ABSTRACT
Quercetin is one type of ergogenic aid and its effects on the neuromuscular system have recently attracted interest, but its dose-effect is not yet fully understood. The aim of this study was to examine the effect of different doses of quercetin ingestion on motor unit firing patterns and muscle contractile properties in humans. Thirteen young males and females conducted neuromuscular performance tests before (PRE) and 60 min after (POST) ingestions of 500 or 200 mg of quercetin glycosides (Qg500/Qg200, respectively) or placebo (PLA) on three different days. At PRE and POST, motor unit firing rates were calculated from high-density surface electromyography of the vastus lateralis muscle during 120-s isometric contraction of knee extension at 10% of maximal voluntary contraction. Electrically elicited forces in knee extensor muscles were also measured. After 60 s of voluntary contraction, motor unit firing rates, normalized by the exerted muscle force at POST, were significantly lower at POST than PRE with Qg500 and Qg200 (p < 0.05), but not with PLA (p > 0.05). Changes in motor unit firing rates normalized by the exerted force from PRE to POST were significantly greater with Qg500 than Qg200 at the end of contraction (p < 0.05). Under all three conditions, the electrically elicited force did not significantly change from PRE to POST (p > 0.05). These results suggest that both 500 and 200-mg quercetin ingestions alter motor unit firing patterns, and that quercetin’s effect is at least partially dose-dependent.
1. Introduction
For various purposes such as to enhance performance in sport activities or general health, quercetin has been used as an ergogenic aid [Citation1–4]. In addition to the significant benefits of quercetin ingestion to promote human endurance exercise capacity [Citation1], recent studies showed the effects of its ingestion on the neuromuscular system [Citation5–7]. Quercetin has the role of blocking the binding of adenosine to A1 receptors, promoting the release of neurotransmitters such as acetylcholine and dopamine, and increasing calcium release from the sarcoplasmic reticulum [Citation2,Citation8–10]. These effects on the neuromuscular system are similar to caffeine, which is the most commonly consumed ergogenic aid [Citation11]. Such neuromuscular effects may also help explain improvements of endurance performance following quercetin ingestion. Patrizio et al. (2018) reported that 8 days of receiving a single dose of quercetin ameliorates any loss of maximal voluntary contraction (MVC) and promotes neuromuscular efficiency after resistance exercise [Citation6]. Bazzucchi et al. (2019) reported an increase of MVC and reduced decay of muscle fiber conduction velocity after fatiguing eccentric contractions following 14 days of quercetin supplementation [Citation5]. Our previous study showed that a single ingestion of quercetin mitigates reduced muscle contractile properties and decreases in the motor unit firing rate and exerted forces, suggesting that there is an increase in neuromuscular efficiency following its ingestion [Citation7].
Most previous studies used more than 1,000 mg of quercetin per day for oral administration to test its physiological effects in humans [Citation1,Citation4–6,Citation12,Citation13]. This amount of quercetin ingestion is known to lead to rapid and persistent elevations in circulating levels of quercetin [Citation1,Citation2]. Our previous study revealed that 500 mg of quercetin glucosides, which is half the amount used in previous studies, can have significant acute effects on the neuromuscular system [Citation7]. However, commercial nutritional supplementations used in previous studies contained 250–300 mg of quercetin in one dosing form, such as a drink or chewable food [Citation3,Citation4]. These amounts of quercetin are approximately a quarter of that used in previous studies (1,000 mg) [Citation1,Citation4–6,Citation12,Citation13] and half of that used in our previous study (500 mg) [Citation7]. For the application of quercetin supplementation, the dose-effect and/or minimum dose needed to achieve sufficient physiological benefits should be tested within the range of amounts contained in one package of commercially available supplements.
The aim of this study was to test the effect of different doses of quercetin ingestion on motor unit firing patterns and muscle contractile properties in humans. This study compared 500 and 200-mg quercetin ingestions, used in our previous study [Citation7] and a clinically feasible dose [Citation3,Citation4], respectively. Previous studies showed that even a smaller amount, ~150 mg, of quercetin ingestion increases the plasma quercetin concentration [Citation14]. Also, dose-effects of quercetin ingestion were reported in the plasma quercetin concentration following oral quercetin ingestion between 250 and 500 mg [Citation2]. We thus hypothesized that 200-mg quercetin ingestion has acute effects on the neuromuscular system, and 500-mg quercetin ingestion has greater effects compared with 200-mg ingestion.
2. Methods
2.1. Participants
Thirteen healthy young adults including eight males and five females (mean ± SD: age: 23.6 ± 5.1 years, height: 170.2 ± 6.2 cm, body weight: 62.9 ± 11.5 kg) participated in this study. Written informed consent was obtained from all participants after providing them with a detailed explanation of the purposes, potential benefits, and risks associated with participation. This study was approved by the Research Ethics Committee of Chukyo University (2019–003).
2.2. Experimental design
The present study was performed as a double-blind cross-over randomized study. Participants visited the laboratory three times, separated by at least 48-h intervals. On each day, one of the three kinds of capsules containing 500 mg (Qg500) or 200 mg (Qg200) of quercetin glycosides or placebo (PLA) was ingested in random order. Thirteen patterns for various combinations of days (1,2, and 3) and ingestions (Qg500, Qg200, and PLA) were made and each pattern was randomly applied to each participant. Neuromuscular performance tests were conducted before (PRE) and 60 min after (POST) ingestions of the capsules (). High-intensity exercise, consumptions of foods or drinks containing quercetin and/or caffeine, and the ingestion of other possible ergogenic aids which are sold as functional foods were prohibited 24 h before testing.
Figure 1. Schematic overview of the experimental protocol. MVC, maximal voluntary contraction; PRE, before ingestion; POST, 60 min after ingestion.
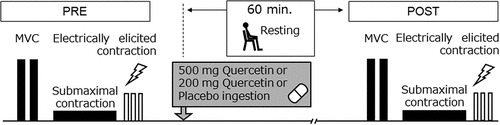
Following warm-up, the participants performed maximal voluntary contraction (MVC) trials involving isometric knee extension of the right leg on a dynamometer. Submaximal sustained contraction to record motor unit activation and electrically elicited contractions to measure muscle contractile properties were performed for knee extensor muscles of the right leg after MVC. These measurements were repeated PRE and POST. Knee extension joint torque during MVC, submaximal contraction, and electrically elicited contraction were measured using a dynamometer (Takei Scientific Instruments Co., Ltd., Niigata, Japan) with a force transducer (LU-100KSE; Kyowa Electronic Instruments, Tokyo, Japan) fixed to the distal part of the shank of the right leg. During these measurements, hip and knee joint angles were fixed at 90°.
With ~300 mL of water, participants took six gelatin-coated and colored capsules containing 500 or 200 mg of quercetin glycosides (San-Ei Gen F.F.I., Inc., Osaka, Japan) [Citation15] with 350 mg of dextrin or 0 mg of quercetin with 350 mg of dextrin after measurements at PRE as trials for Qg500, Qg200, and PLA, respectively. The capsules for Qg500, Qg200, and PLA had the same shape, color, and weight, and randomized, double-blind, placebo-controlled treatment was administered. After ingestion of the capsules, participants sat and rested on a chair for 60 min until the measurements at POST.
2.3. MVC
Two MVCs were performed using the right leg with a 2-min rest interval between them at PRE and POST, respectively. An MVC trial included a gradual increase in the knee extension force to a maximum effort in 2–3 s, and the plateau phase at maximum effort was maintained for 2–3 s with a verbal count given at 1-s intervals. At PRE, prior to MVC measurements, participants performed submaximal contractions at approximately 50%, 70%, and 90% of maximum efforts for familiarization and warm-up. The highest MVC forces of two MVC trials at PRE and POST were used for further analysis, respectively.
2.4. Submaximal contractions for recording motor unit activations
Participants performed submaximal sustained contractions of isometric knee joint extension at 10% of MVC for 120 s. This contraction included a 1–2-s increasing phase from the baseline to 10% of MVC and a 120-s sustained phase at 10% of MVC. At PRE and POST, the MVC force at PRE was used to calculate 10% of MVC, resulting in the application of the same forces for sustained isometric contractions between PRE and POST on the same day.
During contraction, high-density surface electromyography (HDsEMG) signals were recorded from the vastus lateralis (VL) muscle of the right leg using a semi-disposable adhesive grid of 64 electrodes (13 rows and 5 columns with one missing electrode) and a 1-mm diameter and 8-mm inter-electrode distance (ELSCH064NM2, OT Bioelectronica, Torino, Italy). The electrode grids were placed at the midpoint of the line between the head of the greater trochanter and upper lateral edge of the patella, and the long side of grids was aligned along the reference line. A wet electrode strap (WS2, OT Bioelectronica, Torino, Italy) was placed at the right knee. Monopolar surface EMG signals were recorded with a band-pass filter (10–500 Hz), amplified by a factor of 150, sampled at 2,048 Hz, and converted to digital form by a 16-bit analog-to-digital converter (Quattrocento, OT Bioelectronica, Torino, Italy). The signal from the force transducer of the dynamometer was also recorded and synchronized with this analog-to-digital converter.
From the recorded monopolar surface EMG signals, individual motor units were identified by the Convolution Kernel Compensation (CKC) technique using DEMUSE software [Citation16–19]. The procedures for decomposition into individual motor units used in the present study were previously and extensively validated based on HDsEMG signals from various skeletal muscles including the VL muscle [Citation7,Citation19–24]. Based on Holobar et al. (2014), the pulse-to-noise ratio (PNR) was used as an indicator of the motor unit identification accuracy, and only motor units with PNR >30 dB (corresponding to an accuracy of motor unit firing identification >90%) were used for further analysis; all other motor units were discarded [Citation25]. We tracked the same motor units from PRE to POST on the same day, and the motor units that could be tracked through PRE and POST were used for further analysis.
Motor unit tracking was ensured by applying the MU filter estimated from surface EMG at PRE to the EMG signals recorded at POST, as described in the previous studies [Citation26,Citation27]. The previously introduced criterion of PNR >30 dB was also applied to motor unit tracking, ensuring an accuracy of motor unit firing identification >90% at POST.
Discharge timings of the individual tracked motor units were independently examined by one experienced investigator. From discharge timings of individual motor units, instantaneous motor unit firing rates were calculated. Discharge timings with inter-discharge intervals <33.3 or >250 ms were excluded from further analysis, since firing rates calculated from this range of inter-discharge intervals are unusually high (>30 Hz) or low (<4 Hz) for the VL muscle. At 10, 60, and 120 s after the beginning of sustained contractions, median values of normalized firing rates during 10 s were calculated from instantaneous firing rates for individual motor units. In order to normalize the variations in exerted forces, motor unit firing rates were normalized by the exerted muscle force at the firing timings (pps/%MVC). Motor unit firing rates with >30% coefficient of variation were excluded from further analysis [Citation28]. Motor units detected during the trials were merged across all participants for Qg500, Qg200, or PLA. These procedures were the same as those used in our previous study [Citation7].
2.5. Electrically elicited contractions for measuring muscle contractile properties
In order to assess the muscle contractile properties, involuntary electrically elicited twitch contraction forces were measured for knee extensor muscles of the right leg. Two electrodes (2 × 15 cm) were placed at proximal and distal sites of the right quadriceps femoris muscle [Citation7,Citation29]. A constant current stimulator was used for electrical stimulation (DS7AH, Digitimer, Ltd., Hertfordshire, UK) with a 200-µs pulse width. To determine the stimulation intensity, the current intensity for singlet stimulation was increased by 100 mA from 400 mA until the maximal knee extension force was achieved at PRE, and this maximal current intensity at PRE was used for PRE and POST. Five contractions were elicited with 2 ~ 3-s intervals, and peak forces during contractions were averaged and used for further analysis at PRE and POST, respectively.
2.6. Statistics
All data are presented as the mean ± SD. Before the statistical analyses, distribution and homogeneity of the data were tested by the Shapiro–Wilk test and Levene test. Parametric analyses were applied to the data with normal distribution or homogeneity of variance. When normal distribution and homogeneity of variance were rejected, non-parametric analyses were performed. MVC and electrically elicited force were analyzed by two-way repeated-measures ANOVA for testing interaction of condition and time and were compared between PRE and POST by the paired T-test. Normalized firing rate at 10 s, 60 s, and 120 s were tested by two-way factorial ANOVA, and the unpaired T-test was used to compare PRE and POST for each condition. One-way ANOVA was performed in ratio between PRE and POST in MVC and electrically elicited force. Ratio between PRE and POST in normalized firing rate was compared among Qg500, Qg200, and PLA using Dunn’s test with Bonferroni correction when the Friedman test detected a significant effect of the three different conditions. Statistical analysis was performed using SPSS (version 21.0, SPSS, Tokyo, Japan), and the level of significance was set at 0.05.
3. Results
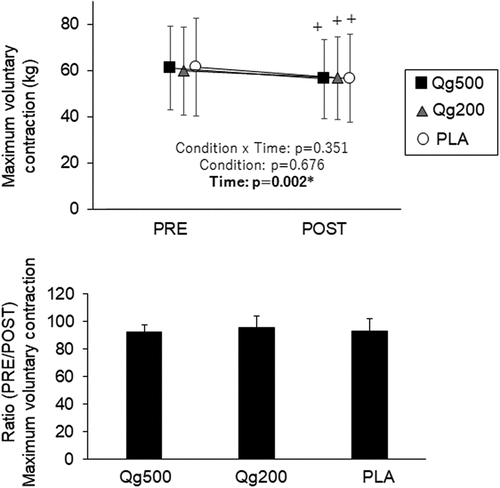
Interaction between condition and time was not detected in MVC (p > 0.05) (, upper panel). Significant decreases in MVC from PRE to POST were found with Qg500, Qg200, and PLA (p < 0.05) (, upper panel). There was no significant effect of condition in the ratio between PRE and POST in MVC under all three conditions (p > 0.05) (, lower panel).
Figure 2. Maximal voluntary contraction before and 60 min after ingestion (top panel) and its ratio between before and 60 min after ingestion (bottom panel). PRE, before ingestion; POST, 60 min after ingestion; Qg500, 500-mg quercetin ingestion; Qg200, 200-mg quercetin ingestion; PLA, placebo ingestion. *p < 0.05 significant effect of time, +p < 0.05 vs PRE.
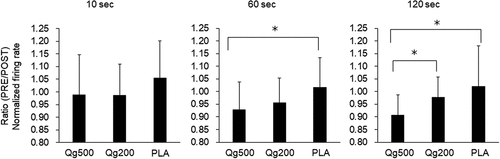
With Qg500, Qg200, and PLA, 68, 34, and 49 motor units were tracked between PRE and POST, respectively, and used for further analyses. Significant interactions between condition and time were found in the normalized firing rate at 10, 60, and 120 s (p < 0.05) (). At 10 s, significant decrease in the normalized firing rate was seen only in PLA (p < 0.05). Significant decreases in the normalized firing rate from PRE to POST were found in both Q500 and Q200 at 60 s (p < 0.05) and in Q500 at 120 s (p < 0.05) (). The ratio between PRE and POST in the normalized firing rate with Qg500 was significantly lower than that with PLA at 60 s and those with Qg200 and PLA at 120 s (p < 0.05) ().
Figure 3. Normalized firing rate during sustained contractions before and 60 min after 500-mg quercetin (left), 200-mg quercetin (center), and placebo (right) ingestions at 10, 60, and 120 s. PRE, before ingestion; POST, 60 min after ingestion. Qg500, 500-mg quercetin ingestion; Qg200, 200-mg quercetin ingestion; PLA, placebo ingestion. *p < 0.05 significant interaction between condition and time, #p < 0.05 significant effect of time, +p < 0.05 vs PRE.
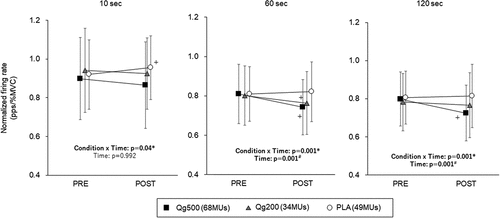
Figure 4. Ratio between before and 60 min after ingestion in normalized firing rate at 10 s (left), 60 s (center), and 120 s (right) during sustained contractions. PRE, before ingestion; POST, 60 min after ingestion. Qg500, 500-mg quercetin ingestion; Qg200, 200-mg quercetin ingestion; PLA, placebo ingestion. *p < 0.05 between conditions.
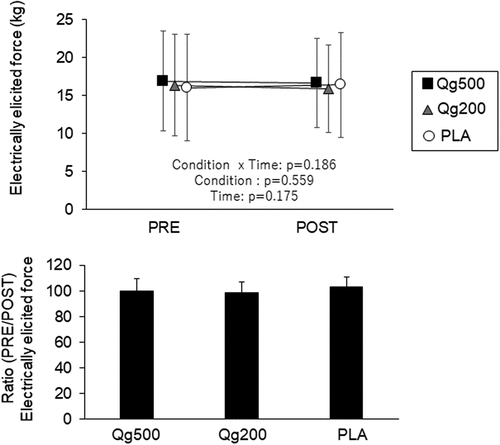
No significant interaction between condition and time was detected in the electrically elicited force with Qg500, Qg200, or PLA (p > 0.05) (, upper panel). The ratios between PRE and POST in the electrically elicited force were not significantly different among the three conditions (p > 0.05) (, lower panel).
Figure 5. Electrically elicited force before and 60 min after ingestion (top panel) and its ratio between before and 60 min after ingestion (bottom panel). PRE, before ingestion; POST, 60 min after ingestion; Qg500, 500-mg quercetin ingestion; Qg200, 200-mg quercetin ingestion; PLA, placebo ingestion.
4. Discussion
We tested the effect of different doses of quercetin ingestion on motor unit firing patterns and muscle contractile properties in humans. Changes in motor unit firing patterns following quercetin ingestion were found with both 500 and 200-mg quercetin ingestion; however, the rate of change was greater with 500 than 200 mg. Also, no effects of quercetin glucoside ingestion or dose-effects on muscle contractile properties were detected in the present study. These results support our hypothesis that 200-mg quercetin ingestion has acute effects on the neuromuscular system, and 500-mg quercetin ingestion has greater effects than 200 mg in terms of the motor unit firing pattern, but not muscle contractile properties.
4.1. Effects of quercetin ingestion on motor unit firing rate
During sustained contraction, normalized firing rates were significantly decreased after ingestions of 500 and 200 mg of quercetin glucosides (p < 0.05) (). On the other hand, the normalized firing rate in PLA, interpreted as the control condition, showed different manners from PRE to POST with Qg500 at 10, 60, and 120 s and with Qg200 at 10 and 60 s (p < 0.05) (). These results were reflected in a significant interaction between condition and time in normalized firing rate (p < 0.05) (). From these results, we consider that quercetin ingestion induces changes in the motor unit firing pattern. The normalized firing rate used in the present study reflects neural inputs from the central nervous system to peripheral muscles to generate an absolute force level. Therefore, decreases in the normalized firing rate suggest a reduction in neural inputs required to generate a given force, in other words, improvement of neuromuscular efficiency. This would be markedly influenced by not only activations of the central nervous system but also peripheral muscle contractile properties. Although the present study did not reveal significant differences in muscle contractile properties between quercetin ingestions and the placebo (p > 0.05) (), our previous study showed the attenuation of electrically elicited muscle contraction decline following quercetin ingestion [Citation7]. Quercetin has actions affecting calcium release from the sarcoplasmic reticulum [Citation2,Citation10] and would alter electro-contraction coupling in skeletal muscle. This action was also confirmed in humans using caffeine, which has a similar action to quercetin. Lopes et al. (1983) reported that caffeine ingestion induces an increase in electrically elicited contraction forces [Citation30]. Moreover, this caffeine-induced alteration in muscle contractile properties was selectively noted at low to middle stimulation frequencies (10–50 Hz) but not a high stimulation frequency (100 Hz) [Citation30]. Since it has been considered that the failure to generate force at low stimulation frequencies is due to an impairment of excitation-contraction coupling, caffeine or quercetin ingestion could improve such coupling. Therefore, we concluded that decreases in normalized firing rates with Qg500 and Qg200 can be partly explained by the action of quercetin on electro-contraction coupling related to calcium release from the sarcoplasmic reticulum [Citation2,Citation10]. We also need to consider motor units not analyzed in the present study. Since we analyzed only motor units recruited from the beginning of contraction that could be tracked between PRE and POST, not all recruited motor units were investigated. During sustained contraction, recruitment or de-recruitment of motor units occurs even with sustained contraction at a lower force level [Citation31]. In our previous study, a decrease in the recruitment threshold of motor units was noted following quercetin ingestion [Citation7]. The possibility that quercetin ingestion enhances the recruitment of motor units with higher recruitment thresholds was reported in a previous study based on an increase in the median frequency of surface EMG [Citation6]. Therefore, we considered that a larger number of motor units may be recruited during contractions after ingestions of Qg500 and Qg200 than those of PLA in the present study. This also explains the decrease in normalized firing rates with Qg500 and Qg200 from PRE to POST due to decreases in the contribution to force production in motor units recruited from the beginning of contractions and recruited in both PRE and POST. Moreover, quercetin may alter neurotransmitters. It is known to act as an antagonist of A1 adenosine receptors [Citation2,Citation8]. This function would attenuate inhibitions of neurotransmitter release and neuronal firing rates and then alter arousal and induce excitations of the central nervous system.
4.2. Effect of quercetin ingestion on muscle contractile properties
In our previous study, the electrically elicited force decreased 60 min after placebo ingestion, and this decline was not detected in the 500-g quercetin ingestion trial [Citation7]. We interpreted these results as showing the function of quercetin to attenuate declines in muscle contractile properties. The present study did not show reductions in electrically elicited force under any of the conditions (). These inconsistent results regarding muscle contractile properties between the present and previous studies may be explained by differences in the number of neuromuscular performance tests. In our previous study [Citation7], more tests were performed in a day, i.e. MVC, tendon reflex, rapid maximal contraction, submaximal isometric contraction, submaximal sustained contraction, and electrical stimulations with two different twitch types. A higher frequency of neuromuscular tests might induce declines in muscle contractile properties [Citation7]. In other previous studies, quercetin ingestion exerted a protective role against exercise-induced declines in physiological functions [Citation1,Citation2]. For example, Patrizio et al. (2018) detected enhancement of neuromuscular performance following a single resistance training session [Citation6]. Thus, any lack of quercetin-related changes in muscle contractile properties may be associated with the given tasks in the present study. Loading exercise-induced physiological changes, i.e. intensive exercises, may be necessary to detect the beneficial effects of quercetin ingestion on muscle contractile properties.
4.3. Dose-effect of quercetin ingestion on motor unit firing pattern
While similar results were observed between Qg500 and Qg200 in normalized firing rates (), degrees of change from PRE to POST were considered to be dose-dependent (). For normalized firing rates, significant differences in the ratio between PRE and POST were detected between Qg500 and PLA, but not between Qg200 and PLA, at 60 s (). Also, the ratio between PRE and POST in the normalized firing rate with Qg500 was significantly lower than that for Qg200 at 120 s (). These results suggest greater effects of 500-mg quercetin ingestion on the normalized firing rate than 200-mg quercetin ingestion, which is an indicator of neuromuscular efficiency. While their study involved chronic intervention, only Nieman et al. (2009) reported the dose-effects of quercetin ingestion on human physiological functions [Citation13]. They tested the chronic effects of 1,000 mg of quercetin ingestion per day with or without additional nutritional supplementation, including 400-mg quercetin, and showed that a higher level of quercetin ingestion (1,400 mg/day) has greater effects of countering exercise-induced inflammation than a smaller amount of quercetin ingestion (1,000 mg/day) following 2 weeks of quercetin supplementation. Dose-effects of oral quercetin ingestion on the plasma quercetin concentration were reported in humans. Egert et al. (2008) reported that the maximum and area under the curve (AUC) of the plasma concentration were significantly greater with a larger dose, and the time to reach the maximum plasma concentration was significantly delayed with a larger dose, although the dose used in this study ranged from 50 to 150 mg, being smaller than the dose used in other previous studies [Citation14]. In the study of Davis et al. (2009), the plasma quercetin concentration was significantly greater with 500 than 250-mg ingestion at 120–180 min after the ingestion [Citation2]. The dose-effects of quercetin ingestion on the motor unit firing pattern in the present study may be partially explained by differences in absorption patterns with different doses of quercetin. However, we could not detect any differences in MVC and electrically elicited contraction between Qg500 and Qg200 (). Previous studies testing the dose-effects of quercetin absorption also reported weaker dose-effects at a relatively earlier epoch after quercetin ingestion, such as 60 min after ingestion, used in the present study. For example, no significant differences were noted in rates of quercetin absorption among 50, 100, and 150-mg ingestions [Citation14] or in the plasma quercetin concentration at 60 min after ingestion between 250 and 500-mg doses [Citation2]. Moreover, Kressler et al. (2011) showed no significant correlation between the effect size of quercetin ingestion on endurance performance and the plasma concentration of quercetin in their review paper [Citation1]. Therefore, we concluded that the dose-effects of quercetin ingestion may not be equal for all functions.
Although 1,000-mg or higher doses have been applied to test the effects of quercetin ingestion on physiological responses in humans [Citation1,Citation4–6,Citation12,Citation13], one package of commercial nutritional supplementations typically contains 250–300 mg [Citation3,Citation4]. The present study showed significant decreases in the normalized firing rate with both 500 and 200-mg quercetin ingestions (), suggesting that quercetin ingestion-induced changes in the motor unit firing pattern can be realized even with 200 mg of quercetin. While this physiological response with a smaller amount of quercetin ingestion cannot be applied to all neuromuscular functions, such as maximal voluntary contraction or muscle contractile properties, our results may help to clarify clinically feasible doses of quercetin ingestion that have beneficial effects on the motor unit firing pattern.
4.4. Limitations
Since neuromuscular responses during exercise could be influenced by various factors, we requested the participants to control physical activity and foods/drinks such as ergogenic aids. However, the present study did not collect their reports or replication of physical activity and foods/drinks before the experiments. Also, the present study examined both males and females. There are no studies investigating the effects of sex on physiological responses to quercetin ingestion. Moreover, for female participants, we did not consider the menstrual cycle. Therefore, the present study is performed with some limitations, and further studies are needed to clarify the dose effects of quercetin ingestions on neuromuscular system under a controlled condition.
5. Conclusion
The present study investigated the effect of 500, 200, and 0-mg quercetin ingestions on motor unit firing patterns and muscle contractile properties in humans. Normalized motor unit firing rates during isometric sustained contraction were significantly decreased from before to after ingestions of both 500 and 200 mg of quercetin, but not placebo. Degrees of decline in normalized motor unit firing rates from before to after quercetin ingestion were significantly greater with 500 than 200 mg. The electrically elicited force, which is an indicator of muscle contractile properties, was not significantly changed from before to after ingestions of 500 and 200 mg of quercetin or placebo. These results suggested that both 500 and 200-mg quercetin ingestions alter the motor unit firing pattern, but quercetin’s effect is at least partially dose-dependent.
Authors’ contributions
KW planned research, conducted experiments, analyzed data, discussed results, and wrote paper. SK conducted experiments, analyzed data, discussed results, and edited and reviewed manuscript. AH analyzed data, discussed results, and edited and reviewed manuscript.
Consent to participate and publication
The participants gave informed consent for the study after receiving a detailed explanation of the purposes, potential benefits, and risks associated with participation.
Ethics approval
This study was approved by the Research Ethics Committee for Human Experimentation at Chukyo University (No. 2019–003).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available from the authors upon reasonable request.
Additional information
Funding
References
- Kressler, J, Millard-Stafford, M, Warren, GL. Quercetin and endurance exercise capacity: a systematic review and meta-analysis. Med Sci Sports Exercise. 2011 Dec;43(12):2396–836. doi: 10.1249/MSS.0b013e31822495a7
- Davis, JM, Murphy, EA, Carmichael, MD. Effects of the dietary flavonoid quercetin upon performance and health [Research support, U.S. Gov’t, non-P.H.S. Review]. Curr Sports Med Rep. 2009 Jul-Aug;8(4):206–213. doi: 10.1249/JSR.0b013e3181ae8959
- MacRae, HS, Mefferd, KM. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance [randomized controlled trial Research support, non-U.S. Gov’t]. Int J Sport Nutr Exercise Metab. 2006 Aug;16(4):405–419. doi: 10.1123/ijsnem.16.4.405
- Quindry, JC, McAnulty, SR, Hudson, MB, et al. Oral quercetin supplementation and blood oxidative capacity in response to ultramarathon competition. Int J Sport Nutr Exercise Metab. 2008 Dec;18(6):601–616. doi: 10.1123/ijsnem.18.6.601
- Bazzucchi, I, Patrizio, F, Ceci, R, et al. The effects of quercetin supplementation on eccentric exercise-induced muscle damage [randomized controlled trial]. Nutrients. 2019 Jan 21;11(1):205. doi: 10.3390/nu11010205
- Patrizio, F, Ditroilo, M, Felici, F, et al. The acute effect of quercetin on muscle performance following a single resistance training session [randomized controlled trial]. Eur J Appl Physiol. 2018 May;118(5):1021–1031. doi: 10.1007/s00421-018-3834-y
- Watanabe, K, Holobar, A. Quercetin ingestion modifies human motor unit firing patterns and muscle contractile properties. Exp Brain Res. 2021 May;239(5):1567–1579. doi: 10.1007/s00221-021-06085-w
- Alexander, SP. Flavonoids as antagonists at A1 adenosine receptors [comparative study]. Phytother Res. 2006 Nov;20(11):1009–1012. doi: 10.1002/ptr.1975
- Konrad, M, Nieman, DC. Evaluation of quercetin as a countermeasure to exercise-induced physiological stress. In: Lamprecht M, editor. Antioxidants in sports nutrition. Boca Raton, FL, USA: CRC Press/Taylor & Francis; 2015. pp. 155–170. doi: 10.1201/b17442-10
- Lee, EH, Meissner, G, Kim, DH. Effects of quercetin on single Ca(2+) release channel behavior of skeletal muscle [Research support, non-U.S. Gov’t Research support, U.S. Gov’t, P.H.S.]. Biophys J. 2002 Mar;82(3):1266–1277. doi: 10.1016/S0006-3495(02)75483-0
- Grgic, J, Mikulic, P, Schoenfeld, BJ, et al. The influence of caffeine supplementation on resistance exercise: a review [review]. Sports Med. 2019 Jan;49(1):17–30. doi: 10.1007/s40279-018-0997-y
- Nieman, DC, Henson, DA, Gross, SJ, et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exercise. 2007 Sep;39(9):1561–1569. doi: 10.1249/mss.0b013e318076b566
- Nieman, DC, Henson, DA, Maxwell, KR, et al. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exercise. 2009 Jul;41(7):1467–1475. doi: 10.1249/MSS.0b013e318199491f
- Egert, S, Wolffram, S, Bosy-Westphal, A, et al. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008 Sep;138(9):1615–1621. doi: 10.1093/jn/138.9.1615
- Makino, T, Shimizu, R, Kanemaru, M, et al. Enzymatically modified isoquercitrin, alpha-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats [Research support, non-U.S. Gov’t]. Biol Pharm Bull. 2009 Dec;32(12):2034–2040. doi: 10.1248/bpb.32.2034
- Merletti, R, Holobar, A, Farina, D. Analysis of motor units with high-density surface electromyography. J Electromyogr Kinesiol. 2008 Dec;18(6):879–890. doi: 10.1016/j.jelekin.2008.09.002
- Holobar, A, Zazula, D. On the selection of the cost function for gradient-based decomposition of surface electromyograms. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:4668–4671.
- Holobar, A, Zazula, D. Correlation-based decomposition of surface electromyograms at low contraction forces. Med Biol Eng Comput. 2004 Jul;42(4):487–495. doi: 10.1007/BF02350989
- Holobar, A, Farina, D, Gazzoni, M, et al. Estimating motor unit discharge patterns from high-density surface electromyogram. Clin Neurophysiol. 2009 Mar;120(3):551–562. doi: 10.1016/j.clinph.2008.10.160
- Farina, D, Holobar, A, Merletti, R, et al. Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol. 2010 Oct;121(10):1616–1623. doi: 10.1016/j.clinph.2009.10.040
- Yavuz, US, Negro, F, Sebik, O, et al. Estimating reflex responses in large populations of motor units by decomposition of the high-density surface electromyogram [Research support, non-U.S. Gov’t]. Journal Of Physiology. 2015 Oct 1;593(19):4305–4318. doi: 10.1113/JP270635
- Gallego, JA, Dideriksen, JL, Holobar, A, et al. The phase difference between neural drives to antagonist muscles in essential tremor is associated with the relative strength of supraspinal and afferent input [Research support, non-U.S. Gov’t]. J Neurosci. 2015 Jun 10;35(23):8925–8937. doi: 10.1523/JNEUROSCI.0106-15.2015
- Gallego, JA, Dideriksen, JL, Holobar, A, et al. Influence of common synaptic input to motor neurons on the neural drive to muscle in essential tremor [Research support, non-U.S. Gov’t]. J Neurophysiol. 2015 Jan 1;113(1):182–191. doi: 10.1152/jn.00531.2014
- Watanabe, K, Holobar, A, Kouzaki, M, et al. Age-related changes in motor unit firing pattern of vastus lateralis muscle during low-moderate contraction [Research support, non-U.S. Gov’t]. Age. 2016 Jun;38(3):48. doi: 10.1007/s11357-016-9915-0
- Holobar, A, Minetto, MA, Farina, D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric [Research support, non-U.S. Gov’t]. J Neural Eng. 2014 Feb;11(1):016008. doi: 10.1088/1741-2560/11/1/016008
- Del Vecchio, A, Negro, F, Holobar, A, et al. You are as fast as your motor neurons: speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans [Research support, non-U.S. Gov’t]. Journal Of Physiology. 2019 May;597(9):2445–2456. doi: 10.1113/JP277396
- Frančič, A, Holobar, A. On the reuse of motor unit filters in high density surface electromyograms recorded at different contraction levels. IEEE Access. 2021;9:115227–115236. doi: 10.1109/ACCESS.2021.3104762
- Fuglevand, AJ, Winter, DA, Patla, AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol. 1993 Dec;70(6):2470–2488. doi: 10.1152/jn.1993.70.6.2470
- Tomita, A, Kawade, S, Moritani, T, et al. Novel perspective on contractile properties and intensity-dependent verification of force-frequency relationship during neuromuscular electrical stimulation. Physiol Rep. 2020 Nov;8(22):e14598. doi: 10.14814/phy2.14598
- Lopes, JM, Aubier, M, Jardim, J, et al. Effect of caffeine on skeletal muscle function before and after fatigue [clinical trial controlled clinical trial]. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1303–1305. doi: 10.1152/jappl.1983.54.5.1303
- Bawa, P, Murnaghan, C. Motor unit rotation in a variety of human muscles [Research support, non-U.S. Gov’t]. J Neurophysiol. 2009 Oct;102(4):2265–2272. doi: 10.1152/jn.00278.2009
