ABSTRACT
Background
In the realm of sports science, nutrition is a well-established pillar for athletes’ training, performance, and post-workout recovery. However, the role of gut microbiota, often overlooked, is a novel and intriguing aspect that can significantly impact athletic performance. With this in mind, our study ventures into uncharted territory, investigating the effect of probiotic and casein supplementation on the aerobic capacity of male soccer players.
Method
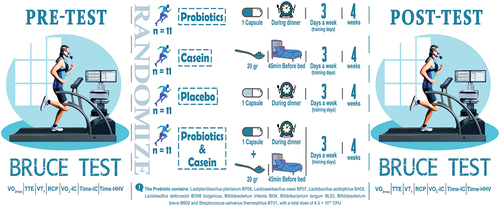
A double-blinded and placebo-controlled study was conducted with 44 male soccer players (Age: 22.81 ± 2.76 yr, Height: 177.90 ± 6.75 cm, Weight: 67.42 ± 8.44 kg). The participants were subjected to the Bruce test in the beginning; then, they were randomly divided into four groups, each consisting of 11 people: probiotics (PRO), casein (CAS), probiotics with casein (PRO+CAS), and placebo (PLA). PRO group was given one probiotic capsule (containing strains of Lactiplantibacillus plantarum BP06, Lacticaseibacillus casei BP07, Lactobacillus acidophilus BA05, Lactobacillus delbrueckii BD08 bulgaricus, Bifidobacterium infantis BI04, Bifidobacterium longum BL03, Bifidobacterium breve BB02 and Streptococcus salivarius thermophilus BT01, with a total dose of 4.5 × 1011 CFU) during dinner, while the CAS group consumed 20 grams of casein powder 45 minutes before bed. The PRO+CAS group was given one probiotic capsule during dinner and 20 grams of casein powder 45 minutes before bed. The participants in the PLA group were given one red capsule (containing 5 grams of starch) during dinner. All participants were instructed to take the supplements only on training days, three times a week for four weeks. The maximal oxygen consumption (VO2max), Ventilatory Threshold (VT), Time-to-exhaustion (TTE), Respiratory Compensation Point (RCP), Isocapnic area Time (Time-IC), Isocapnic area oxygen consumption (VO2-IC), and Hypocapnic Hyperventilation area Time (Time-HHV), after the Bruce test were Measured. All data were analyzed using SPSS Windows software, mixed repeated measure ANOVA, and Bonferroni post hoc test at p < 0.05 level.
Results
The current study’s findings illustrated that, after the intervention, TTE (p = 0.01) and RCP (p = 0.01) were significantly improved in PRO+CAS compared to the PLA group. No significant difference was observed between PRO and PLA (p = 0.52), PRO and CAS (p = 0.999), PRO and PRO+CAS (p = 0.9), CAS and PLA (p = 0.65), CAS and PRO+CAS (p = 0.73) in TTE. In addition, no significant difference was observed between PRO and CAS (p = 0.999), PRO and PLA (p = 0.40), PRO and PRO+CAS (p = 0.999), CAS and PLA (p = 0.263), CAS and PRO+CAS (p = 0.999) in RCP. Time-HHV was significantly higher in PRO+CAS (p = 0.000) and CAS (p = 0.047) compared to the PLA group. However, no significant difference was observed in the Time-HHV between PRO and CAS (p = 0.999), PRO and PRO+CAS (p = 0.25), PRO and PLA (p = 0.12), and CAS and PRO+CAS (p = 0.57). Additionally, all the groups had no significant differences in VO2max, VT1, VO2-IC and Time-IC.
Conclusion
The findings showed that consuming probiotics and casein could relatively improve the aerobic capacity of male soccer players. Nevertheless, simultaneous consumption of probiotics and casein had a more pronounced effect on aerobic capacity indicators, especially TTE and Time-HHV.
1. Introduction
With the increasing popularity of soccer among people, exercise science and sports nutrition science entered this profession, and today, all coaches and athletes have started using the latest scientific materials in these fields to improve players’ individual and team performance [Citation1]. Performing intense training, competitions lasting more than 90 minutes and sometimes up to 120 minutes, busy travel schedules, sleep disorders, and physical and mental pressures indicate the importance of energy and nutrients in this sport. [Citation1]. Depression in soccer players with excessive training load, psychological stress, disturbed sleep, and more can all contribute to the increased risk of gastrointestinal infections. Certain conditions, including exposure to overcrowding, foreign travel, and poor hygiene at home and training or competition venues, may expose these players to pathogens and increase infection rates [Citation2]. Approximately 70% of the human immune system resides in the gut, and it has been shown that probiotic supplements can induce an immune response in the body [Citation2].
Gut microbiota consists of microorganisms that live in the digestive tract, and their estimated number is more than 1014 cells [Citation3]. The microbiota genome is 150 times larger than the human genome, about 10 times larger than bacterial cells [Citation3]. Biodiversity and the overall composition of the intestinal microbiota are essential in maintaining natural homeostasis in the human body [Citation2]. Bacteria are the most abundant population of intestinal microbiota, with more than 1000 different species of bacteria observed [Citation2,Citation3]. Probiotics are living microorganisms that, if present in sufficient quantities in the human intestine, are beneficial for the host’s health, which is very effective for an athlete’s performance [Citation2]. In addition to maintaining gut health, probiotics can increase the absorption of critical nutrients, such as amino acids from proteins [Citation2]. Also, based on the research, supplementing with multi-strain probiotics increases VO2max, aerobic power, training load tolerance, and time to reach fatigue [Citation2]. These indicators are directly related to aerobic performance. In addition, in response to muscle-damaging resistance exercises, probiotic supplements with protein can accelerate recovery and reduce pain and other indicators of musculoskeletal injury [Citation2].
In a four-week study on seven endurance runners who used the probiotic supplement with Lactobacillus salivarius strains, no increase in their performance was observed [Citation4]. However, in 30 endurance athletes who consumed a yogurt drink containing Streptococcus thermophilus or Lactobacillus delbrueckii bulgaricus for 30 days during intense aerobic exercise, a significant increase in VO2max and aerobic power in the Cooper aerobic test was reported [Citation5]. Another study showed that 14 weeks of multi-strain probiotic supplementation did not affect VO2max and maximal performance in endurance-trained men [Citation6]. Still, on the contrary, Huang et al. 2018 found an increase in endurance performance and blood glucose concentration following exercise until exhaustion after six weeks of a high dose of 1 × 1011 Lactobacillus plantarum in healthy adult men [Citation7].
On the other hand, the issue of macronutrient consumption in soccer players has been studied for years, and different recommendations have been provided, both in terms of the type of macronutrient consumed and its dose and amount [Citation8]. The nature of soccer shows that due to the high and irregular running of the soccer player (sometimes up to 13 kilometers) and sudden and fast runs, the aerobic and anaerobic systems are both very widely important in this sport (aerobic energy production appears to account for more than 90% of total energy consumption); however, anaerobic energy production plays an essential role during soccer matches too [Citation9], and this indicates the importance of muscle glycogen reserves [Citation10]. High-carbohydrate diets can supply this muscle glycogen, delaying fatigue and improving soccer player performance [Citation10]. Nevertheless, the importance of protein consumption by soccer players cannot be ignored. There is a general belief that extra protein in soccer players’ diets increases strength, recovery speed, endurance, and non-endurance performance [Citation10]. Several studies have reported that consuming casein protein before sleep can positively affect recovery after physical activity [Citation11,Citation12]. A study on healthy young men showed that 40 grams of casein protein, consumed 30 minutes before sleep after resistance training, is well-digested and absorbed during sleep. In addition, the amino acid level in their blood circulation increases rapidly [Citation12]. It leads to an increase in the rate of protein synthesis in the whole body and an improvement in protein balance, which causes positive effects on muscle recovery [Citation12]. Also, a study involving English soccer players showed that consuming 40 grams of casein protein 30 minutes before sleep after an official match positively affected the recovery of the strength index 12 and 36 hours after the match [Citation11]. Additionally, Salehzadeh et al. in 2015, during a 4-week study on the effects of drinking probiotic yogurt on changes in fat profile, C-reactive protein (CRP), and aerobic fitness in athletes, showed that the VO2max of endurance athletes and aerobic capacity had increased significantly. Regarding digestion and immunity, it significantly decreased serum CRP and increased High-Density Lipoprotein (HDL) [Citation5]. Also, in an 8-week study, Salarkia et al., in 2013, investigated the effect of probiotic yogurt on female endurance swimmers’ performance and respiratory and digestive systems [Citation13]. The findings indicated that in terms of performance, the VO2max of the swimmers had improved significantly, but it had no effect on the swimming duration. It significantly reduced respiratory and ear infections in terms of immunity and digestion. Therefore, it is expected that if soccer players consider consuming casein protein before bed after a match or a training session, they can prepare for the next game or practice with less fear of performance impairment [Citation14].
According to the above, probiotic consumption can increase mitochondrial biogenesis, angiogenesis, and amino acid absorption [Citation3]. Additionally, physiological fatigue, such as extreme fatigue after exercise, is accompanied by poor athletic performance and loss of favorable working conditions for tissues [Citation15]. In response to higher-intensity exercise, the concentration of lactate and hydrogen ions increased markedly, resulting in acidification in muscle and subsequent fatigue [Citation16,Citation17]. Approximately 75% of the total lactate produced is used for oxidative energy production in the exercising body and can be utilized for the de novo synthesis of glucose in the liver [Citation18]. Probiotic supplementation may remove and use blood lactate after exercise [Citation19]. For instance, most Lactobacillus species produce lactic acid, which could facilitate butyrate production by lactate-utilizing bacteria that first produce acetyl-CoA from lactate [Citation19]. On the other hand, casein protein, which is a slow-absorbing protein, improves the recovery speed of soccer players and thus enhances their performance, including endurance performance, by delaying fatigue [Citation11,Citation12]. Altogether, more studies on probiotic supplementation are needed to draw a definite conclusion about their effect on the aerobic performance of athletes. Also, very few studies have investigated the impact of simultaneous consumption of probiotics and casein protein on the aerobic performance of soccer players, and the results obtained from these few studies are also contradictory to those mentioned before.
According to the mentioned materials, it seems that probiotics can affect the endurance performance of soccer players such primary ventilatory threshold (VT1) and respiratory compensation point (RCP), which is considered the Isocapnic area (IC), by reducing lactate secretion, increasing buffering capacity and increasing oxygen consumption [Citation2]. Also, probiotics help improve soccer players’ performance by postponing fatigue and enhancing time to exhaustion (TTE) [Citation19]. These changes in RCP and TTE increase the hypocapnic hyperventilation (HHV) area period. Additionally, it is assumed that the consumption of probiotic supplements with casein protein can affect the endurance performance of soccer players. They are considering the contradictory and limited study on the effects of probiotics and casein protein supplementation on the aerobic capacity indicators of soccer players. Therefore, the current study aimed to investigate the effect of probiotic and casein supplementation on the endurance performance of male soccer players.
2. Methods
2.1. Participants
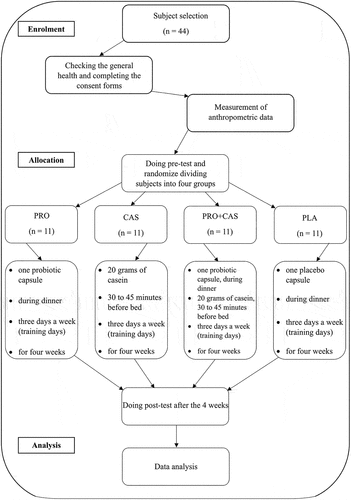
In this study, they participated in 44 male soccer players with approximately 3 years of Iran Soccer League experience. The demographic information of the participants is listed in . Participants had no known diseases or medical issues, no history of allergy to probiotics and casein protein, and were not consuming any supplements or medications. Furthermore, the participants did not smoke or drink alcohol or caffeinated beverages during data collection. According to the above conditions, all 44 participants were selected from the soccer players who volunteered to participate in this study. The current study was reviewed and approved by The Human Research Ethics Committee of Shiraz University (ethics approval code: IR.US.REC.1402.005, 2022) and carried out in accordance with the Declaration of Helsinki.
Table 1. The anthropometric data of participations.
2.2. Sample size calculation
The number of participants in this study was determined based on the study by Huang et al.. (2019), according to which probiotic supplementation led to a significant improvement in endurance performance at 85 % VO2max (effect size = 1.01) compared to placebo [Citation20]. Using G*Power 3.1, considering the confidence interval of 95% and the analysis power of 0.80, it was found that at least 26 participants are needed for this study (13 participants for each group). Therefore, 44 participants were selected for the current study (11 participants for each group).
2.3. Study design
This research was a randomized, double-blinded, placebo-controlled study. After the players had their general health checked by a doctor and completed the PAR-Q test, they filled out a food record questionnaire to report their food and supplement intake that might affect their gut microbiota. They also signed a consent form to participate in the research, which included detailed information about the study’s procedures, benefits, risks, and potential complications. During this visit, they familiarized themselves with the equipment and received instructions on performing the Bruce test. Over three days, the players visited the laboratory at Shiraz University. After warming up, they did the Bruce test on the treadmill (h/p/cosmos, Sports & Medical GMBH). Also, their aerobic capacity and endurance performance (maximum oxygen consumption (VO2max), time to exhaustion (TTE), primary ventilatory threshold (VT1), respiratory compensation point (RCP), isocapnic area time (Time-IC), isocapnic area oxygen consumption (VO2-IC), and hypocapnic hyperventilation area time (Time-HHV)) measured by a Respiratory Gas Analyzer device of Cortex company (METALYZER 3B, Germany). Then, participants were randomly divided into four groups of 11 people: probiotics (PRO), casein (CAS), probiotics with casein (PRO+CAS), and placebo (PLA) (). It should be noted that researchers were blinded to the conditions of each participant in the current study by an expert using shuffling cards. In this method, the expert assigned the participants to four groups with codes 1, 2, 3, and 4. The researchers were unaware of which group was assigned to PLA, CAS, PRO+CAS, or PRO. All participants were informed that they were taking a safe dose of casein or probiotics but did not know what they consumed. Each group received its supplement and was taught how to maintain and consume it. After four weeks, the subjects did the Bruce test again, and the Respiratory Gas Analyzer device re-recorded all the data. All the participants in the study were members of the same training camp, and they followed the same training regimen under the supervision of trainers. The procedures followed in the present study can be seen in .
2.4. Kinetic oxygen conception parameters
Ventilatory Threshold (VT): The VT was visually determined using the modified V-slope method as described by Sue et al. [Citation21], which is a modification of the method described by Beaver et al. [Citation22]. The ventilatory equivalent method (the point at which ventilatory equivalent for oxygen (VE/VO2) begins to rise without an increase in ventilatory equivalent for carbon dioxide (VE/VCO2)) and end-tidal methods (partial pressure of end-tidal oxygen tension (PetO2) starts to grow without a decrease in partial pressure of end-tidal carbon dioxide (PetCO2)) was used as a complement [Citation23] ().
Figure 3. Determination of isocapnic (IC) and hypocapnic hyperventilation (HHV) areas in one of the participants.
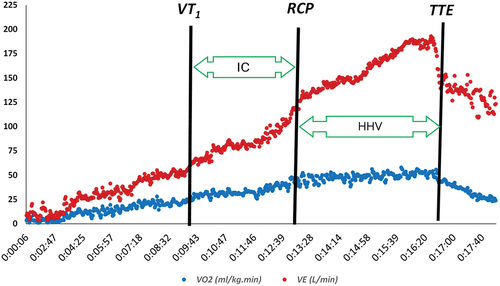
Respiratory Compensation Point (RCP): The respiratory compensation point was comprehensively determined from the point where PetCO2 decreased, VE/VCO2 began to increase, and the inflection point of the VE/VCO2 slope [Citation24] ().
Isoapnic area (IC): In this study, the distance between VT1 and RCP was considered as the Isocapnic area [Citation25,Citation26] (). So, Time-IC was the time of VT1 till RCP, and VO2-IC was VO2 at RCP minus VO2 at VT1.
Hypocapnic hyperventilation (HHV): In this study, the distance between RCP and TTE was considered as the HHV area [Citation25,Citation26] (). So, Time-HHV was the time of RCP till TTE.
2.5. Training protocol
All participants were members of the same training camp (Fajr Shahid Sepasi – Shiraz – Iran), and their training regime was the same under the supervision of trainers. All subjects participated in the following training program: 5 training sessions of 90 min per week, including 10 min of warm-up, 20 min of physical training (Core stability, Speed, Agility, and Quickness), 10 min of technical training, 20 min of tactical training, 25 min of the training game, and at the end there was cooling for 5 min. Strength and power training occurred once per week as part of team training. It consisted of a combination of plyometric (single leg hops, drop jump, box jump, squat jump: 3 sets × 8 repetitions for each) and resistance exercises (3–4 sets, 10–12 repetitions, 75–80% of a maximum repetition). The training program’s type, intensity, load and duration were similar for all participants. Also, one day a week was dedicated to friendly matches, in which each participant played for approximately 10–15 minutes.
2.6. Supplementation protocol
The PRO group was instructed to consume one probiotic capsule (Comflor probiotic supplement-Farabiotic company- Iran- containing strains of Lactiplantibacillus plantarum BP06, Lacticaseibacillus casei BP07, Lactobacillus acidophilus BA05, Lactobacillus delbrueckii BD08 bulgaricus, Bifidobacterium infantis BI04, Bifidobacterium longum BL03, Bifidobacterium breve BB02 and Streptococcus salivarius thermophilus BT01, totally 200 mg in the form of capsule) with a total dose of 4.5 × 1011 CFU during dinner () [Citation27]. Also, the CAS group consumed 20 grams of casein powder (casein pro supplement of EuRho vital company, Germany, 76.8% pure protein per serving) 45 minutes before bed [Citation28]. The PRO+CAS group was instructed to consume one probiotic capsule during dinner and 20 grams of casein powder 45 minutes before bed, and the PLA group participants were instructed to consume one red capsule (containing 5 grams of starch) during dinner. The red color of the placebo capsules was used to create a similarity with the red capsules of the Comflor probiotic supplement. Furthermore, all participants were instructed to take the supplements only on training days, three times a week for four weeks.
Table 2. Strains and dosage per one capsule (200 mg) of the probiotics which used in the present study.
2.7. Bruce protocol
Bruce’s test consists of seven stages, each lasting 3 minutes, and in each stage, in addition to increasing the speed of the treadmill, also added %2 to its slope. How to increase the speed and incline of the treadmill in the Bruce test is as follows: first stage: speed of 2.7 km/h and %10 slopes; second stage: speed of 4 km/h and %12 slopes; third stage: speed of 5.5 km/h and %14 slopes, fourth stage: speed of 6.8 km/h and %16 slopes, fifth stage: speed of 8 km/h and %18 slopes, sixth stage: speed of 8.8 km/h and %20 slopes, and seventh stage: speed of 9.6 km/h and %22 slopes [Citation29]. Additionally, before beginning the test, the first 3 minutes were for respiratory gas checking without movement. After the final stage, the last 3 minutes were for recovery with a 2.7 km/h speed without slope.
2.8. Statistical analyses
All data were analyzed using descriptive and inferential statistical methods. The data distribution normality was determined using the Kolmogorov-Smirnov test. Mixed repeated measure analysis ANOVA test was used to determine the main effect of pretest and posttest on the indicators, and Bonferroni post hoc test was used to determine pairwise differences. Data were analyzed using SPSS software (version 26, IBM-SPSS Inc., Chicago, IL, USA). The level of statistical significance was p ≤ 0.05.
3. Results
Descriptive characteristics (including mean and standard deviation) are reported in . Also, the mean and standard deviation of the measured variables (VO2max, TTE, VT1, RCP, VO2-IC, Time-IC, Time-HHV) and the mixed repeated measure ANOVA test analysis results are reported in .
Table 3. Mean, standard deviation (SD) and ANOVA results of the measured variables.
The results indicate that there was a significant main effect of time on VO2max [F = 24.7, p < 0.001], TTE [F = 33.4, p < 0.001], VT1 [F = 10.2, p = 0.003], RCP [F = 46.4, p < 0.001], VO2-IC [F = 10.4, p = 0.003], and Time-HHV [F = 4.3, p = 0.04]. However, the main effect of time was not significant for Time-IC (p > 0.05) (as shown in ). Moreover, the main effect of interaction (time × group) was significant for TTE [F = 5.3, p = 0.004], RCP [F = 7.2, p = 0.001] and Time-HHV [F = 6.5, p = 0.001]. Additionally, the main effect of the group was significant for Time-HHV [F = 3.7, p = 0.02] but not for VO2max, TTE, VT1, RCP, VO2-IC, and Time-IC (p > 0.05) (as shown in ). There was insignificant interaction observed for VO2max, VT1, RCP, VO2-IC and Time-IC (p > 0.05) (as shown in ).
Post-hoc tests showed that the VO2max of PRO (MD = 2.20, p = 0.001, 95% CI [0.98–3.38]), CAS (MD = 1.46, p = 0.027, 95% CI [0.16–2.56]) and PRO+CAS (MD = 2.21, p = 0.001, 95% CI [0.98–3.38]) increased significantly compared to baseline after supplementation, while the PLA group did not show significant changes (MD = 0.27, p = 0.761, 95% CI [−1.01–1.38]) (). TTE showed a significant increase in the PRO (MD = 1.05, p = 0.000, 95% CI [0.57–1.52]) and PRO+CAS (MD = 1.20, p = 0.000, 95% CI [0.72–1.68]) compared to baseline, while CAS (MD = 0.44, p = 0.065, 95% CI [−0.02–0.92]) and PLA (MD = 0.03, p = 0.899, 95% CI [−0.44–0.51]) had no significant changes (). Compared to baseline, VT1 showed a significant increase in PRO (MD = 1.32, p = 0.011, 95% CI [0.32–2.32]) and PRO+CAS (MD = 1.57, p = 0.003, 95% CI [0.57–2.57]), while no significant changes were observed for CAS (MD = 0.40, p = 0.414, 95% CI [−0.59–1.41]) and PLA (MD = 0.14, p = 0.771, 95% CI [−1.14–0.85]) (). The RCP showed a significant increase in PRO (MD = 2.10, p = 0.001, 95% CI [0.93–3.26]), CAS (MD = 2.50, p = 0.000, 95% CI [1.34–3.67]) and PRO+CAS (MD = 3.43, p = 0.000, 95% CI [2.27–4.60]) compared to baseline, while no significant changes were observed in the PLA group (MD = −0.2, p = 0.730, 95% CI [−1.36–0.96]) (). VO2-IC showed a significant increase in CAS (MD = 2.10, p = 0.006, 95% CI [0.63–3.56]) and PRO+CAS (MD = 1.86, p = 0.014, 95% CI [0.39–3.33]) compared to baseline, while there were no significant changes in the PLA (MD = −0.05, p = 0.940, 95% CI [−1.52–1.41]) and PRO (MD = 0.77, p = 0.294, 95% CI [−0.69–2.24]) (). Time-IC had no significant changes in PRO (MD = 2.72, p = 0.640, 95% CI [−8.98–14.43]), CAS (MD = 1.36, p = 0.815, 95% CI [−10.34–13.07]), PRO+CAS (MD = 11.09, p = 0.063, 95% CI [−0.62–22.80]) and PLA (MD = 4.36, p = 0.456, 95% CI [−7.34–16.07]) compared to baseline (). Although Time-HHV illustrated a significant decrease in the PLA group (MD = −14.6, p = 0.023, 95% CI [−27.10 - −2.17]), a significant increase was observed in PRO+CAS supplementation compared to the baseline (MD = 22.90, p = 0.001, 95% CI [10.44–35.37]); however, PRO (MD = 5.81, p = 0.351, 95% CI [−6.64–18.28]) and CAS (MD = 11.63, p = 0.067, 95% CI [−0.83–24.10]) had no significant changes (). All of these results are reported in .
Figure 4. Means and standard deviation (SD) of VO2max, TTE, VT1 and RCP in pretest and posttest. PRO: probiotics, CAS: casein, PLA: placebo.
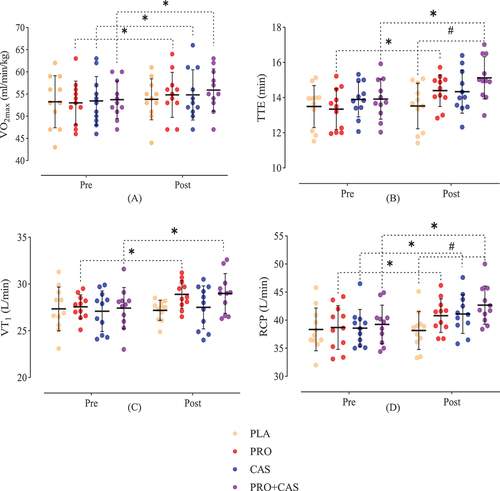
Figure 5. Means and standard deviation (SD) of VO2-IC, time-IC and time-HHV in pretest and posttest. PRO: probiotics, CAS: casein, PLA: placebo.
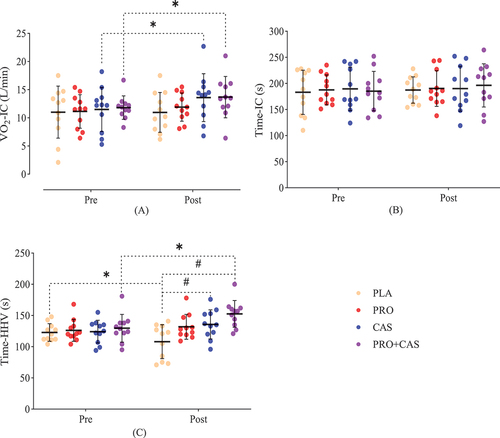
Table 4. Comparison of posttest and pretest variables data in the four groups.
In addition, the results of the Bonferroni test showed that after the intervention, TTE (MD = 1.60, p = 0.01, 95% CI [0.22–2.96]) and RCP (MD = 4.51, p = 0.01, 95% CI [0.60–8.43]) were significantly improved in PRO+CAS compared to the PLA group (). However, no significant difference was observed between PRO and PLA (MD = 0.87, p = 0.52, 95% CI [−0.50–2.24]), PRO and CAS (MD = 0.05, p = 0.999, 95% CI [−1.32–1.43]), PRO and PRO+CAS (MD = −0.72, p = 0.9, 95% CI [−2.10–0.64]), CAS and PLA (MD = 0.81, p = 0.65, 95% CI [−0.56–2.19]), CAS and PRO+CAS (MD = 0.78, p = 0.73, 95% CI [−2.16–0.59]) in TTE (). In addition, no significant difference was observed between PRO and CAS (MD = −0.29, p = 0.999, 95% CI [−4.20–3.62]), PRO and PLA (MD = 2.64, p = 0.40, 95% CI [−1.27–6.56]), PRO and PRO+CAS (MD = −1.87, p = 0.999, 95% CI [−5.79–2.04]), CAS and PLA (MD = 2.93, p = 0.263, 95% CI [−0.98–6.85]), CAS and PRO+CAS (MD = −1.58, p = 0.999, 95% CI [−5.49–2.33]) in RCP (). Also, Time-HHV was significantly higher in PRO+CAS (MD = 44.45, p = 0.000, 95% CI [17.08–71.82]) and CAS (MD = 27.63, p = 0.047, 95% CI [0.26–55.00]) compared to PLA group. However, no significant difference was observed in the Time-HHV between PRO and CAS (MD = −3.81, p = 0.999, 95% CI [−31.19–23.55]), PRO and PRO+CAS (MD = 20.63, p = 0.25, 95% CI [−6.73–48.00]), PRO and PLA (MD = 23.81, p = 0.12, 95% CI [−3.55–51.19]), CAS and PRO+CAS (MD = 16.81, p = 0.57, 95% CI [−10.55–44.19]) (). Additionally, there were no significant differences in VO2max, VT1, VO2-IC and Time-IC between groups after the intervention. At baseline, no significant differences were observed between the groups in any of the measured dependent variables (p > 0.05).
4. Discussion
This study aimed to investigate the effect of probiotic and casein supplementation on the endurance performance of male soccer players. Seven indicators were measured and analyzed to investigate the aerobic performance of the soccer players. The results showed that: 1) VO2max was significantly higher than baseline in PRO, CAS, and PRO+CAS. 2) TTE increased significantly in PRO and PRO+CAS compared to their baselines and significantly increased in PRO+CAS compared to PLA after intervention. 3) VT1 significantly increased in PRO and PRO+CAS compared to the baseline. 4) RCP was significantly higher than the baseline in PRO, CAS, and PRO+CAS, and there was a significant increase in PRO+CAS compared to PLA after intervention. 5) VO2-IC increased significantly in CAS and PRO+CAS compared to the baseline. 6) There was no significant increase in Time-IC in all groups of this study. 7) Time-HHV significantly improved in PRO+CAS compared to the baseline; also, there was a significant increase in CAS and PRO+CAS compared to PLA after the intervention.
Some studies are consistent with the present research results regarding these findings [Citation5,Citation7,Citation13]. For example, it has been reported that consuming a yogurt drink containing Streptococcus thermophilus or Lactobacillus delbrueckii bulgaricus for 30 days during intense aerobic exercise can increase VO2max and aerobic power in the Cooper aerobic test of 30 endurance athletes [Citation5]. Also, the investigation by Salarkia et al. showed that the swimmers’ aerobic fitness had improved significantly after eight weeks of probiotic yogurt consumption by teenage female swimmers [Citation13]. In addition, Huang et al. found an increase in endurance performance after six weeks of a high dose of 1 × 1011 Lactiplantibacillus plantarum TWK10 in healthy adult men [Citation7]. However, some other studies have reported that probiotic consumption does not affect athletes’ endurance performance [Citation4,Citation6]. For example, the dose of probiotic supplement used by Lamprecht et al. (1010 CFU) and also the used strains (Bifidobacterium bifidum, Bifidobacterium lactis, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus breve, and Lactococcus lactis), was different compared to the present study. They have shown that multi-strain probiotic supplementation did not affect VO2max and maximal performance in endurance-trained men [Citation6]. In another study, there was a difference compared to this research in the number of participants (seven endurance runners) who took a probiotic supplement, and no increase in their endurance performance was observed [Citation4]. Methodological differences, demographic characteristics of the participants and their numbers, the type of supplements consumed and their dosage, or the duration of the consumption can all be the reasons for the contradictory findings.
Increasing intestinal probiotic content in athletes is associated with reducing blood lipopolysaccharide (LPS) levels, oxidative stress, and inflammation [Citation30–32], which increases the mammalian target of rapamycin (mTOR) and decreases nuclear factor kappa B (NF-KB) [Citation3]. Also, specific probiotic strains have been shown to increase amino acid appearance in the blood [Citation33]. Additionally, probiotic administration increases amino acid absorption from plant protein, too [Citation34]. This contributes to higher muscle glycogen storage, increasing mitochondrial biogenesis and its function, increasing angiogenesis and blood supply, and suppressing anabolic signaling pathways [Citation3,Citation35–37], increasing the athlete’s aerobic capacity. Additionally, probiotics may enable better performance capabilities and training adherence when the risk of upper respiratory tract infection (URTI) development is reduced, as individuals with fewer episodes of infections such as common colds can train more often and more complex [Citation2]. Further, Strasser et al. [Citation38] noted that the multi-species probiotic limited exercise-induced reductions in circulating tryptophan concentration [Citation38]. Higher serum tryptophan levels may enhance the tryptophan transport into the brain and support serotonin metabolism, which can influence an individual’s sensation of fatigue and thus potentially affect training adherence and performance [Citation39]. Interestingly, VO2max was positively correlated with pre-exercise serum tryptophan levels at a moderate magnitude, supporting the role of tryptophan metabolism in training performance [Citation39]. Therefore, it seems that the increase of aerobic capacity indicators, such as VO2max, VT1, and RCP in the PRO group in the current study, can be for these reasons, which, of course, due to not measuring the probiotic content of the participant’s intestines, it is impossible to make a definite comment. In addition, the imposition of an excessive training load and inadequate regeneration periods can lead to physical exhaustion and short-term attenuation of athletic performance. Hence, the adequate restoration of the body’s physiological state post-exercise constitutes a crucial constituent of the training adaptation process [Citation40]. Excessive physical training can lead to tissue damage that elicits an immediate and circumscribed inflammatory response, characterized by an upsurge of several cytokines, predominantly Interleukin-1b (IL-1b), Tumor Necrosis Factor-alpha (TNF-α), interleukin-8 (IL-8), and interleukin-6 (IL-6), to restore the destroyed structures and foster muscle adjustments [Citation41,Citation42]. For this purpose, taking a supplement containing slow-absorbing protein, such as casein, before going to bed can help recover muscle damage [Citation11,Citation12]. Therefore, for these reasons, it is possible to increase the aerobic capacity indicators, such as VO2max and RCP, in the present study’s CAS group. Of course, all these reasons may have caused the improvement in aerobic capacity indicators, such as VO2max, VT1, and RCP in the PRO+CAS group.
Probiotics may affect exercise performance mainly because Lactobacillus bacteria produce lactic acid, which could facilitate butyrate production by lactate-utilizing bacteria that first produce acetyl-CoA from lactate [Citation19]. In the so-called classical pathway, the enzymes phosphotransbutyrylase and butyrate kinase convert butyryl-CoA to butyrate and coenzyme A with the concomitant formation of adenosine triphosphate (ATP) [Citation43]. In addition, the gut microbiota has been proposed to influence muscle metabolism and may constitute a future therapeutic target in managing muscle wasting [Citation43]. Thus, the probiotic and gut microbiota could play essential roles in maintaining normal physiology and energy production during exercise [Citation43]. So, it seems that the increase in VO2-IC in the CAS and PRO+CAS groups and the increase in oxygen consumption in the isocapnic area (VO2-IC) can be because of increasing intestinal probiotic content in athletes, which is associated with delaying lactate secretion, reducing blood LPS levels, oxidative stress and inflammation [Citation30–32], and increasing mTOR and decreasing NF-KB [Citation3], that can make a higher muscle glycogen storage, increase mitochondrial biogenesis and its function, increasing angiogenesis and blood supply, and also, suppression of anabolic signaling pathways [Citation3,Citation35–37].
On the other hand, probiotic supplementation may potentially remove and utilize blood lactate after exercise [Citation44]. For instance, most Lactobacillus species produce lactic acid, which could facilitate butyrate production by lactate-utilizing bacteria that first produce acetyl-CoA from lactate [Citation44]. In the classical pathway, the enzymes phosphotransbutyrylase and butyrate kinase convert butyryl-CoA to butyrate and coenzyme A with concomitant formation of ATP [Citation43]. Thus, probiotics and the gut microbiota could play essential roles in maintaining normal physiology and energy production during exercise [Citation43]. Several animal studies have been conducted with promising results [Citation27,Citation45]. In mice who consumed probiotic kefir daily over four weeks, swimming TTE was significantly longer, forelimb grip strength was higher, and serum lactate, ammonia, blood urea nitrogen (BUN), and creatine kinase levels were lower after the swimming test [Citation45]. In mice supplemented with L. plantarum TWK10 over six weeks, supplementation dose-dependently increased grip strength and endurance swimming time and decreased serum lactate, ammonia, creatine kinase, and glucose levels after an acute exercise challenge [Citation27]. Furthermore, the number of types I fibers in the gastrocnemius muscle significantly increased with LP10 treatment [Citation27]. In a six-week human double-blind placebo-controlled clinical study, young, healthy amateur runners supplemented with L. plantarum TWK10 underwent exhaustive treadmill exercise measurements and related biochemical indexes. The TWK10 group had significantly higher endurance performance and glucose content in a maximal treadmill running test than the placebo group, indicating that TWK10 supplementation may benefit energy harvest [Citation7]. Together, these studies suggest that certain probiotics may enhance energy harvesting and have health promotion, performance improvement, and anti-fatigue effects. It may impair sports performance through endocrine or immune system disorders, insufficient muscle glycogen storage, and microbiome imbalance [Citation46]. Certain probiotic strains (Streptococcus thermophilus FP4 and Bifidobacterium breve BR03) have antioxidant and anti-inflammatory properties, improving exercise recovery [Citation47]. In addition, as mentioned before, the consumption of protein supplements can be adequate for the recovery and improvement of an athlete’s performance [Citation41,Citation42]. Therefore, it seems that the abovementioned reasons caused an increase in TTE in the PRO and PRO+CAS groups of the present study. Additionally, with the rise of TTE, the time interval in the HHV area (Time-HHV) naturally increases. In addition to the reasons above, this improvement in Time-HHV in PRO+CAS groups can be because probiotics can improve exercise performance by delaying fatigue in athletes by producing short-chain fatty acids (SCFAs). Furthermore, species within the Lactobacillus genus synthesize lactic acid, converted to butyrate and later to acetyl-CoA, used in the Krebs Cycle to generate adenosine triphosphate (ATP) [Citation48]. Also, we had no significant increase in Time-IC in all the study groups. Although Time-IC increased in PRO, CAS, and PRO+CAS groups, it was insignificant. It seems that if the number of participants was more or if the duration of the intervention was longer, these changes could be significant. By the way, it seems that increasing the time to reach the accumulation of lactate and the delay in its secretion makes it possible to increase the Time-IC and the duration of the athlete’s activity in this area.
It is essential to acknowledge the limitations of our investigation. Firstly, we could not measure the probiotic content of the participants’ intestines due to financial limitations. Additionally, we could not create a placebo group for the casein protein due to a lack of participants from the same team. It is important to note that our study only involved male soccer players, so our findings should be used with caution when applied to other athletes. Lastly, we could not measure the participants’ lactate response during the Bruce test due to our lab’s lack of a lactometer. Therefore, in future studies, it is important to measure the gut’s probiotic content, consider a placebo group for casein, and measure the participants’ lactate response to the Bruce test.
5. Conclusion
The present study’s findings indicate that the intake of probiotics and casein can enhance the aerobic capacity of male soccer players. However, it was observed that the simultaneous consumption of probiotics and casein had a more significant impact on aerobic endurance indicators, particularly TTE and Time-HHV. Based on these results, it can be suggested that soccer players incorporate probiotic and casein supplements in their diet to improve their endurance performance.
Abbreviations
| PRO | = | Probiotics Group |
| CAS | = | Casein Group |
| PRO+CAS | = | Probiotic with Casein Group |
| PLA | = | Placebo Group |
| CFU | = | Colony-Forming Unit |
| VO2max | = | Maximal oxygen consumption |
| VT | = | Ventilatory Threshold |
| TTE | = | Time-to-exhaustion |
| RCP | = | Respiratory Compensation Point |
| Time-IC | = | Isocapnic area Time |
| VO2-IC | = | Isocapnic area oxygen consumption |
| Time-HHV | = | Hypocapnic Hyperventilation area Time |
| CRP | = | C-reactive protein |
| HDL | = | High-Density Lipoprotein |
| VE/VO2 | = | Ventilatory Equivalent for oxygen |
| VE/VCO2 | = | Ventilatory Equivalent/Carbon dioxide production |
| PetO2 | = | Partial pressure of end-tidal oxygen tension |
| PetCO2 | = | Partial pressure of end-tidal carbon dioxide |
| LPS | = | lipopolysaccharide |
| mTOR | = | Mammalian target of rapamycin |
| NF-KB | = | Nuclear factor kappa B |
| URTI | = | Upper respiratory tract infection |
| IL-1b | = | Interleukin-1b |
| TNF-α | = | Tomour Necrosis Factor-alpha |
| IL-8 | = | Interleukin-8 |
| IL-6 | = | Interleukin-6 |
| ATP | = | Adenosine triphosphate |
| GABA | = | Gamma-aminobutyric acid |
| CNS | = | Central nervous system |
| 5-HT | = | 5-hydroxytryptamine |
| BUN | = | Blood urea nitrogen |
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Ethics approval and consent to participate
The current study was reviewed and approved by The Human Research Ethics Committee of Shiraz University (ethics approval code: IR.US.REC.1402.005, 2022) and carried out in accordance with the Declaration of Helsinki. All participants gave written informed consent before taking part in the study.
Acknowledgments
We want to thank all the participants who supported us in the examination and the implementation of the current study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Keen R. Nutrition-related considerations in soccer: a review. Am J Orthopedics (Belle Mead, NJ). 2018;47(12):12–20. doi: 10.12788/ajo.2018.0100
- Jäger R, Mohr AE, Carpenter KC, et al. International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr. 2019;16(1):62. doi: 10.1186/s12970-019-0329-0
- Przewłócka K, Folwarski M, Kaźmierczak-Siedlecka K, et al. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients. 2020;12(5):1451. doi: 10.3390/nu12051451
- Heath EH. ACSM’s guidelines for exercise testing and prescription. Med Sci Sports Exercise. 2005;37(11):2018. doi: 10.1249/01.mss.0000189073.33400.04
- Salehzadeh K. The effects of probiotic yogurt drink on lipid profile, CRP and record changes in aerobic athletes. Int J Life Sci. 2015;9(4):32–37. doi: 10.3126/ijls.v9i4.12672
- Lamprecht M, Bogner S, Schippinger G, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9(1):45. doi: 10.1186/1550-2783-9-45
- Huang W-C, Hsu Y-J, Li H, et al. Effect of lactobacillus plantarum TWK10 on improving endurance performance in humans. Chin J Physiol. 2018;61(3):163–170. doi: 10.4077/CJP.2018.BAH587
- Steffl M, Kinkorova I, Kokstejn J, et al. Macronutrient intake in soccer players—A meta-analysis. Nutrients. 2019;11(6):1305. doi: 10.3390/nu11061305
- Bangsbo J. Energy demands in competitive soccer. J Sports Sci. 1994;12(sup1):S5–S12. doi: 10.1080/02640414.1994.12059272
- Caruana Bonnici D, Greig M, Akubat I, et al. Nutrition in soccer: a brief review of the issues and solutions. J Sci In Sport Exercise. 2019;1(1):3–12. doi: 10.1007/s42978-019-0014-7
- Ranchordas MK, Dawson JT, Russell M. Practical nutritional recovery strategies for elite soccer players when limited time separates repeated matches. J Int Soc Sports Nutr. 2017;14(1):35. doi: 10.1186/s12970-017-0193-8
- Res PT, Groen B, Pennings B, et al. Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exercise. 2012;44(8):1560–1569. doi: 10.1249/MSS.0b013e31824cc363
- Salarkia N, Ghadamli L, Zaeri F, et al. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: a randomized controlled trial. Med J Islam Repub Iran. 2013;27(3):141.
- Kim J. Pre-sleep casein protein ingestion: new paradigm in post-exercise recovery nutrition. Phys Activity Nutr. 2020;24(2):6. doi: 10.20463/pan.2020.0009
- Gauchard GC, Gangloff P, Vouriot A, et al. Effects of exercise-induced fatigue with and without hydration on static postural control in adult human subjects. Int J Neurosci. 2002;112(10):1191–1206. doi: 10.1080/00207450290026157
- Green H, Halestrap A, Mockett C, et al. Increases in muscle MCT are associated with reductions in muscle lactate after a single exercise session in humans. Am J Physiol-Endocrinol Metab. 2002;282(1):E154–E60. doi: 10.1152/ajpendo.2002.282.1.E154
- Hobson RM, Saunders B, Ball G, et al. Effects of β-alanine supplementation on exercise performance: a meta-analysis. Amino Acids. 2012;43(1):25–37. doi: 10.1007/s00726-011-1200-z
- Brooks SP, Storey KB. A quantitative evaluation of the effect of enzyme complexes on the glycolytic rate in vivo: mathematical modeling of the glycolytic complex. J Theor Biol. 1991;149(3):361–375. doi: 10.1016/S0022-5193(05)80311-X
- Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004
- Huang W-C, Wei C-C, Huang C-C, et al. The beneficial effects of lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients. 2019;11(2):353. doi: 10.3390/nu11020353
- Sue DY, Wasserman K, Moricca RB, et al. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease: use of the V-slope method for anaerobic threshold determination. Chest. 1988;94(5):931–938. doi: 10.1378/chest.94.5.931
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020
- Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69
- Kominami K, Imahashi K, Katsuragawa T, et al. The ratio of oxygen uptake from ventilatory anaerobic threshold to respiratory compensation point is maintained during incremental exercise in older adults. Front Physiol. 2022;13:769387. doi: 10.3389/fphys.2022.769387
- Chicharro JL, Hoyos J, Lucía A. Effects of endurance training on the isocapnic buffering and hypocapnic hyperventilation phases in professional cyclists. Br J Sports Med. 2000;34(6):450–455. doi: 10.1136/bjsm.34.6.450
- Lucía A, Hoyos J, Chicharro JL. Physiology of professional road cycling. Sports Med. 2001;31(5):325–337. doi: 10.2165/00007256-200131050-00004
- Chen Y-M, Wei L, Chiu Y-S, et al. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients. 2016;8(4):205. doi: 10.3390/nu8040205
- Kerksick CM, Wilborn CD, Roberts MD, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2018;15(1):38. doi: 10.1186/s12970-018-0242-y
- Hanson NJ, Scheadler CM, Lee TL, et al. Modality determines VO 2max achieved in self-paced exercise tests: validation with the Bruce protocol. Eur J Appl Physiol. 2016;116(7):1313–1319. doi: 10.1007/s00421-016-3384-0
- Ni Lochlainn M, Bowyer RC, Steves CJ. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients. 2018;10(7):929. doi: 10.3390/nu10070929
- Martarelli D, Verdenelli MC, Scuri S, et al. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol. 2011;62(6):1689–1696. doi: 10.1007/s00284-011-9915-3
- Lamprecht M, Frauwallner A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Acute topics in sport nutrition. Acute Topics Sport Nutr. 2012;59:47–56.
- Stecker RA, Moon JM, Russo TJ, et al. Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein. Nutr Metab (Lond). 2020;17(1):1–11. doi: 10.1186/s12986-020-00515-2
- Jäger R, Zaragoza J, Purpura M, et al. Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob Proteins. 2020;12(4):1330–1339. doi: 10.1007/s12602-020-09656-5
- Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43(1):12–21. doi: 10.1007/s12020-012-9751-7
- Liu H-W, Chen Y-J, Chang Y-C, et al. Oligonol, a low-molecular weight polyphenol derived from lychee, alleviates muscle loss in diabetes by suppressing atrogin-1 and MuRF1. Nutrients. 2017;9(9):1040. doi: 10.3390/nu9091040
- Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Disease Model Mech. 2013;6(1):25–39. doi: 10.1242/dmm.010389
- Strasser B, Geiger D, Schauer M, et al. Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8(11):752. doi: 10.3390/nu8110752
- Meeusen R. Exercise, nutrition and the brain. Sports Med. 2014;44(1):47–56. doi: 10.1007/s40279-014-0150-5
- da Rocha AL, Pinto AP, Kohama EB, et al. The proinflammatory effects of chronic excessive exercise. Cytokine. 2019;119:57–61. doi: 10.1016/j.cyto.2019.02.016
- Angeli A, Minetto M, Dovio A, et al. The overtraining syndrome in athletes: a stress-related disorder. J Endocrinol Invest. 2004;27(6):603–612. doi: 10.1007/BF03347487
- Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exercise. 2000;32(2):317. doi: 10.1097/00005768-200002000-00011
- Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? The Int J Biochem Cell Biol. 2013;45(10):2186–2190. doi: 10.1016/j.biocel.2013.06.021
- Dolan P, Witherbee KE, Peterson KM, et al. Effect of carbohydrate, caffeine, and carbohydrate+ caffeine mouth rinsing on intermittent running performance in collegiate male lacrosse athletes. The J Strength Cond Res. 2017;31(9):2473–2479. doi: 10.1519/JSC.0000000000001819
- Hsu Y-J, Huang W-C, Lin J-S, et al. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients. 2018;10(7):862. doi: 10.3390/nu10070862
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491–497. doi: 10.1136/bjsports-2014-093502
- Jäger R, Purpura M, Stone JD, et al. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients. 2016;8(10):642. doi: 10.3390/nu8100642
- Coqueiro AY, de Oliveira Garcia AB, Rogero MM, et al. Probiotic supplementation in sports and physical exercise: does it present any ergogenic effect? Nutr Health. 2017;23(4):239–249. doi: 10.1177/0260106017721000