ABSTRACT
Background
Sarcopenia and knee osteoarthritis are common age-related diseases that have become important public health issues worldwide. Few studies have reported the association between muscle mass loss and knee osteoarthritis. This may be due to the high level of heterogeneity between studies stemming from different definitions of muscle mass loss.
Methods
The systematic searches were carried out in PubMed and Web of Science from the inception of the databases until 13 January 2023, by two independent researchers. Pooled odds ratios (ORs) for overall and subgroup analyses were obtained using either a random effects model (I2 >50%) or fixed effects model (I2 ≤50%) in Stata.
Results
Of the 1,606 studies identified, we ultimately included 12 articles on the association between muscle mass and knee osteoarthritis (prospective: n = 5; cross-sectional: n = 7). Low-quality evidence indicated that low muscle mass index and sarcopenic obesity increase the odds of knee osteoarthritis (low muscle mass index OR: 1.36, 95% CI: 1.13–1.64; sarcopenic obesity OR: 1.78, 95% CI: 1.35–2.34). However, no association was observed between general sarcopenia or low muscle mass with knee osteoarthritis.
Conclusion
This systematic review and meta-analysis revealed that low muscle mass index and sarcopenic obesity were associated with an increased risk of developing knee osteoarthritis.
1. Introduction
With the aging of the worldwide population, osteoarthritis and sarcopenia are becoming important public health issues. Osteoarthritis is a chronic joint disease that is closely related to aging. It is characterized by cartilage degeneration, abnormal remodeling of subchondral bone, and formation of osteophytes at the edge of the joint, which cause pain and eventual disability. Osteoarthritis is the most common joint disease worldwide, affecting approximately 10% of men and 18% of women aged 60 and above [Citation1]. The prevalent cases of osteoarthritis increased by 113.25% globally, from 247.51 million in 1990 to 527.81 million in 2019. Among these cases, knee osteoarthritis contributed the most to the overall healthcare burden [Citation2]. The treatments for knee osteoarthritis include pain management, interventional therapy, regenerative therapies (cell & cell-free), and joint replacement [Citation3]. Given the diverse responses to non-surgical treatments and the limited lifespan of joint replacement prosthesis, it is crucial to identify modifiable risk factors and novel treatment targets.
Recent studies have highlighted various modifiable risk factors associated with the occurrence of knee osteoarthritis, including obesity, sedentary lifestyle, joint injury, high-intensity sports activities, and muscle mass loss [Citation4,Citation5]. However, the findings of the relationship between muscle mass and knee osteoarthritis remain controversial. Sarcopenia, a progressive and generalized skeletal muscle disorder, affecting 10% to 16% of the elderly globally, is linked to a series of adverse outcomes including falls and increased risk of mortality [Citation6,Citation7]. In 2019, the European Working Group on Sarcopenia in Old People (EWGSOP) updated the consensus on sarcopenia, emphasizing low muscle strength as a primary characteristic of sarcopenia and suggesting the assessment of low muscle mass to confirm the diagnosis of sarcopenia. Additionally, poor physical performance is identified as an indicator of severe sarcopenia [Citation8]. Muscle mass loss is an important sign of the aging process in human beings. With increased age, an imbalance between muscle protein anabolism and catabolism leads to the loss of skeletal muscle mass. This loss of muscle mass involves a reduction in the size and number of myofibres , particularly type II fibers, with intramuscular and intermuscular fat infiltration [Citation6]. Muscle mass loss is a major determinant of muscle strength decline [Citation9]. This muscle weakness compromises the stability of joints, making the joint tissue vulnerable to damage and ultimately contributing to the development and progression of knee osteoarthritis. A systematic review and meta-analysis have shown that knee extensor muscle weakness increases the risk of symptomatic knee osteoarthritis and radiographic knee osteoarthritis in both men and women [Citation10]. Another newly published meta-analysis demonstrated that people with osteoarthritis have an increased risk of sarcopenia, although only four eligible studies were included [Citation11]. In contrast, more studies have focused on the adverse impact of sarcopenia or low muscle mass on knee osteoarthritis [Citation12–15].
Given the common co-existence and shared risk factors of muscle mass loss and knee osteoarthritis, it is important to comprehend their connection. Therefore, this study aims to conduct a systematic review and meta-analysis to investigate the relationship between muscle mass loss and knee osteoarthritis.
2. Methods
2.1. Literature and search strategy
This systematic review and meta-analysis followed the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (PRISMA 2020). The systematic searches were carried out in PubMed and Web of Science from the inception of the databases until 13 January 2023, by two independent investigators (Zhuyan Xu and Qiming Wu). The search strategy included the following keywords: “sarcopenia” or “sarcopenic obesity” or “muscle atrophy” or “muscle wasting” or “muscle mass” or “lean mass” and “knee osteoarthritis” or “osteoarthritis.” Only papers written in English were considered. The title, abstract, and full articles were reviewed as needed to determine the eligiblity. In case of discrepancy, a third researcher (Xiaomin Ma) was consulted. illustrates the selection process of the included studies.
2.2. Inclusion criteria and data extraction
The inclusion criteria were as follows: 1) the original article assessed the association between muscle mass and knee osteoarthritis; 2) the articles used a cross-sectional, case-control, or cohort design; 3) knee osteoarthritis confirmed by X-ray or MRI and symptomatic knee osteoarthritis were used as outcome variables.
2.3. Data extraction
The main result of interest was the association between muscle mass loss and knee osteoarthritis, expressed as OR or relative risk (RR), along with a 95% CI. When both crude and adjusted effect sizes and a 95% CI were reported, adjusted effect sizes and 95% CI were selected for data synthesis. The following information was also extracted from each study: 1) name of the first author; 2) year of publication; 3) study country; 4) study design; 5) age and sex distribution of the study population; 6) number of participants; 7) definition of exposure; 8) definition of knee osteoarthritis; 9) adjusted covariates; 10) key findings.
2.4. Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the overall quality of each case-control or cohort study (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). NOS scores rate the quality of evidence as low (0–3), moderate (4–6), and high (7–9). For each cross-sectional study, the quality of evidence was evaluated using the 11-item checklist recommended by the Agency for Healthcare Research and Quality (AHRQ) [Citation16]. AHRQ scores rate the quality of evidence as low (0–3), moderate (4–7), and high (8–11). Consensus on the quality of evidence was reached by two independent researchers. In addition, we graded the strength of evidence for the overall findings based on GRADE (Grading of Recommendations Assessment, Development and Evaluation) guidance, with the strength of evidence categorized as high, moderate, low, and very low [Citation17].
2.5. Data analysis
Statistical analysis was carried out using Stata 16. The majority of the included studies used OR as the effect size, and thus, we presented the pooled OR as the final outcome. Overall/subgroup pooled OR and corresponding 95% CI were calculated through different subgroup analyses. Specifically, two studies evaluated the OR of knee osteoarthritis with low muscle mass versus high muscle mass, while the other two studies examined the protective effect of high muscle mass for knee osteoarthritis compared with low muscle mass. In this situation, estimates were reversed for the reduced OR in the latter two studies. Statistical heterogeneity among the studies was evaluated with I2 values. For studies with high heterogeneity (I2 >50%), we prioritized the random effects model over the fixed effects model. Egger’s test was employed to assess publication bias (p < 0.05). And sensitivity analysis was performed when a sufficient number of studies were included (>2) to test the robustness of the pooled result.
3. Results
3.1. Search results
As shown in , we identified a total of 1,426 articles after removing duplicates, and 1,351 articles were considered irrelevant after screening the titles and abstracts. After a full-text assessment of the 75 remaining articles, 63 did not meet the inclusion criteria and 12 articles were finally included. Among the 12 articles (some were included for multiple outcomes), four focused on sarcopenia, three on sarcopenic obesity, four on muscle mass index, and four on muscle mass. shows the detailed study characteristics.
Table 1. Characteristics of included studies (n = 12).
3.2. The association between sarcopenia, sarcopenic obesity and knee osteoarthritis
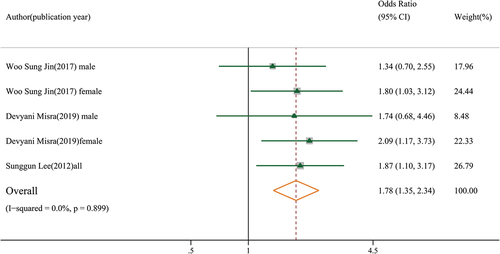
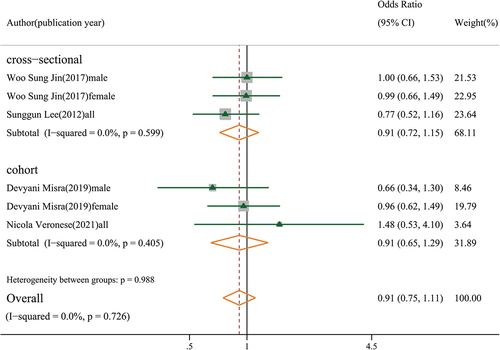
Four moderate-quality studies with 8,903 participants indicated that sarcopenia was not significantly associated with knee osteoarthritis in both cross-sectional and cohort studies (OR: 0.91, 95% CI: 0.75–1.11, GRADE = low) (). However, in Veronese’s cohort study, sarcopenia was associated with symptomatic knee osteoarthritis (defined by the presence of a combination of painful knee osteoarthritis) (OR: 2.29, 95% CI: 1.42–3.71), but not with radiographic knee osteoarthritis [Citation14]. Three moderate-quality studies showed that sarcopenic obesity was associated with knee osteoarthritis (OR: 1.78, 95% CI: 1.35–2.34, GRADE = low) (). A study by Lee et al. found that sarcopenic obesity was associated with knee osteoarthritis (OR: 1.87, 95% CI: 1.10–3.17) in both sexes [Citation12]. However, two other studies reported a significant association between sarcopenic obesity and knee osteoarthritis in females (OR: 1.93, 95% CI: 1.30–2.89), but not in males (OR: 1.46, 95% CI: 0.86–2.48) (Figure S1).
Figure 2. Association between sarcopenia and knee osteoarthritis (subgroup analysis based on study design).
In summary, an insufficient number of studies assessed the association between sarcopenia and knee osteoarthritis, and the evidence remains inconclusive. Although evidence supports an association between sarcopenic obesity and knee osteoarthritis, further studies are required to confirm this association in different sexes.
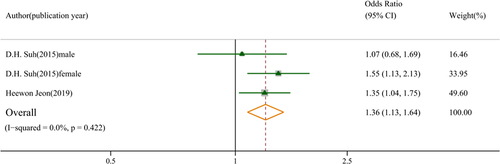
3.3. The association between low muscle mass index and knee osteoarthritis
Two studies revealed that a low muscle mass index was associated with knee osteoarthritis (OR: 1.36, 95% CI: 1.13–1.64, GRADE = low) (). A study by Suh et al. showed that low lower limb muscle mass index was associated with knee osteoarthritis in females (OR: 1.55, 95% CI: 1.13–2.13) but not in males (OR: 1.07, 95% CI: 0.68–1.69) [Citation19].
Figure 4. Association between low muscle mass index and knee osteoarthritis. Low muscle mass index defined by whose muscle mass divided by weight was lower than the reference population.
In the Visser study, muscle mass index was negatively associated with knee osteoarthritis in females (OR: 0.51, 95% CI: 0.29–0.88) but not in males (OR: 0.74, 95% CI: 0.50–1.09) when muscle mass index was regarded as a continuous variable [Citation23]. In the study by Lee et al., it was found that although total muscle mass index was not related to knee osteoarthritis (OR: 0.98, 95% CI: 0.95–1.01), lower limb muscle mass index was negatively associated with knee osteoarthritis (OR: 0.94, 95% CI: 0.90–0.98) [Citation22].
Overall, the cross-sectional evidence supports an association between low muscle mass index and increased risk of knee osteoarthritis. Muscle mass index was inversely associated with knee osteoarthritis, with a specific emphasis on lower limb muscle mass index. Further research is needed to study the effect of muscle mass in distinct anatomical regions on knee osteoarthritis.
3.4. The association between low muscle mass and knee osteoarthritis
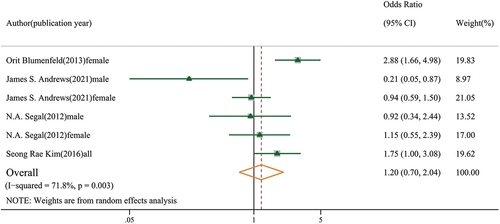
We included four articles on muscle mass and knee osteoarthritis, consisting of two articles on low muscle mass and two articles on high muscle mass. Regarding low muscle mass, the Blumenfeld’s 10-year cohort study showed that decreased lower limb muscle mass was associated with an increased risk of knee osteoarthritis [Citation20]. Conversely, Andrew’s 5-years cohort study found that low muscle mass was associated with a decreased risk of knee osteoarthritis in males [Citation26]. Regarding high muscle mass, Kim et al. found that high muscle mass was negatively associated with knee osteoarthritis, but this relationship shifted after adjusting for BMI [Citation24]. No relationship between high muscle mass and knee osteoarthritis was found in either sex in the study by Segal et al. [Citation25]. After converting the effect size for high muscle mass into that for low muscle mass, no association was found with knee osteoarthritis (OR: 1.20, 95% CI: 0.70–2.04, GRADE = very low) (). In conclusion, we found no sufficient evidence of the relationship between low or high muscle mass and knee osteoarthritis.
3.5. Publication bias and sensitivity analyses
No evidence of publication bias was found during the meta-analysis process (sarcopenia: p = 0.697; sarcopenic obesity: p = 0.622; low muscle mass index: p = 0.574; low muscle mass: p = 0.284). Sensitivity analysis revealed that the pooled OR was not significantly affected by any individual study.
4. Discussion
A total of 12 articles were included in this systematic review and meta-analysis on the association between muscle mass loss and the risk of knee osteoarthritis. The findings with moderate-quality evidence indicated that sarcopenic obesity increases the odds of knee osteoarthritis by approximately 90% in females, but not in males. Low muscle mass index (defined as muscle mass divided by weight falling below the mean or lowest quartile value for sex-stratified reference population) also increases the risk of knee osteoarthritis by approximately 40%. This relationship was more pronounced in lower limb skeletal muscles compared to four limb skeletal muscles. However, no association was found between sarcopenia, low muscle mass and knee osteoarthritis.
Our findings demonstrated that a lower muscle mass index is associated with an increased risk of knee osteoarthritis. Several studies that examined muscle mass index as a continuous variable, have shown that the risk of knee osteoarthritis decreases as muscle mass index increases. Muscle mass index (defined as the value of the appendicular skeletal muscle mass of four limbs divided by the square of height or weight) provides a comparative gauge of individual skeletal muscle mass [Citation8]. However, in the four studies we included regarding the association between muscle mass and knee osteoarthritis, muscle mass was calculated as the summation of the appendicular skeletal muscle mass of limbs, representing the absolute level of individual muscle mass. Among these four studies, three studies adjusted for weight in the analysis [Citation21,Citation24,Citation25], while one did not [Citation20], potentially leading to inconsistent results. It has been established that overweight or obesity is one of the risk factors for knee osteoarthritis. Increased body weight places a greater load on the knee joint, exceeding its physiological capabilities and making it more susceptible to injury [Citation27]. Kim et al. discovered that high muscle mass was negatively associated with knee osteoarthritis. However, this relationship shifted after adjusting for BMI [Citation24]. Therefore, weight is a confounding factor that needs to be considered in studies to examine the association between muscle mass loss and knee osteoarthritis.
Three articles were included in this study that evaluated the effect of sarcopenia and sarcopenic obesity on knee osteoarthritis concurrently. Sarcopenic obesity refers to the co-occurrence of muscle mass loss and obesity in individuals [Citation12,Citation13,Citation15]. The results showed that sarcopenic obesity increased the risk of knee osteoarthritis, whereas no significant correlation was observed for sarcopenia alone. The association between obesity and knee osteoarthritis has been extensively established [Citation4]. Adipose tissue secretes various adipokines, including leptin, adiponectin, resistin, and visfatin, which can lead to adipocyte infiltration and muscle inflammation, leading to decreased muscle mass and articular cartilage breakdwon [Citation28]. Together with obesity, sarcopenia can activate multiple pathophysiological pathways and is more closely associated with knee osteoarthritis, as shown in our results.
In a four-year cohort study, the author used the EWGSOP criteria to define sarcopenia, which encompasses not only muscle mass loss (SMI <7.0 kg/m2 for men and <5.5 kg/m2 for women) but also low muscle strength and poor physical performance [Citation14]. The results showed that sarcopenia increased the risk of symptomatic knee osteoarthritis. However, most studies on sarcopenia solely focus on low muscle mass, overlooking the importance of muscle strength and physical performance. In addition, four institutions (the Asian Working Group for Sarcopenia [Citation29], the European Working Group on Sarcopenia in Older People [Citation8], the International Working Group on Sarcopenia [Citation30], and the Foundation for the National Institutes of Health Sarcopenia Project [Citation31]) have formulated different diagnostic criteria and cutoff values for sarcopenia, posing chanllenges in analyzing the association between sarcopenia and knee osteoarthritis.
We also found that the decline in lower limb muscle mass appears to be a more reliable indicator for knee osteoarthritis compared to the total muscle mass in all four limbs. This is likely because knee osteoarthritis is directly affected by the muscles in the lower limb. Therefore, it is necessary to further analyze the impact of skeletal muscle in different body areas on specific joints. Simply summing the muscle mass of the four limbs to evaluate the effect of muscle mass loss on specific joints may result in negative results. Additionally, there is a lack of studies investigating the correlation between skeletal muscle mass and osteoarthritis in the hip and lumbar spine.
Currently, an increasing number of studies support the association between joint health and the adjacent skeletal muscle. However, there is still a need for relatively more research on the molecular mechanisms involved. A former study showed that myokines, peptides, and growth factors interact with structures such as synovial tissue, fat, cartilage, and bone at a molecular level, through a paracrine mechanism. These molecules also have autocrine and endocrine functions and thus may affect the signaling cascades implicated in knee osteoarthritis [Citation32].
Nonetheless, this study has several limitations. Firstly, a majority of the articles included had a cross-sectional design, which limited the analysis of causal inference on the effect of muscle mass loss on knee osteoarthritis. Reverse causality was observed, as lower muscle mass might be related to the long-term movement difficulties caused by knee osteoarthritis [Citation33,Citation34]. Secondly, the included articles were mainly conducted in Asian countries, with only half of them coming from Europe or America. Therefore, it may be challenging to generalize our results to different populations. Thirdly, there was no unified definition of muscle loss and the measurement of muscle mass. To address this issue, subgroup analyses were conducted based on different methodologies for defining muscle loss. Moreover, the evaluation of muscle should not be limited to muscle mass alone, muscle strength and physical performance should also be taken into consideration. Fourthly, the primary outcome variable used in this study was radiographic osteoarthritis, while arthritis symptoms such as motion pain, swelling, or joint stiffness were not confirmed.
5. Conclusions
In this systematic review and meta-analysis of 12 studies, we demonstrated a significant association between low muscle mass index, sarcopenic obesity and knee osteoarthritis. However, more studies are needed to validate these estimates across different sexes and body parts. In addition, further exploration of the molecular mechanism between skeletal muscle and knee osteoarthritis is needed to provide evidence for clinical diagnosis and treatment.
Supplemental Material
Download MS Word (215.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2024.2352393
Additional information
Funding
References
- Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–415. doi: 10.1016/S0140-6736(14)60802-3
- Long H, Liu Q, Yin H, et al. Prevalence trends of site‐specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol. 2022;74(7):1172–1183. doi: 10.1002/art.42089
- Jang S, Lee K, Ju JH. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int J Mol Sci. 2021;22(5):2619. doi: 10.3390/ijms22052619
- Kulkarni K, Karssiens T, Kumar V, et al. Obesity and osteoarthritis. Maturitas. 2016;89:22–28. doi: 10.1016/j.maturitas.2016.04.006
- Palazzo C, Nguyen C, Lefevre-Colau M-M, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. doi: 10.1016/j.rehab.2016.01.006
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9
- Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabol. 2023;144:155533. doi: 10.1016/j.metabol.2023.155533
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059
- Øiestad BE, Juhl CB, Culvenor AG, et al. Knee extensor muscle weakness is a risk factor for the development of knee osteoarthritis: an updated systematic review and meta-analysis including 46 819 men and women. Br J Sports Med. 2022;56(6):349–355. doi: 10.1136/bjsports-2021-104861
- Pegreffi F, Balestra A, De Lucia O, et al. Prevalence of sarcopenia in knee osteoarthritis: a systematic review and meta-analysis. JCM. 2023;12(4):1532. doi: 10.3390/jcm12041532
- Lee S, Kim T-N, Kim S-H. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum. 2012;64(12):3947–3954. doi: 10.1002/art.37696
- Misra D, Fielding RA, Felson DT, et al. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol. 2019;71(2):232–237. doi: 10.1002/art.40692
- Veronese N, Stefanac S, Koyanagi A, et al. Lower limb muscle strength and muscle mass are associated with incident symptomatic knee osteoarthritis: a longitudinal cohort study. Front Endocrinol. 2021;12:804560. doi: 10.3389/fendo.2021.804560
- Jin WS, Choi EJ, Lee SY, et al. Relationships among obesity, sarcopenia, and osteoarthritis in the elderly. JOMES. 2017;26(1):36–44. doi: 10.7570/jomes.2017.26.1.36
- Zeng X, Zhang Y, Kwong JSW, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evidence Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015
- Jeon H, Lee S-U, Lim J-Y, et al. Low skeletal muscle mass and radiographic osteoarthritis in knee, hip, and lumbar spine: a cross-sectional study. Aging Clin Exp Res. 2019;31(11):1557–1562. doi: 10.1007/s40520-018-1108-5
- Suh DH, Han KD, Hong JY, et al. Body composition is more closely related to the development of knee osteoarthritis in women than men: a cross-sectional study using the fifth Korea national health and nutrition examination survey (KNHANES V-1, 2). Osteoarthritis Cartilage. 2016;24(4):605–611. doi: 10.1016/j.joca.2015.10.011
- Blumenfeld O, Williams FMK, Hart DJ, et al. Lower limbs composition and radiographic knee osteoarthritis (RKOA) in Chingford sample—a longitudinal study. Archives Gerontol Geriatr. 2013;56(1):148–154. doi: 10.1016/j.archger.2012.09.006
- Andrews JS, Gold LS, Nevitt M, et al. Appendicular lean mass, grip strength, and the development of knee osteoarthritis and knee pain among older adults. ACR Open Rheumatol. 2021;3(8):566–572. doi: 10.1002/acr2.11302
- Lee SY, Ro HJ, Chung SG, et al. Low Skeletal Muscle Mass in the Lower Limbs Is Independently Associated to Knee Osteoarthritis.PLoS One. 2016;11(11):e0166385. doi:10.1371/journal.pone.0166385
- Visser AW, de Mutsert R, Loef M, et al. The role of fat mass and skeletal muscle mass in knee osteoarthritis is different for men and women: the NEO study. Osteoarthritis Cartilage. 2014;22(2):197–202. doi: 10.1016/j.joca.2013.12.002
- Kim SR, Choi K-H, Jung G-U, et al. Associations between fat mass, lean mass, and knee osteoarthritis: the fifth Korean national health and nutrition examination survey (KNHANES V). Calcif Tissue Int. 2016;99(6):598–607. doi: 10.1007/s00223-016-0190-y
- Segal NA, Findlay C, Wang K, et al. The longitudinal relationship between thigh muscle mass and the development of knee osteoarthritis. Osteoarthritis Cartilage. 2012;20(12):1534–1540. doi: 10.1016/j.joca.2012.08.019
- Andrews JS, Gold LS, Nevitt M, et al. Appendicular lean mass, grip strength, and the development of knee osteoarthritis and knee pain among older adults. ACR Open Rheuma. 2021;3(8):566–572. doi: 10.1002/acr2.11302
- Georgiev T, Angelov AK. Modifiable risk factors in knee osteoarthritis: treatment implications. Rheumatol Int. 2019;39(7):1145–1157. doi: 10.1007/s00296-019-04290-z
- Belluzzi E, El Hadi H, Granzotto M, et al. Systemic and local adipose tissue in knee osteoarthritis. J Cell Physiol. 2017;232(8):1971–1978. doi: 10.1002/jcp.25716
- Chen L-K, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012
- Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003
- Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A. 2014;69(5):547–558. doi: 10.1093/gerona/glu010
- Krishnasamy P, Hall M, Robbins SR. The role of skeletal muscle in the pathophysiology and management of knee osteoarthritis. Rheumatol. 2018;57(suppl_4):iv22–33. doi: 10.1093/rheumatology/kex515
- Karlsson MK, Magnusson H, Cöster M, et al. Patients with knee osteoarthritis have a phenotype with higher bone mass, higher fat mass, and lower lean body mass. Clin Orthop Relat Res. 2015;473(1):258–264. doi: 10.1007/s11999-014-3973-3
- Dhannakulsakti P, Roopsawang I, Aree-Ue S. Sarcopenia among older adults with knee osteoarthritis: a cross-sectional study of prevalence and its associated factors. Pac Rim Int J Nurs Res. 2022;26:121–134.