Abstract
Background: Hepatoblastoma (HB) is malignant embryonal tumor typically arising in infants and young children. Yes-associated protein (YAP) is aberrantly activated in various tumors; however, the role of YAP in hepatoblastoma is still unexplored. Methods: We assessed YAP expression in hepatoblastoma using immunohistochemistry. The relationships to clinicopathology and survival were analyzed. Results: Positive rate of YAP expression was higher in hepatoblastoma than in adjacent tissues. YAP overexpression was significantly correlated with lymph node metastasis and vascular invasion. Both epithelial and mixed histological types expressed YAP, but high expression was more frequent in MT. YAP expression correlated with VEGF expression, high microvascular density and low overall survival. Multivariable Cox regression analysis revealed that YAP was an independent prognostic factor for survival in children with hepatoblastoma. Conclusion: In hepatoblastoma, YAP may promote VEGF induced angiogenesis and metastases, with resulting poorer prognosis, representing a potential adverse prognostic marker.
Introduction
Hepatoblastoma (HB) is a kind of malignant embryonal tumor with multiple differentiation patterns that accounts for 50%−60% of malignant liver tumors in infants and young children [Citation1,Citation2]. The early symptoms of hepatoblastoma are not obvious, and the disease is often not diagnosed until it invades multiple liver segments or forms an obvious abdominal tumor [Citation3]. A considerable number of children with hepatoblastoma experience vascular invasions, distant metastasis and even tumor rupture. In recent years, the prognosis of patients with hepatoblastoma has improved to some extent due to the optimized combination of liver tumor resection and chemotherapy, as well as the development of individualized preoperative and postoperative chemotherapy [Citation4]; however, the prognosis of children with hepatoblastoma still significantly varies from person to person. Once metastasis occurs, it significantly affects the prognosis of children [Citation5]. Further studies are still needed of the molecular mechanisms of hepatoblastoma development and metastases.
YAP is a downstream effector of the Hippo pathway that cooperates with the transcription factor enhanced association domain (TEAD) family to regulate gene expression and promote cell proliferation and tissue homeostasis [Citation6,Citation7]. In recent years, YAP has received a great deal of attention in tumor malignant biological behavior and has been considered a candidate oncogene [Citation6–8]. YAP protein levels were reported to be highly expressed by various types of human malignancies and are associated with poor prognosis in multiple cancer types, such as hepatocellular carcinoma [Citation9,Citation10], breast cancer [Citation11], colorectal cancer [Citation12,Citation13], ovarian cancer [Citation14], esophageal cancer [Citation15], prostate cancer [Citation16], and pancreatic cancer [Citation17]. These studies demonstrated that YAP overexpression is associated with tumor cell proliferation, metastasis, and epithelial-mesenchymal transition (EMT) [Citation12,Citation16,Citation18]. Research on YAP alterations in pediatric tumors is still limited, but a recent study identified YAP activation in a subset of HBs, which promoted disease progression [Citation19]. At present, the role of YAP in hepatoblastoma remains poorly defined.
In this study, we investigated the immunohistochemical expression of YAP, VEGF and microvessel density (MVD) in 64 cases of hepatoblastoma and compared its relationship to histological parameters, metastasis, and survival. The aim of this study was to measure YAP expression in hepatoblastoma and to determine whether YAP plays an important role in angiogenesis and predicting prognosis.
Material and methods
Clinical data
Sixty-four hepatoblastoma specimens from patients admitted to the Department of Pathology of Tianjin Medical University Cancer Institute and Hospital from 2010 to 2019 were included in this study. Tumor tissue samples were obtained by surgery or ultrasound-guided biopsy. Two independent senior pathologists reviewed all HB cases according to WHO criteria. Clinical information and tumor characteristics, including patient age, sex, survival status, survival time, lymph node metastasis, vascular invasion, and PRETEXT stage were available for all patients. This study was performed and authorized by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Immunohistochemistry
IHC sections were sequentially deparaffinized in xylene and gradient alcohol. After blocking endogenous peroxidase activity, the sections were pretreated in a microwave oven for 12 min at 95 °C for antigen retrieval. Then, serum was used to block the antigen, and the slides were incubated with primary antibodies for 15 h at 4 °C. YAP, VEGF, and CD34 expression was detected using the staining systems PicTure PV6001 and PV6002 (Zhongshan Chemical Co., Beijing, China), which were performed according to the manufacturer’s instructions.
Immunohistochemical analyses
Positive staining was evaluated semi-quantitatively by two pathologists who were unaware of the clinicopathological parameters of the cases. Staining was interpreted using a staining index (SI) with the following standards: ten randomly selected fields at 400x magnification were analyzed for 100 tumor cells each and classified into 4 categories: 0 (insignificant staining); 1 (weak staining); 2 (intermediate staining); and 3 (intense staining). The mean percentage of the 10 fields was classified into 4 categories: 0 (negative cells); 1 (≤ 25%); 2 (25 to 50%); or 3 (≥ 50%). The sum of intensity and percentage (SI) scores was used to evaluate the results. An SI score ≥3 was considered high expression, and an SI score <3 was considered low expression. CD34 staining was located in the cell membrane and cytoplasm, which was utilized to identify vascular endothelial cells and to quantify angiogenesis microvessel density (MVD). Microvessels were measured under 10 high-power microscope fields (200×), and the cutoff value for discriminating MVDhigh and MVDlow was the median of the MVD.
Statistical analysis
SPSS 25.0 software was used for statistical analysis, and differences between groups were analyzed using independent sample t-test. The difference in immunohistochemical expression in paraffin tissue and its relationship with clinicopathological features were analyzed using Pearson’s chi-squared test or Fisher’s exact test. The relationship between YAP expression or MVD in hepatoblastoma and survival time was analyzed using Kaplan-Meier analysis. The survival rate curve was analyzed using Log-rank test. A multivariate Cox regression model was used for risk factor analysis. P < 0.05 indicated the difference was statistically significant.
Results
Yap expression in hepatoblastoma
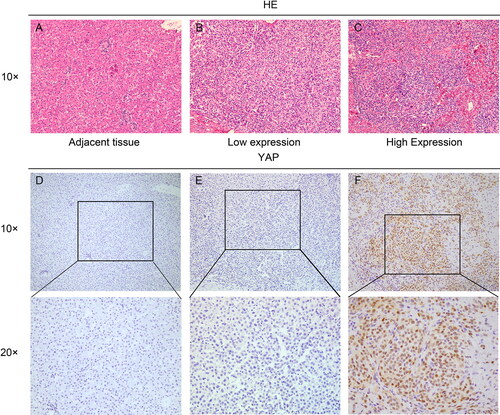
YAP immunohistochemical staining was performed in 64 hepatoblastoma tissues and 20 adjacent tissues. YAP was positive in 35 cases (54.7%) and negative in 29 cases of hepatoblastoma (). YAP protein was weakly positive in 2 cases (10%) and negative in 18 cases in adjacent tissues. Positive YAP expression was significantly higher in hepatoblastoma than in paraneoplastic tissues (P < 0.01).
Analysis of MVD in hepatoblastoma
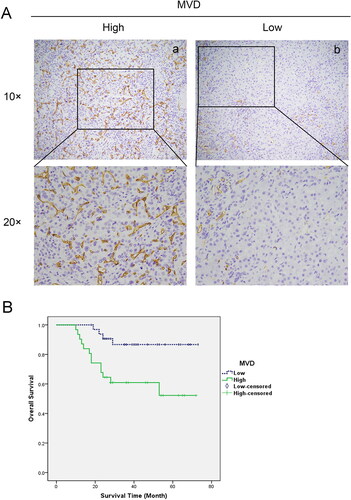
In 64 cases of hepatoblastoma, hematoxylin-eosin staining (HE) sections were observed and combined with immunohistochemical sections to calculate microvessel density (MVD). The median MVD of 64 cases of hepatoblastoma was 38.95. Thus, taking 38.95 as the cutoff value, the group was divided into a high MVD group (MVDHigh) and a low MVD group (MVDLow) (). Survival analysis showed that the overall survival rate of the MVDHigh group was dramatically lower than that of the MVDLow group, and the difference was statistically significant (χ2=7.34, P = 0.009) ().
Relationship between Yap, MVD and VEGF expression in hepatoblastoma
The results are shown in . Correlation analysis revealed that the expression of YAP in hepatoblastoma was significantly correlated with MVD (χ2 = 10.65, P = 0.002), and there was a positive correlation between YAP expression and VEGF expression () (χ2 = 6.01, P = 0.014). The results showed that 24/35 cases in the YAP high expression group exhibited high MVD, with an average of 60.72 ± 28.22. In the YAP low expression group, 8/29 cases displayed high MVD, with an average of 37.71 ± 18.54, and the difference was statistically significant (t = 3.66, P = 0.001) ().
Figure 3. MVD and VEGF expression in hepatoblastoma. (A) Immunohistochemistry for VEGF in hepatoblastoma tissues (x100 and x200). (B) YAP high expression cases exhibited higher MVD (60.72 ± 28.22) than YAP low expression cases (37.71 ± 18.54) (P = 0.001).
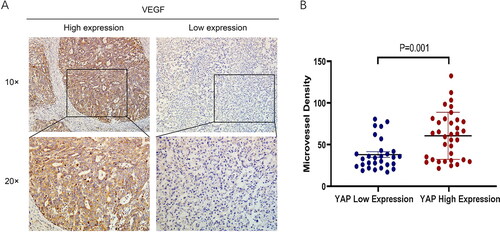
Table 1. Correlations between YAP, MVD and VEGF expression in HBs.
Correlation between Yap expression and clinicopathological features of hepatoblastoma
As shows, expression of YAP in hepatoblastoma was not correlated with sex or age (P > 0.05), but was significantly correlated with lymph node metastasis (P = 0.035), vascular invasion (P = 0.015), histological type (P = 0.031) and PRETEXT stage (P = 0.044). The results also showed that YAP overexpression was detected in both epithelial type (ET) and mixed type (MT) histologies, but high expression was more frequent in MT (70.4%) compared with ET (43.2%).
Table 2. Relationship between YAP expression and clinicopathological features of HBs.
Relationship between Yap expression and hepatoblastoma prognosis
Among 64 patients with hepatoblastoma, 35 cases exhibited high YAP expression and 29 cases had low YAP expression. The median survival time was 27 months and the 5-year survival rate was 60.0% in the high YAP expression group compared with the low YAP expression group whose median survival time was 39 months and the 5-year survival rate was 89.7%. Survival analysis demonstrated that the overall survival rate of HB patients in the high YAP expression group was significantly lower than that of patients in the low YAP expression group (P = 0.007) (). Multivariable Cox regression analysis demonstrated that lymph node metastasis, PRETEXT stage and YAP expression were independent prognostic factors for hepatoblastoma (P < 0.05) ().
Figure 4. Kaplan–Meier analysis of the overall survival of patients with hepatoblastoma based on YAP expression (P = 0.007).
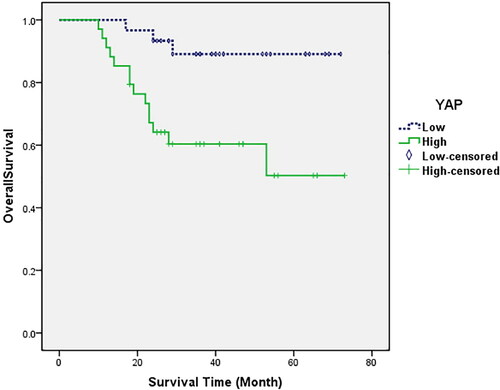
Table 3. Multivariate Cox regression analysis for overall survival in HBs.
Discussion
The present study assessed YAP expression in tumor tissues and adjacent tissues of 64 patients with hepatoblastoma. The positive expression rate of YAP in tumor tissues was significantly higher than that in paraneoplastic tissues (P < 0.05), and the positive expression rate of YAP was correlated with lymph node metastasis, vascular invasion, histological type and PRETEXT stage (P < 0.05).
Although the prognosis of children with hepatoblastoma has improved in recent years, the prognosis of some children with metastasis remains poor [Citation1,Citation2,Citation5]. Additional diagnostic and prognostic markers for hepatoblastoma are needed to identify those tumors with more aggressive biological behavior. Unlike adult hepatocellular carcinoma, hepatoblastoma pathogenesis remains obscure, but it is not related to hepatitis B virus infection and liver cirrhosis [Citation20].
The YAP protein is a crucial transcriptional coactivator protein that is downstream of Hippo signaling and the WNT/β-catenin pathway [Citation21,Citation22]. It executes functions such as promoting tissue regeneration, and maintaining stem cell self-renewal and repair [Citation23–25]. Given the critical role of YAP in cell proliferation, apoptosis regulation and tumor formation have gradually been revealed to be related to YAP, and studies on YAP have increased. Previous findings indicated that YAP is highly expressed in many malignant tumors and promotes the invasion and metastasis of tumor cells [Citation11–17]. YAP represents an independent prognostic marker in hepatocellular carcinoma, and p-YAP dephosphorylation results in nuclear localization, where YAP regulates the malignant biological behavior of tumors [Citation26,Citation27]. Recent studies reported that Wnt/β-catenin collaborated with YAP signaling to induce hepatoblastoma formation and development in the mouse liver [Citation28–30], whereas it had been well known that aberrant β-catenin expression was associated with hepatoblastoma pathogenesis [Citation31–34]. Supportively, the present study demonstrated that overexpression of YAP was significantly correlated with the malignant biological behavior of hepatoblastoma. The limitation of this study is the relatively small number of cases, and we hope that additional study will be performed in multiple centers with more cases.
Tumor growth is closely related to the formation of blood vessels, and tumor angiogenesis is regulated by various factors and tumor environmental factors [Citation35,Citation36]. Tumor neovascularization is closely associated with the appearance of metastases. Neovascularization not only provides nutrients for the growth of the primary tumor but also provides a channel for tumor metastases [Citation35,Citation37,Citation38]. The survival rate of children with hepatoblastoma in the MVDHigh group was significantly lower than that in the MVDLow group, indicating that angiogenesis conveys poor prognosis in hepatoblastoma. Consistent with our results, previous study showed that hypervascularity was correlated with hepatoblastoma progression [Citation39].
Our results showed that YAP expression was significantly correlated with VEGF expression and high microvessel density, with P values of 0.014 and 0.002, respectively. These results demonstrated that YAP high expression cases had significantly higher MVD (60.72 ± 28.22) than compared with YAP low expression cases (37.71 ± 18.54) (t = 3.66, P = 0.001). Consistent with these results, it has been demonstrated that the transcription factor YAP is crucial for promoting angiogenesis during the embryonic stage [Citation40]. YAP nuclear translocation and interaction with the PI3K/Akt signaling pathway can affect synovial angiogenesis in rheumatoid arthritis [Citation41]. The effect of YAP in promoting angiogenesis in hepatoblastoma and its potential mechanism were required to be further explored in future experiments. In the present study, survival analysis demonstrated that HB patients with high YAP expression had a worse prognosis. We demonstrated that YAP expression may represent an independent prognostic marker in children with HB through multivariable Cox regression. Consequently, we speculate that in hepatoblastoma, YAP may promote the expression of VEGF followed by angiogenesis, ultimately promoting tumor metastasis and poor prognosis.
In conclusion, this study determined that YAP expression was up-regulated in hepatoblastoma, which was related to tumor metastasis and vascular invasion. We show that tumor tissues with high expression of YAP exhibited higher tumor microvessel density, which may provide a basis for the further elucidation of the regulatory mechanism of the occurrence and development of hepatoblastoma and angiogenesis and is expected to offer new ideas for predicting the prognosis of children with hepatoblastoma.
Disclosure statement
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Funding
References
- De Ioris M, Brugieres L, Zimmermann A, Keeling J, Brock P, Maibach R, Pritchard J, Shafford L, Zsiros J, Czaudzerna P, et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer. 2008;44(4):545–50. doi:10.1016/j.ejca.2007.11.022.
- D’Antiga L, Vallortigara F, Cillo U, Talenti E, Rugge M, Zancan L, Dall’Igna P, De Salvo G, Perilongo G. Features predicting unresectability in hepatoblastoma. Cancer. 2007;110(5):1050–8. doi:10.1002/cncr.22876.
- Dong Q, Xu W, Jiang B, Lu Y, Hao X, Zhang H, Jiang Z, Lu H, Yang C, Cheng Y, et al. Clinical applications of computerized tomography 3-D reconstruction imaging for diagnosis and surgery in children with large liver tumors or tumors at the hepatic hilum. Pediatr Surg Int. 2007;23(11):1045–50. doi:10.1007/s00383-007-1910-1.
- Czauderna P, Otte JB, Roebuck DJ, Schweinitz DV, Plaschkes J. Surgical treatment of hepatoblastoma in children. Pediatr Radiol. 2006;36(3):187–91. doi:10.1007/s00247-005-0067-0.
- Meyers RL, Rowland JR, Krailo M, Chen Z, Katzenstein HM, Malogolowkin MH. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;53(6):1016–22. doi:10.1002/pbc.22088.
- Qiao Y, Li T, Zheng S, Wang H. The Hippo pathway as a drug target in gastric cancer. Cancer Lett. 2018;420:14–25. doi:10.1016/j.canlet.2018.01.062.
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–9. doi:10.1038/nrc3876.
- Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi:10.1016/j.ccell.2016.05.005.
- Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144(7):1530–42. doi:10.1053/j.gastro.2013.02.009.
- Zhang S, Zhou D. Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin Cell Biol. 2019;61:64–71. doi:10.1016/j.ceb.2019.07.006.
- Tufail R, Jorda M, Wei Z, Reis I, Nawaz Z. Treatment. Loss of Yes-associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2012;131(3):743–50. doi:10.1007/s10549-011-1435-0.
- Ling HH, Kuo CC, Lin BX, Huang YH, Lin CW. Elevation of YAP promotes the epithelial-mesenchymal transition and tumor aggressiveness in colorectal cancer. Exp Cell Res. 2017;350(1):218–25. doi:10.1016/j.yexcr.2016.11.024.
- Zhang S, Wei Q, Yang Y, Qin H, Li X, Cai S, Ma Y. Loss of Yes-associated protein represents an aggressive subtype of colorectal cancer. J Cancer. 2019;10(3):689–96. doi:10.7150/jca.28333.
- Cho SY, Kim K, Park MS, Jang MY, Choi YH, Han S, Shin HM, Chung C, Han HY, Yang JB, et al. Expression of Yes-associated protein 1 and its clinical significance in ovarian serous cystadenocarcinoma. Oncol Rep. 2017;37(5):2620–32. doi:10.3892/or.2017.5517.
- Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, Wu TT, et al. The hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res. 2015;21(11):2580–90. doi:10.1158/1078-0432.CCR-14-2191.
- Jin X, Zhao W, Zhou P, Niu T. YAP knockdown inhibits proliferation and induces apoptosis of human prostate cancer DU145 cells. Mol Med Rep. 2018;17(3):3783–8.
- Yoo W, Lee J, Jun E, Noh KH, Lee S, Jung D, Jung KH, Kim JS, Park YY, Kim SC, et al. The YAP1–NMU axis is associated with pancreatic cancer progression and poor outcome: identification of a novel diagnostic biomarker and therapeutic target. Cancers (Basel). 2019;11:1477. doi:10.3390/cancers11101477.
- Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17(8):2130–9. 15. doi:10.1158/1078-0432.CCR-10-2467.
- Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32(1):38–47. doi:10.1111/j.1478-3231.2011.02646.x.
- Tomlinson GE, Kappler R. Genetics and epigenetics of hepatoblastoma. Pediatr Blood Cancer. 2012;59(5):785–92. Novdoi:10.1002/pbc.24213.
- Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015; 47(3):250–6. doi:10.1038/ng.3218.
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70. doi:10.1016/j.cell.2014.06.013.
- Li C, Wang S, Xing Z, Lin A, Liang K, Song J, Hu Q, Yao J, Chen Z, Park PK, et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19(2):106–19. doi:10.1038/ncb3464.
- Dai XY, Zhuang LH, Wang DD, Zhou TY, Chang LL, Gai RH, Zhu DF, Yang B, Zhu H, He QJ. Nuclear translocation and activation of YAP by hypoxia contributes to the chemoresistance of SN38 in hepatocellular carcinoma cells. Oncotarget. 2016;7(6):6933–47. doi:10.18632/oncotarget.6903.
- Wang Z, Liu P, Zhou X, Wang T, Feng X, Sun YP, Xiong Y, Yuan HX, Guan KL. Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Res. 2017;77(9):2413–23. doi:10.1158/0008-5472.CAN-16-3229.
- Perra A, Kowalik MA, Ghiso E, Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore E, Roncalli M, Giordano S, et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61(5):1088–96. doi:10.1016/j.jhep.2014.06.033.
- Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63(6):1491–501. doi:10.1016/j.jhep.2015.07.008.
- Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger S, et al. Activation of β-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147(3):690–701. Sepdoi:10.1053/j.gastro.2014.05.004.
- Min Q, Molina L, Li J, Adebayo Michael AO, Russell JO, Preziosi ME, Singh S, Poddar M, Matz-Soja M, Ranganathan S, et al. β-Catenin and Yes-associated protein 1 cooperate in hepatoblastoma pathogenesis. Am J Pathol. 2019;189(5):1091–104. doi:10.1016/j.ajpath.2019.02.002.
- Wang H, Lu J, Mandel JA, Zhang W, Schwalbe M, Gorka J, Liu Y, Marburger B, Wang J, Ranganathan S, et al. Patient-derived mutant forms of NFE2L2/NRF2 drive aggressive murine hepatoblastomas. Cell Mol Gastroenterol Hepatol. 2021;12(1):199–228. doi:10.1016/j.jcmgh.2021.02.004.
- Koch A, Denkhaus D, Albrecht S, Leuschner I, Schweinitz Pietsch vDT. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59(2):269–73.
- Cairo S, Armengol C, De Reyniès A, Wei Y, Thomas E, Renard CA, Goga A, Balakrishnan A, Semeraro M, Gresh L, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14(6):471–84. doi:10.1016/j.ccr.2008.11.002.
- López-Terrada D, Alaggio R, de Dávila MT, Czauderna P, Hiyama E, Katzenstein H, Leuschner I, Malogolowkin M, Meyers R, Ranganathan S, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014;27(3):472–91. doi:10.1038/modpathol.2013.80.
- Adesina AM, Lopez-Terrada D, Wong KK, Gunaratne P, Nguyen Y, Pulliam J, Margolin J, Finegold MJ. Gene expression profiling reveals signatures characterizing histologic subtypes of hepatoblastoma and global deregulation in cell growth and survival pathways. Hum Pathol. 2009;40(6):843–53. doi:10.1016/j.humpath.2008.10.022.
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi:10.1038/nrc1093.
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–64. doi:10.1038/nrm3176.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi:10.1016/j.cell.2011.02.013.
- Schweizer MT, Carducci MA. From bevacizumab to tasquinimod: angiogenesis as a therapeutic target in prostate cancer. Cancer J. 2013;19(1):99–106. doi:10.1097/PPO.0b013e31827e0b86.
- Dong R, Liu GB, Liu BH, Chen G, Li K, Zheng S, Dong KR. Targeting long non-coding RNA-TUG1 inhibits tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis. 2016;7(6):e2278. doi:10.1038/cddis.2016.143.
- Wang X, Freire Valls A, Schermann G, Shen Y, Moya IM, Castro L, Urban S, Solecki GM, Winkler F, Riedemann L, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell. 2017;42(5):462–78.e7. doi:10.1016/j.devcel.2017.08.002.
- Chen Q, Fan K, Chen X, Xie X, Huang L, Song G, Qi W. Ezrin regulates synovial angiogenesis in rheumatoid arthritis through YAP and Akt signalling. J Cell Mol Med. 2021;25(19):9378–89. doi:10.1111/jcmm.16877. Epub 2021 Aug 29.