates HR, oxygen consumption, RER, EE, RPE, and exercise time values after the mixed and ketogenic diets during the graded exercise protocol. During the graded exercise from low to high intensity, HR and oxygen consumption were significantly higher, and RER was lower in the KD compared with the MD (). A two-way repeated measures ANOVA and post hoc tests revealed that significant effect are observed at rest on the oxygen consumption and RER (P = .002 and P = .001), during the LIE (P = .001 and P = .001), MIE (both P = .001), HIE (both P = .001), and the recovery period (P = .018 and P = .001) after the KD vs. MD. Furthermore, a significant interaction in the carbohydrate oxidation was detected (P = .004). After the KD, EE was higher at rest and high-intensity exercise compared to the MD (both P < .05) (). RPE was higher during low- and high-intensity exercise after the KD vs. MD (). There was no difference in exercise time detected between the two diet groups.
Table 2. Metabolic values after a mixed and 3-day ketogenic diet during graded exercise protocol.
3.4. Hormonal response at rest and during graded exercise
With respect to FFA concentrations, reveals main effects for diet intervention (P = .001) and exercise (P = .001), and an interaction effect (P = .001). Post hoc tests showed that KD groups have higher serum FFA concentrations at rest (P = .001), during the exercise and at recovery (all P = .001) compared to the MD group because of the nature of diet composition in the two diet arms. Our within-group analysis showed that serum FFA concentrations maintain constant during the graded exercise testing from low-, to moderate-intensity. However, a significant decrease in FFA concentrations was observed during high-intensity exercise in individuals after the KD, and an increase in FFA concentrations was noted during the recovery period in both diet groups (). For BOHB concentrations, main effects for diets (P = .001) and exercise (P = .001) were observed, while there was no interaction effect between diets and exercise (P = .071). Post hoc tests exhibited that BOHB concentrations are higher at rest (P = .001), during exercise (all P < .01), and recovery period (P = .002) in the KD vs. the MD groups (). BOHB concentrations decreased gradually over the exercise testing with a subsequent rebound of BOHB concentrations at the recovery period (). In contrast, after the MD, BOHB concentrations did not change over the exercise testing, yet a significant increase in BOHB was observed at the recovery period.
Figure 4. Fasting blood parameters. Abbreviation: MD; mixed diet, KD; ketogenic diet. Free fatty acids (a); BOHB (b); glucose (c); insulin (d); cortisol (e). *: significant difference of P < .05, between MD and KD. #: significant difference of P < .05, between compared to baseline value.
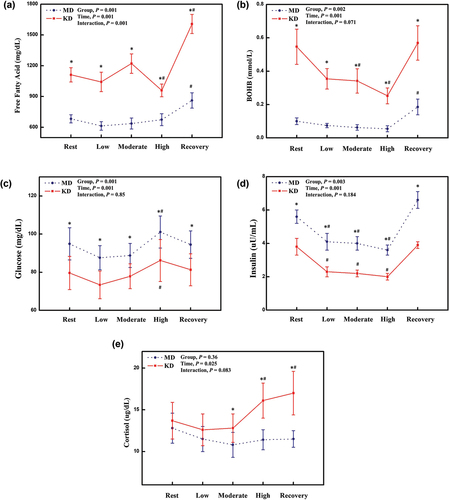
With respect to glucose concentrations, shows significant main effect for diet intervention (P = .001), and exercise (P = .001), but no interaction effect was observed (P = .850). Our between-group analysis (by post hoc tests) showed that glucose concentrations of KD group decreased at rest (P = .001), during the graded exercise (all P = .001), and the recovery (P = .001) compared to those concentrations of MD group (). For the glucose metabolism, within-group analysis showed that glucose concentrations increase at high-intensity exercise compared to the resting period in both diet groups, with no change in glucose concentrations during low- to moderate-intensity exercise compared to resting period in both diet groups (). For insulin concentrations, main effects for diets (P = .003) and exercise (P = .001) were observed, while there was no interaction effect between diets and exercise (P = .184). Our between-group analysis showed that individuals after the KD have lower insulin concentrations at rest (P = .004), during low-moderate- and high-intensity exercise (all P < .05), and the recovery period (P = .001) compared to MD group (). For the insulin dynamics, our within-group analysis showed that insulin concentrations decrease along with elevation of exercise intensity in both diet groups, with no differences in insulin concentrations between rest vs. recovery periods ().
In terms of cortisol concentrations, main effects for exercise (P = .025) were observed, while there were no diets (P = .36) and interaction effect (P = .184). Between-group analysis showed that KD group has higher cortisol concentrations at moderate-, high-intensity exercise (all P < .05), and recovery period (P = .009) compared to the MD group (). Within-group analysis showed that cortisol concentrations increase during high-intensity exercise and the recovery period after the KD compared to the resting period. In contrast, after the MD, cortisol concentrations did not change over the exercise testing ().
For the T3 and T4 dynamics, our between-group analysis showed that T3 concentrations were generally lower over exercise testing after KD compared to the MD (all P = .001), and T4 concentrations were higher at rest (P = .024) and the recovery period (P = .003) after the compared to the MD (). Our within-group analysis showed that T3 and T4 concentrations does not change over the graded exercise testing from low-, to moderate-, to high-intensity, and recovery period compared to the resting period in both diet groups ().
Table 3. Hormonal response to ketogenic diet and during exercise protocol.
Lastly, between-groups analysis showed that epinephrine concentrations increase during high-intensity exercise after the KD compared to the MD (P = .041); however, norepinephrine concentrations did not change after KD compared to the MD (). Our within-group analysis showed epinephrine concentrations does not change in response to exercise in either diet group; however, norepinephrine concentrations significantly increase at recovery period compared to the resting group ().
4. Discussion
The present study compared the effects of 3 days of KD vs. MD on body composition, exercise efficiency, substrate utilization, and metabolic/hormonal regulation during a graded exercise test. After the KD compared with the MD, participants had: 1) significant weight loss, most of it due to a reduction in muscle mass, 2) impaired exercise efficiency as indicated by 16% decrease in gross efficiency during high-intensity exercise; 3) decreased RER, indicative of increased fat utilization as a fuel, during the graded exercise; 4) increased ketosis as evidenced by a two-fold increase in FFAs and BOHB; and 5) increased exercise stress measured by higher RPE during the exercise and higher cortisol concentrations during the recovery period, respectively.
Our data showed a significant body weight reduction in response to the 3-day KD vs. MD resulting from muscle mass loss, without any change in fat mass. In line with our study, a recent systematic review showed that effects of a KD on body mass changes in individuals with normal weight over a period of 3–12 weeks in the 18 studies [Citation28]. Fifteen studies observed reductions in body mass, averaging approximately 2 kg. Additionally, the 14 studies that examined body composition, 12 of them reported decreases in fat mass [Citation28]. However, previous studies implementing long-term ketogenic diets demonstrated a significant reduction in fat mass [Citation29,Citation30]. Compared to the long-term KD, the decrease in muscle observed in our short-term KD might be attributed to the reduction in total body water caused by glycogen depletion. Glycogen stores are typically depleted within 48 h during carbohydrate restriction diet, the process entails the mobilization of glycogen stored in the liver and muscles [Citation31]. Each gram of glycogen mobilized is accompanied by approximately 2 g of water. Consequently, it is estimated that the total mobilization of glycogen stores leads to a weight loss of approximately 1 kg [Citation32]. Despite the potential limitations in the assessment of body composition, the reduction in body weight is significant for endurance athletes, given that carrying weight distally during exercise increase the aerobic demand during exercise [Citation33].
Our major novelty of the present study is that the short-term KD reduced exercise efficiency during high-intensity exercise compared with the MD despite having similar concentrations of exercise efficiency until mid-intensity exercise between the two diet arms. This is further supported by the observation of increased oxygen consumption and higher EE during high-intensity exercise after the short-term KD. This results coincide with a study [Citation12] demonstrating exercise inefficiency measured by predicted VO2 during exercise after 31-days of KD. Shaw et al. [Citation12] showed that 31-days of the KD derive impaired exercise efficiency, particularly at >70% VO2max, as evidenced by increased EE in eight male elite runners with a higher oxidative capacity measured by VO2max from treadmill test. However, Cole et al. [Citation11] reported that there were no differences in exercise efficiency between moderate-carbohydrate and 3-days of low-carbohydrate diet in 15 trained male cyclists. The study observed reduction of exercise efficiency (3.5%) during submaximal cycling (60% maximal minute power) following a 3-days of low-carbohydrate diet compared with a 3-days of high-carbohydrate diet. Collectively, above inconsistent findings from multiple studies including ours in terms of KD effects on exercise efficiency would be stemmed from differences in EE. The increase in EE when consuming a KD, compared to a MD, may be due to the thermic effect of food [Citation34], uncoupling protein [Citation35], and increased hepatic oxygen consumption proportional rate to the rate of ketone production [Citation36]. However, the mechanism regarding KD and increased EE remains unclear [Citation37]. Furthermore, another potential difference is study design, i.e. exercise intensity and target participants.
In the present study, we could verify that all participants were in ketosis after the KD from a series of our observations regarding lower RER, FFAs, BOHB together with higher fat oxidation and lower carbohydrate oxidation at rest, during and after the graded exercise testing. Those observations may provide insights into potential mechanism responsible for the relationship between the KD and exercise inefficiency. First, a significant decrease in RER in participants after the KD vs. the MD attests ~1.3-fold higher fat oxidation and ~2.3-fold lower carbohydrate oxidation during the graded exercise testing. Our observation is in line with previous studies showing that KD increased fat oxidation and reduced the carbohydrate oxidation during exercise [Citation5,Citation8,Citation9,Citation12,Citation16–19]. In a recent study by Prins et al. [Citation17] found that the consumption of a short-term KD (4-days) resulted in a significant ~4.5-fold increase in the rate of fat oxidation, from ~0.14 to ~0.63 g min−1 during a 5-km time trial performed at an intensity of about 82–84% of VO2max in seven male competitive recreational athletes. Additionally, a longer period of 7 days on the KD led to ~1.8-fold higher rates of fat oxidation and reduced carbohydrate utilization in nine endurance-trained males [Citation19]. This suggested that a KD decreases availability of carbohydrate substrate, potentially followed by additional decrease in muscle and hepatic glycogen stores during exercise [Citation38]. Considering the important role of carbohydrates as a major energy resource during high-intensity increases (>65% VO2max) [Citation39] and a source of carbon for biosynthesis and anaplerosis [Citation40], it is physiologically natural that the KD derives elevation of fat availability along with reduced muscle glycogen content, thereby potentially causing impaired endurance performance during exercise [Citation41,Citation42]. Furthermore, given that fat oxidation requires more oxygen for combustion to generate the equivalent yield compared to carbohydrate oxidation, oxygen uptake would have been expected to increase after consuming the KD. This increased oxygen cost of exercise after ketosis can then impair exercise efficiency especially during high-intensity endurance exercise [Citation9]. Therefore, an elevated fat oxidation rate and reduced carbohydrate oxidation rate after the KD is likely to increase exercise inefficiency during high-intensity exercise.
Our findings of increased concentrations of circulating FFA and BOHB during a graded exercise after the short-term KD compared with the MD further support that participants were in ketosis. FFA and BOHB concentrations significantly decreased during high-intensity exercise but significantly increased during the recovery period in individuals after the KD. As previously noted (i.e. KD led increased fat oxidation), it could be speculated that the KD derives an increase in FFA contribution to the total EE over exercise testing compared with the MD. Taken together, due to the lack of optimal carbohydrate availability, increased FFA oxidation results in the formation of ketone bodies (BOHB, acetoacetate, and acetone) as an additional fuel source of metabolism [Citation5], and spares endogenous carbohydrate stores to maximize fat oxidation [Citation5,Citation10,Citation11]. It could be postulated that the mechanisms provided above (i.e. increased fat availability relative to carbohydrate together with our ketosis theory) can be promising drivers for exercise inefficiency during the high-intensity exercise.
Several studies investigated the metabolic and hormonal responses to aerobic exercise in a glycogen-depleted state resulting from adaptation to a mid to long-term KD in well-trained male endurance athletes [Citation43–46]. These studies showed that a KD increases activation of the sympathetic nervous system, decreases insulin concentrations, and enhances the secretion of glucagon, adrenaline, cortisol, and growth hormone [Citation43–46]. Although our participants consumed only 3 days of the KD, we also observed significant decreases in circulating glucose and insulin concentrations and an increase in cortisol concentrations during a graded exercise testing, which were in line with previous studies [Citation43–46]. These hormonal changes were probably due to short-term ketosis enhancing the glucostatic mechanism. Thus, depletion of muscle and liver glycogen due to the KD could stimulate lipolysis and glucose production, derived by potential alternations in the secretion of glucoregulatory hormones such as glucagon [Citation47]. Another debating finding from ours and others would be a significant increase in EE in individuals who took the KD vs. the MD. We observed a decrease in circulating T3 concentrations in our participants after the KD. Despite the fact that thyroid hormones could significantly and negatively affect EE [Citation48,Citation49], we did not observe reduction in EE during exercise testing. In fact, there was a significant increase in EE accompanied by an increased HR, without any changes in epinephrine or norepinephrine concentrations which could affect sympathetic tone and metabolic rate. Our findings suggest that other factors besides thyroid hormones and sympathetic response to the KD may play a role in EE regulation during exercise.
Our observations of elevated cortisol concentrations and increased RPE scores at high-intensity exercise in participants who took the KD vs. MD support the relationship between KD and exercise stress. This was the same case in recreational male athletes who took a 2-weeks of KD [Citation50]. Increased concentrations of cortisol, which is a neuroendocrine marker of stress, reflected the stress [Citation51], and highly correlated with RPE level and pain perception during exercise [Citation52]. It is confirmed by us showing increased levels of RPE at high-intensity exercise together with elevation of cortisol concentrations. According to the previous reports [Citation50,Citation53,Citation54], participants who consumed very low-carbohydrate diets had higher concentrations of cortisol during endurance exercise compared with those who took normal or high carbohydrate diet. We speculate that low-carbohydrate availability during high-intensity exercise could derive increased burden of protein breakdown and cortisol promoting gluconeogenesis in the liver [Citation55,Citation56]. Additional mechanistic studies should be warranted to investigate relationship between changes in stress and ketosis over a variety of KD periods.
Our study has strengths and limitations. First of all, the present investigation provides new evidence of very short term (3-days) KD effects on substrate utilization and metabolic regulations during exercise in untrained healthy adults. Moreover, the current study also investigated exercise efficiency according to the different exercise intensities (low, moderate, high) between participants consumed MD vs. KD. Our study has several limitations as well. First, we could not control for potential confounding factors including physical activity, habitual diet, and menstrual cycle/hormonal contraception. Second, although we had 3-days of wash-out periods between the two diet arms, it is possible that participants had learning effect after MD prior to the KD. Lastly, because we had a relatively small sample size, a larger trial with comprehensive examinations of exercise performances, such as exercise time trial, sprint test, and critical power test should be warranted.
5. Conclusions
In summary, a very short-term KD is favorable for fat metabolism leading to increased circulation of FFAs and BOHB during low to moderate exercise in untrained healthy adults. However, a KD may cause exercise inefficiency manifested by increased EE during exercise and elevated exercise stress during high-intensity exercise and recovery as evidenced by the RPE and high cortisol concentration.
Authors’ contributions
Conceptualization, J.Y.J., W.L., Y.C., W.C., H.J.; funding acquisition, W.L., J.Y.J., investigation, W.C., H.J., H.I.Y., D.P., S.H., methodology, W.C., formal analysis, W.C., supervision, J.Y.J., W.L., W.C., writing-original draft, W.C., J.Y.J., writing – review and editing, D.H.L., Y.C., S.H.S., J.Y.K.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions
Ethics approval and consent to participate
This study was approved by the Yonsei University Institutional Review Board, Seoul, South Korea (Yonsei IRB no. 201701-HR-744-03). A signed consent form was collected from each participant.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–822.
- Pradhan, A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nut Rev. 2007;65(suppl_3):S152–S6. doi: 10.1301/nr.2007.dec.S152-S156
- O’Neill, BJ. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):301–307. doi: 10.1097/MED.0000000000000569
- Kolb, H, Martin, S. Environmental/Lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15(1):1–11. doi: 10.1186/s12916-017-0901-x
- Phinney, SD, Bistrian, BR, Evans, W, et al. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32(8):769–776. doi: 10.1016/0026-0495(83)90106-3
- Brehm, BJ, Seeley, RJ, Daniels, SR, et al. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88(4):1617–1623. doi: 10.1210/jc.2002-021480
- Yancy, WS, Foy, M, Chalecki, AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metabol. 2005;2(1):1–7. doi: 10.1186/1743-7075-2-34
- McSwiney, FT, Wardrop, B, Hyde, PN, et al. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism. 2018;81:25–34. doi: 10.1016/j.metabol.2017.10.010
- Burke, LM, Ross, ML, Garvican‐Lewis, LA, et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. Journal Of Physiology. 2017;595(9):2785–2807. doi: 10.1113/JP273230
- Burke, LM, Whitfield, J, Heikura, IA, et al. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. Journal Of Physiology. 2021;599(3):771–790. doi: 10.1113/JP280221
- Cole, M, Coleman, D, Hopker, J, et al. Improved gross efficiency during long duration submaximal cycling following a short-term high carbohydrate diet. Int J Sports Med. 2014;35(3):265–269. doi: 10.1055/s-0033-1348254
- Shaw, DM, Merien, F, Braakhuis, A, et al. Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sports Exercise. 2019;51(10):2135–2146. doi: 10.1249/MSS.0000000000002008
- Sjödin, A, Hellström, F, Sehlstedt, E, et al. Effects of a ketogenic diet on muscle fatigue in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. 2020;12(4):955. doi: 10.3390/nu12040955
- Dostal, T, Plews, DJ, Hofmann, P, et al. Effects of a 12-week very-low carbohydrate high-fat diet on maximal aerobic capacity, high-intensity intermittent exercise, and cardiac autonomic regulation: non-randomized parallel-group study. Front Physiol. 2019;10:912. doi: 10.3389/fphys.2019.00912
- Cao, J, Lei, S, Wang, X, et al. The effect of a ketogenic low-carbohydrate, high-fat diet on aerobic capacity and exercise performance in endurance athletes: a systematic review and meta-analysis. Nutrients. 2021;13(8):2896. doi: 10.3390/nu13082896
- Webster, CC, Noakes, TD, Chacko, SK, et al. Gluconeogenesis during endurance exercise in cyclists habituated to a long‐term low carbohydrate high‐fat diet. Journal Of Physiology. 2016;594(15):4389–4405. doi: 10.1113/JP271934
- Prins, PJ, Noakes, TD, Welton, GL, et al. High rates of fat oxidation induced by a low-carbohydrate, high-fat diet, do not impair 5-km running performance in competitive recreational athletes. J Sports Sci Med. 2019;18(4):738.
- Volek, JS, Freidenreich, DJ, Saenz, C, et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2016;65(3):100–110. doi: 10.1016/j.metabol.2015.10.028
- McSwiney, FT, Fusco, B, McCabe, L, et al. Changes in body composition and substrate utilization after a short-term ketogenic diet in endurance-trained males. Biol Sport. 2021;38(1):145–152. doi: 10.5114/biolsport.2020.98448
- Burke, LM. Ketogenic low‐CHO, high‐fat diet: the future of elite endurance sport? Journal Of Physiology. 2021;599(3):819–843. doi: 10.1113/JP278928
- Kymberly, AW, TROTT, MN, SCHWEITZER, GG, et al. Low-carbohydrate, ketogenic diet impairs anaerobic exercise performance in exercise-trained women and men: a randomized-sequence crossover trial. J Sports Med Phys Fitness. 2019;59(4):600–607. doi: 10.23736/S0022-4707.18.08318-4
- Bruce, R, Blackmon, JR, Jones, JW. Exercising testing in adult normal subjects and cardiac patients. Pediatrics. 1963;32(4):742. doi: 10.1542/peds.32.4.742
- Weir Jd, V. New methods for calculating metabolic rate with special reference to protein metabolism. Journal Of Physiology. 1949;109(1–2):1. doi: 10.1113/jphysiol.1949.sp004363
- Gibson, AL, Wagner, D, Heyward, V. Advanced fitness assessment and exercise prescription. Human Kinetics: 2018. doi: 10.5040/9781718220966
- Borg, GA. Psychophysical bases of perceived exertion. Medicine & Science In Sports & Exercise. 1982;14(5):377???381. doi: 10.1249/00005768-198205000-00012
- Freeman, JM, Kossoff, EH, Hartman, AL. The ketogenic diet: one decade later. Pediatrics. 2007;119(3):535–543. doi: 10.1542/peds.2006-2447
- Amati, F, Dubé, JJ, Shay, C, et al. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol. 2008;105(3):825–831. doi: 10.1152/japplphysiol.90384.2008
- Kang, J, Ratamess, NA, Faigenbaum, AD, et al. Ergogenic properties of ketogenic diets in normal-weight individuals: a systematic review. J Am Coll Nutr. 2020;39(7):665–675. doi: 10.1080/07315724.2020.1725686
- Volek, JS, Phinney, SD, Forsythe, CE, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44(4):297–309. doi: 10.1007/s11745-008-3274-2
- Volek, JS, Sharman, MJ, Love, DM, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism. 2002;51(7):864–870. doi: 10.1053/meta.2002.32037
- Adam‐Perrot, A, Clifton, P, Brouns, F. Low‐carbohydrate diets: nutritional and physiological aspects. Obesity Rev. 2006;7(1):49–58. doi: 10.1111/j.1467-789X.2006.00222.x
- Denke, MA. Metabolic effects of high-protein, low-carbohydrate diets. Am J Cardiol. 2001;88(1):59–61. doi: 10.1016/S0002-9149(01)01586-7
- Thomas, DT, Erdman, KA, Burke, LM. Position of the academy of Nutrition and dietetics, dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116(3):501–528. doi: 10.1016/j.jand.2015.12.006
- Hochstenbach-Waelen, A, Veldhorst, MA, Nieuwenhuizen, AG, et al. Comparison of 2 diets with either 25% or 10% of energy as casein on energy expenditure, substrate balance, and appetite profile. Am J Clin Nutr. 2009;89(3):831–838. doi: 10.3945/ajcn.2008.26917
- Arkinstall, MJ, Tunstall, RJ, Cameron-Smith, D, et al. Regulation of metabolic genes in human skeletal muscle by short-term exercise and diet manipulation. Am J Physiol Endocrinol Metab. 2004;287(1):E25–31. doi: 10.1152/ajpendo.00557.2003
- SCHOLZ, R, SCHWABE, U, SOBOLL, S. Influence of fatty acids on energy metabolism: 1. Stimulation of oxygen consumption, ketogenesis and CO2 production following addition of octanoate and oleate in perfused rat liver. Eur J Biochem. 1984;141(1):223–230. doi: 10.1111/j.1432-1033.1984.tb08179.x
- Paoli, A, Rubini, A, Volek, JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–796. doi: 10.1038/ejcn.2013.116
- Bergström, J, Hermansen, L, Hultman, E, et al. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2‐3):140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x
- Purdom, T, Kravitz, L, Dokladny, K, et al. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr. 2018;15(1):1–10. doi: 10.1186/s12970-018-0207-1
- Soeters, MR, Soeters, PB, Schooneman, MG, et al. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am J Physiol Endocrinol Metab. 2012;303(12):E1397–E1407. doi: 10.1152/ajpendo.00397.2012
- Karlsson, J, Saltin, B. Diet, muscle glycogen, and endurance performance. J Appl Physiol. 1971;31(2):203–206. doi: 10.1152/jappl.1971.31.2.203
- Walker, JL, Heigenhauser, GJ, Hultman, E, et al. Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J Appl Physiol. 2000;88(6):2151–2158. doi: 10.1152/jappl.2000.88.6.2151
- Bjorkman, O, Eriksson, L. Splanchnic glucose metabolism during leg exercise in 60-hour-fasted human subjects. Am J Physiol Endocrinol Metab. 1983;245(5):E443–E8. doi: 10.1152/ajpendo.1983.245.5.E443
- GALBO, H, Holst, J, Christensen, N. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand. 1979;107(1):19–32. doi: 10.1111/j.1748-1716.1979.tb06438.x
- Langfort, J, Zarzeczny, R, Pilis, W, et al. The effect of a low-carbohydrate diet on performance, hormonal and metabolic responses to a 30-s bout of supramaximal exercise. Eur J Appl Physiol Occup Physiol. 1997;76(2):128–133. doi: 10.1007/s004210050224
- Pequignot, J, Peyrin, L, Peres, G. Catecholamine-fuel interrelationships during exercise in fasting men. J Appl Physiol. 1980;48(1):109–113. doi: 10.1152/jappl.1980.48.1.109
- Wooten, JS, Biggerstaff, KD, Ben-Ezra, V. Responses of LDL and HDL particle size and distribution to omega-3 fatty acid supplementation and aerobic exercise. J Appl Physiol. 2009;107(3):794–800. doi: 10.1152/japplphysiol.91062.2008
- Elliott, KH, Welcker, J, Gaston, AJ, et al. Thyroid hormones correlate with resting metabolic rate, not daily energy expenditure, in two charadriiform seabirds. Biol Open. 2013;2(6):580–586. doi: 10.1242/bio.20134358
- Zurlo, F, Larson, K, Bogardus, C, et al. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Investig. 1990;86(5):1423–1427. doi: 10.1172/JCI114857
- Terink, R, Witkamp, RF, Hopman, MT, et al. A 2 week cross-over intervention with a low carbohydrate, high fat diet compared to a high carbohydrate diet attenuates exercise-induced cortisol response, but not the reduction of exercise capacity, in recreational athletes. Nutrients. 2021;13(1):157. doi: 10.3390/nu13010157
- Baechle, T, Earle, R, Wathen, D. Essentials of strength and conditioning: National Strength and Conditioning Association (NSCA). Champaign IL: Human Kinetics; 2000.
- Hollander, DB, Durand, RJ, Trynicki, JL, et al. RPE, pain, and physiological adjustment to concentric and eccentric contractions. Med & Sci In Sports & Ex. 2003;35(6):1017–1025. doi: 10.1249/01.MSS.0000069749.13258.4E
- Bishop, NC, Walsh, NP, Haines, DL, et al. Pre-exercise carbohydrate status and immune responses to prolonged cycling: II. Effect on plasma cytokine concentration. Int J Sport Nutr Exercise Metab. 2001;11(4):503–512. doi: 10.1123/ijsnem.11.4.503
- Gleeson, M, Blannin, AK, Walsh, NP, et al. Effect of low-and high-carbohydrate diets on the plasma glutamine and circulating leukocyte responses to exercise. Int J Sport Nutr Exercise Metab. 1998;8(1):49–59. doi: 10.1123/ijsn.8.1.49
- Lemon, P, Mullin, J. Effect of initial muscle glycogen levels on protein catabolism during exercise. J Appl Physiol. 1980;48(4):624–629. doi: 10.1152/jappl.1980.48.4.624
- Hackney, AC, Walz, EA. Hormonal adaptation and the stress of exercise training: the role of glucocorticoids. Trends Sport Sci. 2013;20(4):165.