?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: Schizophrenia, cocaine-related disorder, antisocial personality disorder, and psychopathy share biological bases, but few studies discriminate between these disorders by means of prepulse inhibition. This work studies the phenotype of patients with cocaine-related disorders who are vulnerable to presenting a dual diagnosis of schizophrenia or antisocial personality disorder, by evaluating their prepulse inhibition, impulsivity and psychopathy personality traits. Methods: The sample (n = 38) was divided into three groups: (1) cocaine-related disorder (8 individuals diagnosed with cocaine-related disorder who did not present any other mental disorder), (2) cocaine-related disorder and schizophrenia (n = 14), and (3) cocaine-related disorder and antisocial personality disorder (n = 16). Results: The prepulse inhibition in the two groups with dual diagnosis was lower than that in the cocaine-related disorder group, F(2, 35) = 6.52, p = .004, while there was no significant differences between the two dual-diagnosis groups. Psychopathy was evaluated with the revised Hare Psychopathy Checklist and showed no correlation with the prepulse inhibition. Secondary psychopathy (impulsivity and poor behavior control), as evaluated with Levenson Self-Report Psychopathy Scale, was related to the prepulse inhibition. Two discriminating functions were obtained that allowed prediction of patient inclusion in the groups using the prepulse inhibition and the revised Hare Psychopathy Checklist with a success rate of 81.6% (cocaine-related disorder = 62.5%; cocaine-related disorder and schizophrenia = 78.6%; cocaine-related disorder and antisocial personality disorder = 93.8%). These results are discussed in regard to the neurobiological implications of prepulse inhibition in dual diagnosis. Conclusions: The results suggest that the prepulse inhibition is a promising dual-diagnosis vulnerability marker in individuals with cocaine addiction, because prepulse inhibition deficits are related both to schizophrenia and antisocial personality disorder. In addition, prepulse inhibition, which is considered a good endophenotype for studies on the genetic and neurobiological basis of cocaine-related disorder and schizophrenia, could be used in the same way in studies on antisocial personality disorder.
Introduction
Cocaine is responsible for more hospital admissions for treatment (36.5%) and emergencies (43.7%) than any other drug (Observatorio Español de la Droga y las Toxicomanías (OEDT), Citation2016). Schizophrenia and antisocial personality disorder (ASPD) are very prevalent in cocaine users (Araos et al., Citation2014; Arias et al., Citation2013). Prepulse inhibition (PPI; Blumenthal, Reynolds, & Spence, Citation2015) is a robust measure of sensorimotor gating (Kohl, Heekeren, Klosterkötter, & Kuhn, Citation2013), is a cross-species phenomenon (Zhang, Forkstam, Engel, & Svensson, Citation2000), is significantly heritable (Greenwood et al., Citation2016), and is a useful schizophrenia endophenotype (Siegel, Talpos, & Geyer, Citation2013). PPI is reduced in patients with psychiatric disorders compared with healthy controls (Arenas, Caballero-Reinaldo, Navarro-Francés, & Manzanedo, Citation2017). Since animal studies suggest the unitary nature of the neurocircuits that govern both major psychiatric syndromes and enhanced addiction vulnerability, it is probable that many genetic and environmental risk factors for mental illness, substance use disorders, or dual diagnosis conditions could be identical (Chambers, Citation2007). PPI, given its usefulness as an endophenotype, seems a good starting point to study these common etiological bases.
The regulation of PPI depends on the correct functioning and interaction of different neurotransmission systems (dopaminergic, glutamatergic, serotoninergic, and cholinergic), which are altered in schizophrenia (Geyer, Krebs-Thomson, Braff, & Swerdlow, Citation2001). The acoustic startle response is sensitive to dopaminergic modulation (Efferen et al., Citation2000). Cocaine users seem to have a lower startle reaction to single pulse trials and a more marked PPI, but PPI alterations could also be the result of a lower baseline startle reactivity (Kohl et al., Citation2013). The overlap of reward circuits shown to be altered in cocaine users (in particular the ventral striatum), and cortico-striato-pallido-pontine circuits regulating PPI, suggests that PPI may be altered in cocaine addiction (Arenas et al., Citation2017; Preller et al., Citation2013).
Very little research has used PPI to study personality disorders. Kumari et al. (Citation2005) found that PPI was lower in patients with ASPD or schizophrenia and a history of violence and with schizophrenia and no history of violence, than in healthy volunteers, but that this parameter could not distinguish between these three groups. In contrast, Sedgwick et al. (Citation2017) found significantly lower PPI in violent men with diagnoses of both psychosis and dissocial personality disorder, relative to the control and psychosis-only groups. In addition, across all three clinical groups, PCL-R factor-2 (behavioral, impulsive, and lifestyle factor) scores were negatively correlated with PPI. Furthermore, Loomans, Tulen, and Van Marle (Citation2015) used the startle reflex to differentiate between patients with ASPD and psychopathy and those with ASPD only. Thus, the neural substrates of PPI, specifically the hippocampus, amygdala, thalamus, and basal ganglia, are all implicated in ASPD and schizophrenia (Kumari et al., Citation2005). However, very little research has analyzed the PPI in patients with ASPD and/or psychopathy.
Given the biological bases shared by schizophrenia, cocaine-related disorder, ASPD, and psychopathy, and the scarcity of studies that discriminate between these disorders using PPI, the main objective of this work was to use PPI to study the characteristics of patients with cocaine-related disorder who presented a dual diagnosis of schizophrenia or ASPD, and to relate it to impulsivity personality traits and psychopathy. The specific objectives were to: (a) evaluate the presence of mental disorders, impulsivity, psychopathy, and PPI in individuals with cocaine-related disorder; (b) assess the relationship between PPI and psychopathy in participants with cocaine-related disorder; and (c) study if there are any differences in impulsivity, psychopathy, or PPI between patients with dual diagnosis (cocaine-related disorder + schizophrenia and cocaine-related disorder + ASPD) and those with only cocaine-related disorder. Our hypotheses were that: (1) individuals with cocaine-related disorder with a dual diagnosis (with ASPD or schizophrenia) present lower PPIs than those who only have cocaine-related disorder; and (2) PPI negatively correlates with the degree of psychopathy in patients with cocaine-related disorder.
Materials and methods
Design
This was an observational, cross-sectional, multi-center study in participants aged over 18 years who were admitted to the Addictive Behavior Unit, Hospital Detoxification Unit, or Severe Dual Diagnosis Program at the Provincial Hospital Consortium of Castellón (Spain), for treatment for cocaine-related disorder.
Participants
The sample comprised 38 participants, all male, obtained by intentional consecutive sampling of patients who underwent treatment for cocaine-related disorder in 2017. Criteria for inclusion in the study were: (a) males aged 18 years or older; (b) diagnosis of cocaine-related disorder, cocaine-related disorder and schizophrenia, or cocaine-related disorder and ASPD; and (c) consent to participation by signing the informed consent form. Criteria for exclusion were: (a) mental disorders other than those of the inclusion criteria; (b) a neurological disorder that could interfere in the PPI; and (c) illiteracy or being unable to understand sufficient Spanish to participate.
Three groups were created: (a) cocaine-related disorder (eight individuals diagnosed with cocaine-related disorder who did not present any other mental diagnoses); (b) cocaine-related disorder + schizophrenia (14 individuals diagnosed with both cocaine-related disorder and schizophrenia); and (c) cocaine-related disorder + ASPD (16 individuals diagnosed with both cocaine-related disorder and ASPD).
Procedure
There was a complete discussion of the study with potential participants. Written informed consent was obtained after this discussion. The confidentiality of the participants and the data was guaranteed. This research complied with the guidelines set out at the World Medical Association Declaration of Helsinki. The Ethical Committee of Hospital Provincial of Castellón approved this research (ref. 21122017). The sample participants were recruited by the healthcare professionals attending the patients. These volunteers were required to sign their informed consent in order to participate in the study. The researchers conducting these psychometric tests were trained in their administration and in the evaluation of PPI. The tests were administered after 7–10 days of detoxification treatment when psychopathological stabilization had been reached.
Instruments
Sociodemographic questionnaire.
Dual Diagnosis Screening Interview (Mestre-Pintó, Domingo-Salvany, Martín-Santos, Torrens, & PsyCoBarcelona Group, Citation2013), according to the DSM-IV criteria for people with a substance-use disorder.
Psychiatric Research Interview for Substance and Mental Disorders (PRISM-IV; Torrens, Serrano, Astals, Pérez-Domínguez, & Martín-Santos, Citation2004), which evaluates past and current disorders caused by substance consumption and psychiatric disorders, borderline personality disorder, and ASPD.
Barratt Impulsiveness Scale (Patton, Stanford, & Barratt, Citation1995), which assesses total, cognitive, motor, and unplanned impulsivity.
Sensitivity to Punishment and Sensitivity to Reward Questionnaire (Torrubia, Avila, Moltó, & Caseras, Citation2001).
The Levenson Self-Report Psychopathy Scale (LSRP; Levenson, Kiehl, & Fitzpatrick, Citation1995), which determines two dimensions: manipulation/insensitivity and impulsivity/conduct-controlled behavior.
The revised Hare Psychopathy Checklist (PCL-R; Hare, Citation2003), which evaluates psychopathy and two other factors: interpersonal/affective and social deviance. Psychopathy was considered to be present if the total score was 26 or more (Loomans et al., Citation2015).
Evaluation of the PPI of the acoustic startle reflex, using the BIOPAC MP 150 QUICK START (Mark II, SR-Lab, San Diego, California). This system generates alarm stimuli by emitting sounds that are played through headphones. The response is measured by facial changes in the periorbital area with surface electromyography. In our study, the participants received white noise at 70 dB, followed by three blocks of stimuli. The first block consisted of five single pulses. The second block was comprised of eight single pulses and 24 pulses preceded by a prepulse at 30, 60, or 120 ms. The third block was the same as the first one. The pulse intensities were 105 dB and lasted 40 ms and the prepulse intensities were 85 dB and lasted 20 ms. A total of 42 trials were performed in approximately 15 minutes.
There was no restriction in relation to tobacco consumption prior to the PPI test, but the test was not carried out until at least 20 minutes after the participant’s last cigarette. The main dependent variable was the PPI percentage calculated as: {[(response strength for the single pulse) – (response strength for the pulse preceded by the prepulse)] ÷ (response strength for the single pulse)} × 100. The mean latency, amplitude (mean response to the single pulse tests), and habituation (difference between the average response to the single-pulse tests from the first and last block) were also calculated.
Data analysis
The SPSS (v21) program was used for the statistical analyses. After the exploratory and descriptive study, the sociodemographic and clinical variables were compared by analysis of variance (ANOVA) for quantitative and for categorical variables. We used multivariate analysis of variance (MANOVA) to verify if there were differences between the groups in the dependent variables: impulsivity, behavioral activation and inhibition, psychopathy and PPI; specifying these differences using one-way ANOVAs and Tukey tests. The effect size (ES) as the partial eta squared and the observed power (1 − β) were calculated. Mixed ANOVA (Prepulse
Group) was used to check the influence of the prepulse administration interval and repeated-measures ANOVA was used for intragroup comparison according to the prepulse interval. The correlation between PPI and the other variables was calculated using Spearman’s rho and the difference in PPI between individuals with and without psychopathy, those who consumed substances or not, and who took antipsychotics or not were assessed using Student t-tests. The data were modeled with discriminant functions to check if the dependent variables were able to predict patient inclusion in the diagnostic groups.
Results
The average participant age was 41.47 years (SD = 6.56), and there were no differences between the groups ( cocaine-related disorder = 43.13, SD = 5.46;
cocaine-related disorder + schizophrenia = 39.14, SD = 7.18;
cocaine-related disorder + ASPD = 42.69, SD = 6.27). Sociodemographic descriptions and comparisons between groups are shown in .
Table 1. Sociodemographic Descriptions and Comparisons Between Groups
The participants were addicted to an average of 3.26 substances (SD = 1.63). There were differences in the number of addictions, F(2, 35) = 3.61, p = .038, with cocaine-related disorder + ASPD patients presenting more addictions than cocaine-related disorder + schizophrenia individuals ( cocaine-related disorder = 3.1, SD = 1.45;
cocaine-related disorder + schizophrenia = 2.5, SD = 1.09;
cocaine-related disorder + ASPD = 4, SD = 1.86). All (100%) of the participants were smokers and they all had an addiction to cocaine (N = 38); 68.4% to cannabis (n = 26), 55.3% to alcohol (n = 21), 47.4% to heroin (n = 18), 31.6% to sedatives (n = 12), 10.5% to stimulants (n = 4), 7.9% to methadone (n = 3), and 5.3% to other opioids (n = 2). Cocaine-related disorder + ASPD individuals presented a higher rate of addiction to heroin than cocaine-related disorder + schizophrenia participants
= 16.476, p < .001.
Antipsychotics were used by 54.1% (n = 20) of the study cohort and the average daily chlorpromazine-converted dose was 64.30 mg (SD = 59.32). The majority of patients taking antipsychotics were in the cocaine-related disorder + schizophrenia group, = 23.91, p < .001, and so this group had the highest average daily doses of these drugs (
cocaine-related disorder = 0.45, SD = 0.39;
cocaine-related disorder + schizophrenia = 88.91, SD = 51.21;
cocaine-related disorder + ASPD = 0.10, SD = 0.07, F(2, 15) = 6.75, p = .008). The clinical descriptions and comparisons for these patients are shown in .
Table 2. Clinical Descriptions and Comparisons Between Groups
There were differences between the groups in terms of the dependent variables, F(34, 32) = 2.529, p = .005, ES = 0.729, 1 − β = 0.990. shows the scores and comparisons for dependent variables of impulsivity and behavioral activation and inhibition. shows the scores and comparisons for the psychopathy variables.
Table 3. Scores and Comparisons Between the Groups in the Dependent Variables: Impulsivity, Activation, and Behavioral Inhibition
Table 4. Scores and Comparisons Between the Groups in the Psychopathy-Dependent Variables
There were no differences between the groups in terms of the latency (F(2, 35) = 1.966, p = .155), amplitude (F(2, 35) = 0.793, p = .460), or habituation (F(2, 35) = 0.182, p = .835) of the response reflex. shows the percentages of PPI at 30, 60, and 120 ms and the comparisons between the groups.
Table 5. Percentages of Prepulse Inhibition and Comparisons Between Groups
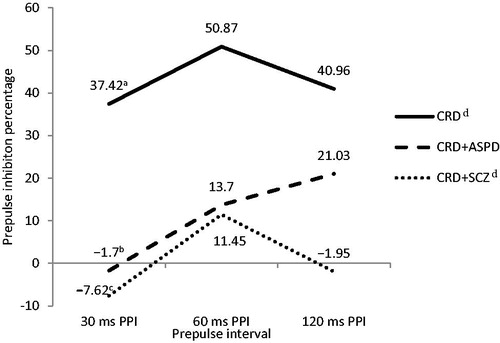
No interactions were observed for the Group Prepulse ANOVA (F(4, 66) = 0.554, p = .697, ES = 0.032, 1 − β = 0.176), the main effects of the group (F(2, 32) = 2.539, p = .094, ES = 0.133, 1 − β = 0.472), or the prepulse interval (F (2, 32)= 1.717, p = .196, ES = 0.097, 1 − β = 0.334) in the total sample. However, there were differences according to the prepulse interval in cocaine-related disorder (F(2, 4) = 9.381, p = .031, ES = 0.824, 1 − β = 0.728) and cocaine-related disorder + schizophrenia (F(2, 12) = 6.013, p = .016, ES = 0.501, 1 − β = 0.784) groups, although these differences did not reach significance in the pairwise comparisons. There were no differences according to the prepulse interval in the cocaine-related disorder + ASPD group, (F(2, 14) = 2.524, p = .116, ES = 0.265, 1 − β = 0.422). shows the average PPI of each group in each prepulse interval.
Figure 1. Prepulse inhibition percentage. Note. CRD = cocaine related disorder; SCZ = schizophrenia; ASPD = antisocial personality disorder; PPI = prepulse inhibition; ms = milliseconds. The a value was significantly higher than b (p = .011) and c (p = .004). The d variables had differences according PPI interval (CRD p = .031 and CRD + SCZ p = .016), that did not reach significance in the pairwise comparisons.
The PPI at 30 ms was related to the motor impulsivity score (rho = −0.334, p = .041) and secondary psychopathy on the LSRP (rho = −0.434, p = .006), while the PPI at 60 ms and 120 ms was not related to any of the variables we studied. PPI was not related to the daily amount consumed for any of the substances studied or to the average daily dose of antipsychotics. No differences were found in PPI between individuals with or without psychopathy, between those addicted or not to different substances, or between those who did or did not take antipsychotics.
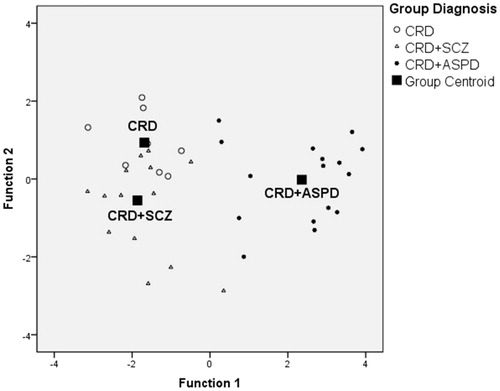
Two discriminating functions were obtained that allowed prediction of patient inclusion in the groups using the PPI and the PCL-R with a success rate of 81.6% (cocaine-related disorder = 62.5%; cocaine-related disorder + schizophrenia = 78.6%; cocaine-related disorder + ASPD = 93.8%). The first had an eigenvalue of 4.612 and explained 93.5% of the variance and the second had an eigenvalue of 0.321 and explained 6.5% of the variance. These equations are:
shows the scatter diagram for these discriminant functions.
Discussion
The objective of this work was to study the characteristics of patients with cocaine-related disorder who have a dual diagnosis with schizophrenia or ASPD, and these results confirm our initial hypothesis. The main finding is that the two groups with a dual diagnosis have a lower PPI than the group which only had a cocaine addiction. That is, the PPI is altered both in patients with cocaine-related disorder who have comorbid schizophrenia and in those with an ASPD comorbidity.
PPI is very useful as a schizophrenia endophenotype which links this disorder with its genetic vulnerability (Greenwood et al., Citation2016). However, this hypothesis had not been tested in patients with schizophrenia who also have a cocaine addiction. In addition, very few studies have shown the usefulness of PPI as an endophenotype in ASPD, although antisocial features have been associated with a lower PPI (Sedgwick et al., Citation2017). Our results indicate that this is also true in patients with ASPD and cocaine addiction.
However, as in the study by Kumari et al. (Citation2005), the PPI does not differentiate individuals with schizophrenia from those with ASPD. This suggests that alterations in PPI could be a marker of nonspecific mental disorder vulnerability. Alterations in the PPI would affect the functionality of inhibitory mechanisms, vulnerability to stress (García-Sánchez, Martínez-Gras, Rodríguez-Jiménez, & Rubio, Citation2011), information processing, some executive functions, lateralized attention, and reasoning (Morales-Muñoz et al., Citation2015), and depending on the individual and environmental factors, would result in one or another mental disorder. Alterations in PPI are derived from neurobiological changes, which may be common in the different disorders that present them. Complex psychiatric syndromes such as schizophrenia, with an increasingly well-known neurobiological basis, can help us to understand the neurobiological basis of other syndromes such as ASPD, which have been studied less but that also has PPI alterations. The orbitofrontal cortex and striatal dopamine have been identified as a neurobiological substrate of ASPD (Preller et al., Citation2013; Rosenbluth & Sinyor, Citation2012). PPI has an established neural basis in the cortico-pallido-striatal-thalamic circuitry, and these neural substrates are implicated in the pathophysiology of schizophrenia. Sharing neurobiological substrates makes it difficult to use the PPI for differential diagnosis (Kohl et al., Citation2013); however, it can facilitate psychopharmacological studies, for example with antipsychotics (Ettinger & Kumari, Citation2015)—with demonstrated efficacy in schizophrenia and improvement of PPI deficits—although there is little evidence for its use in the treatment of ASPD.
Atypical antipsychotics are effective for treating impulsivity, aggression, and anger in personality disorders (Rosenbluth & Sinyor, Citation2012); quetiapine decreases impulsivity, hostility, aggressiveness, irritability, and rage reactions in ASPD (Walker, Thomas, & Allen, Citation2003) and clozapine reduces aggression in schizophrenia and ASPD (Brown et al., Citation2014). Thus, integrating psychopharmacology with the neurobiological effects of psychotherapy may produce synergistic and long-lasting benefits (Ripoll, Triebwasser, & Siever, Citation2011). PPI could serve as a psychotropic drug or psychotherapy treatment-response marker (Kohl et al., Citation2013; Swerdlow, Braff, & Geyer, Citation2016) among people with schizophrenia and/or ASPD. That is, PPI seems to be a promising endophenotype for improving our knowledge about ASPD, a disorder whose prevalence is almost the same as that of schizophrenia, and which generates a tremendous personal, social, and economic cost for which few effective treatments are available (Walker et al., Citation2003).
Our second hypothesis was only partially confirmed. The psychopathy evaluated with the PCL-R did not correlate with PPI, although secondary psychopathy (impulsivity and poor behavioral control) evaluated with LSRP was related to PPI. No differences were found in PPI between individuals with cocaine-related disorder with and without psychopathy. However, through discriminant functions, the psychopathy evaluated with PCL-R allowed the group with ASPD to be distinguished from the other two groups (probably because all of the participants with psychopathy are in the ASPD group), while PPI discriminates between those who only have cocaine-related disorder and those who present a dual pathology, especially those with schizophrenia.
Traditionally, a distinction has been made between two factors of psychopathy: one interpersonal–affective (charm, grandiosity, deceitfulness/conning, lack of remorse, empathy, and emotional depth) and another related to impulsivity and antisocial acts (Anton, Baskin-Sommers, Vitale, Curtin, & Newman, Citation2012). Evidence has been found that the neurobiological underpinnings to psychopathy-specific features are distinct and separate from those associated with general antisocial-externalizing tendencies (Drislane, Vaidyanathan, & Patrick, Citation2013). Furthermore, Loomans et al. (Citation2015) hypothesized that the aversive reactivity deficits were specifically related to the affective/interpersonal factor. In addition, Drislane et al. (Citation2013) hypothesized that in addition to the deficits in impulse control that also occurred in ASPD, psychopathy is specifically characterized by impairments in the brain’s defensive motivational system reactivity and structures involved in processing fear (e.g., the amygdala).
On the one hand, unlike our study, Ehlers, Phillips, Criado, and Gilder (Citation2011) found no relationship between the startle response and the diagnosis of ASPD/conduct disorder. On the other hand, the results of Sedgwick et al. (Citation2017), which showed that the impulsive/antisocial factor is related to poor PPI, do coincide with our findings. Kumari et al. (Citation2005) also found a relationship between high violence ratios and the impact of PPI. These divergent results may be due to the differential characteristics of the samples or because the distinction between both factors is not very clear given the high degree of overlap between the two; there may be underlying common factors such as violence and criminal acts or they may share biological bases. The striatum has been linked to both interpersonal/affective and impulsive/antisocial features of psychopathy (Glenn & Yang, Citation2012), and both are marked by dysfunction in the frontal regions of the brain required for impulse control, executive function, and planning (Drislane et al., Citation2013). Likewise, reduced amygdala volume has been implicated in the development of severe persistent aggression and the development of psychopathic personality (Pardini, Raine, Erickson, & Loeber, Citation2014). In addition, the association between violent behavior and impaired PPI suggests that the neural structures and functions underlying PPI are implicated in the inhibition of violence (Kumari et al., Citation2005).
Another interesting point is that the differences in PPI between the groups only appeared for the 30 ms interval. There is a lot of variety in the literature about PPI regarding the intervals in which the differences occur. In schizophrenia, deficits occur mainly at 60 ms, although they have also been found at other intervals (Swerdlow et al., Citation2014). For cocaine, Efferen et al. (Citation2000) found differences at 100 ms, and Preller et al. (Citation2013) found differences in the average PPI of the four intervals used (30, 60, 120, and 240 ms) and also showed that the PPI increased with longer intervals. In ASPD, Sedgwick et al. (Citation2017) observed them at 60 ms, whereas Kumari et al. (Citation2005) found that the PPI did not increase as the interval increased. In our study, no significant differences were found for ASPD according to the interval (although the PPI did tend to increase with longer interval lengths), while in the two groups where there were differences according to the interval (cocaine-related disorder and cocaine-related disorder + schizophrenia), the highest PPI was recorded at 60 ms. These differences may be because of methodological factors related to PPI evaluation. Studies from different laboratories differ slightly in the precise temporal “sweet spot” for this inhibitory deficit, and this might reflect differences in stimulus characteristics or response acquisition hardware and/or software (Swerdlow et al., Citation2014).
However, the different intervals could have a clinical significance. Pre-attentive stimulus, detection, and evaluation occur at a prepulse lead time of about 60 ms, stimulus discrimination and further attentional allocation occur at lead times of about 120 ms, and transition from stimulus evaluation to judgment occurs at lead times of about 240 ms (Li, Du, Li, Wu, & Wu, Citation2009). Therefore, we propose that deficits at 60 ms could constitute a marker of vulnerability to mental disorders with involvement of higher functions, while deficits at 30 ms could be markers of vulnerability to mental disorders with involvement of more pre-attention functions. Another explanatory proposal is that because all the patients studied had cocaine-related disorder, statistical differences in the 60 ms PPI would not have been found because they would all have deteriorated in a similar way because of this addiction. The groups with a dual diagnosis could present a ‘double dose’ of PPI deterioration and hence differ from the group without dual pathology at 30 ms, therefore indicating higher basic pre-attention process deterioration.
This double effect could be interpreted as an indicator of greater severity of the pathology. The concept of a double dose has been described by Sedgwick et al. (Citation2017) with another connotation. These authors postulated that the existence of a double dose means that the PPI would be more strongly affected in individuals with comorbid psychosis and ASPD than those with only one of these disorders, without taking into account the different prepulse intervals. Morales-Muñoz et al. (Citation2014) proposed a third explanation of the double dose effect, suggesting that deficits induced by the consumption of cannabis or cocaine should be added to the congenital deficits in PPI function (Arenas et al., Citation2017). Thus, in their model, while the deficit at 30 ms would be related to vulnerability, deficits at 60 ms and 120 ms would be the result of neurodegeneration caused by a dual diagnosis and would reflect long-term psychosocial deterioration (Morales-Muñoz et al., Citation2016). Following this line, it has also been found that psychosocial deprivation in childhood (Sedgwick et al., Citation2017) and maternal deprivation or social isolation cause PPI deficits (Fendt & Koch, Citation2013).
We cannot conclude our discussion without considering the main limitation of our study: we did not compare the patients with cocaine-related disorder to healthy volunteers. This was because, in order to test our first hypothesis, we had to use cocaine-related disorder participants without a dual diagnosis as a comparison group. In addition, the effects of cocaine on PPI are not clear. It appears that acute administration of cocaine decreases PPI by increasing dopamine, although depending on the period of abstinence, the PPI could be restored in chronic cocaine users (Arenas et al., Citation2017). However, a design which also included a group of healthy volunteers would have allowed the hypothesis of double-dose deterioration caused by the consumption of substances to be better tested. Another limitation is that only men were included in the study. It is important to remember that lower PPI have been observed both in women with schizophrenia and in healthy women (Javitt & Freedman, Citation2015). Although tests were administered when participants were psychophysiological stabilized, they only spent 7–10 days without using cocaine. Therefore, one limitation could be the potential effects of withdrawal in PPI. Finally, we took into account the capacity that antipsychotic medication has to reduce the PPI (Swerdlow et al., Citation2014). There were no differences in the PPI between those taking antipsychotics and those who did not, nor in the relationship between their dose and the PPI. We also studied all the substances used by the patients, but noted no differences in the PPI between those who did or did not use substances, nor any relationship between the amount consumed and the PPI. Thus, we consider that the use of antipsychotics or the consumption of abuse substances does not influence the PPI results obtained.
In conclusion, the PPI represents a promising marker of vulnerability to dual diagnosis in patients with cocaine addiction because taking into account the “double dose” hypothesis of PPI deterioration, deficits in PPI are related to addiction and both schizophrenia and ASPD. In addition, PPI, which is considered a good endophenotype for studies on the genetic and neurobiological basis of cocaine-related disorder and schizophrenia, could also play the same role in studies on ASPD.
Disclosure statement
All authors declare no conflicts of interest.
Additional information
Funding
References
- Anton, M. E., Baskin-Sommers, A. R., Vitale, J. E., Curtin, J. J., & Newman, J. P. (2012). Differential effects of psychopathy and antisocial personality disorder symptoms on cognitive and fear processing in female offenders. Cognitive, Affective, and Behavioral Neuroscience, 12(4), 761–776. doi:10.3758/s13415-012-0114-x
- Araos, P., Vergara-Moragues, E., Pedraz, M., Pavón, F. J., Campos Cloute, R., Calado, M., … Rodríguez de Fonseca, F. (2014). Psychopathological comorbidity in cocaine users in outpatient treatment. Adicciones, 26(1), 15–26. doi:10.20882/adicciones.124
- Arenas, M. C., Caballero-Reinaldo, C., Navarro-Francés, C. I., & Manzanedo, C. (2017). Efecto de la cocaína sobre la inhibición por prepulso de la respuesta de sobresalto. [Cocaine effect on prepulse inhibition of startle response]. Revista de Neurología, 65(11), 507–519. doi:10.33588/rn.6511.2017298
- Arias, F., Szerman, N., Vega, P., Mesias, B., Basurte, I., Morant, C., … Babin, F. (2013). Abuso o dependencia a la cocaína y otros trastornos psiquiátricos. Estudio Madrid sobre la prevalencia de la patología dual. [Cocaine abuse or dependence and other psychiatric disorders. Madrid trial on dual pathology prevalence]. Revista de Psiquiatría y Salud Mental, 6(3), 121–128. doi:10.1016/j.rpsm.2012.09.002
- Blumenthal, T. D., Reynolds, J. Z., & Spence, T. E. (2015). Support for the interruption and protection hypotheses of prepulse inhibition of startle: Evidence from a modified Attention Network Test. Psychophysiology, 52(3), 397–406. doi:10.1111/psyp.12334
- Brown, D., Larkin, F., Sengupta, S., Romero-Ureclay, J. L., Ross, C. C., Gupta, N., … Das, M. (2014). Clozapine: An effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectrums, 19(5), 391–402. doi:10.1017/S1092852914000157
- Chambers, R. A. (2007). Animal modeling and neurocircuitry of dual diagnosis. Journal of Dual Diagnosis, 3(2), 19–29. doi:10.1300/J374v03n02_04
- Drislane, L. E., Vaidyanathan, U., & Patrick, C. J. (2013). Reduced cortical call to arms differentiates psychopathy from antisocial personality disorder. Psychological Medicine, 43(4), 825–835. doi:10.1017/S0033291712001547
- Efferen, T. R., Duncan, E. J., Szilagyi, S., Chakravorty, S., Adams, J. U., Gonzenbach, S., …Rotrosen, J. (2000). Diminished acoustic startle in chronic cocaine users. Neuropsychopharmacology, 22(1), 89–96. doi:10.1016/S0893-133X(99)00089-5
- Ehlers, C. L., Phillips, E., Criado, J. R., & Gilder, D. A. (2011). N4 component responses to pre-pulse startle stimuli in young adults: Relationship to alcohol dependence. Journal of Psychiatry Research, 188(2), 237–244. doi:10.1016/j.psychres.2011.04.010
- Ettinger, U., & Kumari, V. (2015). Effects of sleep deprivation on inhibitory biomarkers of schizophrenia: Implications for drug development. The Lancet Psychiatry, 2(11), 1028–1035. doi:10.1016/S2215-0366(15)00313-2
- Fendt, M., & Koch, M. (2013). Translational value of startle modulations. Cell and Tissue Research, 354(1), 287–295. doi:10.1007/s00441-013-1599-5
- García-Sánchez, F., Martínez-Gras, I., Rodríguez-Jiménez, R., & Rubio, G. (2011). Inhibición prepulso del reflejo de la respuesta de sobresalto en los trastornos neuropsiquiátricos. [Prepulse inhibition of startle response reflex in neuropsychiatric disorders]. Revista de Neurología, 53, 422–432.
- Geyer, M. A., Krebs-Thomson, K., Braff, D. L., & Swerdlow, N. R. (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology, 156(2–3), 117–154. doi:10.1007/s002130100811
- Glenn, A. L., & Yang, Y. (2012). The potential role of the striatum in antisocial behavior and psychopathy. Biological Psychiatry, 72(10), 817–822. doi:10.1016/j.biopsych.2012.04.027
- Greenwood, T. A., Light, G. A., Swerdlow, N. R., Calkins, M. E., Green, M. F., Gur, R. E., … Braff, D. L. (2016). Gating deficit heritability and correlation with increased clinical severity in schizophrenia patients with positive family history. American Journal of Psychiatry, 173(4), 385–391. doi:10.1176/appi.ajp.2015.15050605
- Hare, R. D. (2003). Manual for the Hare Psychopathy Checklist-Revised. (2nd ed.). Toronto, ON: Canada.
- Javitt, D. C., & Freedman, R. (2015). Sensory processing dysfunction in the personal experience and neural machinery of schizophrenia. American Journal of Psychiatry, 172(1), 17–31. doi:10.1176/appi.ajp.2014.13121691
- Kohl, S., Heekeren, K., Klosterkötter, J., & Kuhn, J. (2013). Prepulse inhibition in psychiatric disorders–apart from schizophrenia. Journal of Psychiatry Research, 47(4), 445–452. doi:10.1016/j.jpsychires.2012.11.018
- Kumari, V., Das, M., Hodgins, S., Zachariah, E., Barkataki, I., Howlett, M., & Sharma, T. (2005). Association between violent behaviour and impaired prepulse inhibition of the startle response in antisocial personality disorder and schizophrenia. Behavioural Brain Research, 158(1), 159–166. doi:10.1016/j.bbr.2004.08.021
- Levenson, M. R., Kiehl, K. A., & Fitzpatrick, C. M. (1995). Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology, 68(1), 151–158. doi:10.1037//0022-3514.68.1.151
- Li, L., Du, Y., Li, N., Wu, X., & Wu, Y. (2009). Top–down modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience & Biobehavioral Reviews, 33(8), 1157–1167. doi:10.1016/j.neubiorev.2009.02.001
- Loomans, M. M., Tulen, J. H. M., & Van Marle, H. J. C. (2015). The startle paradigm in a forensic psychiatric setting: Elucidating psychopathy. Criminal Behaviour and Mental Health, 25(1), 42–53. doi:10.1002/cbm.1906
- Mestre-Pintó, J. I., Domingo-Salvany, A., Martín-Santos, R., Torrens, M, & PsyCoBarcelona Group. (2013). Dual diagnosis screening interview to identify psychiatric comorbidity in substance users: Development and validation of a brief instrument. European Addiction Research, 20(1), 41–48. doi:10.1159/000351519
- Morales-Muñoz, I., Jurado-Barba, R., Caballero, M., Rodríguez-Jiménez, R., Jiménez-Arriero, M. A., Fernández-Guinea, S., & Rubio, G. (2015). Cannabis abuse effects on prepulse inhibition in patients with first episode psychosis in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences, 27(1), 48–53. doi:10.1176/appi.neuropsych.12120398
- Morales-Muñoz, I., Jurado-Barba, R., Fernández-Guinea, S., Rodríguez-Jiménez, R., Jiménez Arriero, M. A., Criado, J. R., & Rubio, G. (2016). Sensory gating deficits in first-episode psychosis. Evidence from neurophysiology, psychophysiology, and neuropsychology. Journal of Nervous and Mental Disease, 204(12), 877–884. doi:10.1097/NMD.0000000000000572
- Morales-Muñoz, I., Jurado-Barba, R., Ponce, G., Martínez-Gras, I., Jiménez-Arriero, M. A., Moratti, S., & Rubio, G. (2014). Characterizing cannabis-induced psychosis: A study with prepulse inhibition of the startle reflex. Psychiatry Research, 220(1–2), 535–540. doi:10.1016/j.psychres.2014.08.010
- Observatorio Español de la Droga y las Toxicomanías (OEDT), (2016). 12th July 2017. Informe Alcohol, tabaco y drogas ilegales en España. [2016 report: Alcohol, tobacco and illegal drugs in Spain]. Retrieved from: http://www.pnsd.msssi.gob.es/profesionales/publicaciones/catalogo/catalogoPNSD/publicaciones/pdf/INFORME_OEDT_2016.PDF
- Pardini, D. A., Raine, A., Erickson, K., & Loeber, R. (2014). Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits and future violence. Biological Psychiatry, 75(1), 73–80. doi:10.1016/j.biopsych.2013.04.003
- Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology, 51(6), 768–774.
- Preller, K. H., Ingold, N., Hulka, L. M., Vonmoos, M., Jenni, D., Baumgartner, M. R., … Quednow, B. B. (2013). Increased sensorimotor gating in recreational and dependent cocaine users is modulated by craving and attention-deficit/hyperactivity disorder symptoms. Biological Psychiatry, 73(3), 225–234. doi:10.1016/j.biopsych.2012.08.003
- Ripoll, L. H., Triebwasser, J., & Siever, L. J. (2011). Evidence-based pharmacotherapy for personality disorders. The International Journal of Neuropsychopharmacology, 14(9), 1257–1288. doi:10.1017/S1461145711000071
- Rosenbluth, M., & Sinyor, M. (2012). Off-label use of atypical antipsychotics in personality disorders. Expert Opinion on Pharmacotherapy, 13(11), 1575–1585. doi:10.1517/14656566.2011.608351
- Sedgwick, O., Young, S., Greer, B., Arnold, J., Parsons, A., Puzzo, I., …Kumari, V. (2017). Sensorimotor gating characteristics of violent men with comorbid psychosis and dissocial personality disorder: Relationship with antisocial traits and psychosocial deprivation. Schizophrenia Research, 198, 21–27. doi:10.1016/j.schres.2017.06.045
- Siegel, S. J., Talpos, J. C., & Geyer, M. A. (2013). Animal models and measures of perceptual processing in schizophrenia. Neuroscience and Biobehavioral Reviews, 37(9), 2092–2098. doi:10.1016/j.neubiorev.2013.06.016
- Swerdlow, N. R., Braff, D. L., & Geyer, M. A. (2016). Sensorimotor gating of the startle reflex: What we said 25 years ago, what has happened since then, and what comes next. Journal of Psychopharmacology, 30(11), 1072–1081. doi:10.1177/0269881116661075
- Swerdlow, N. R., Light, G. A., Sprock, J., Calkins, M. E., Green, M. F., Greenwood, T. A., … Braff, D. L. (2014). Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophrenia Research, 152(2–3), 503–512. doi:10.1016/j.schres.2013.12.004
- Torrens, M., Serrano, D., Astals, M., Pérez-Domínguez, G., & Martín-Santos, R. (2004). Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. American Journal of Psychiatry, 161(7), 1231–1237. doi:10.1176/appi.ajp.161.7.1231
- Torrubia, R., Avila, C., Moltó, J., & Caseras, X. (2001). The sensitivity to punishment and sensitivity reward questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. doi:10.1016/S0191-8869(00)00183-5
- Walker, C., Thomas, J., & Allen, T. S. (2003). Treating impulsivity, irritability, and aggression of antisocial personality disorder with Quetiapine. International Journal of Offender Therapy and Comparative Criminology, 47(5), 556–567. doi:10.1177/0306624X03253027
- Zhang, J., Forkstam, C., Engel, J. A., & Svensson, L. (2000). Role of dopamine in prepulse inhibition of acoustic startle. Psychopharmacology, 149(2), 181–188. doi:10.1007/s002130000369