Abstract
Introduction
We describe five abnormal crania which may provide more diagnostic data for assessment of abnormal crania in newborns.
Methods
Five malformed perinatal crania from the Saxtorphian Collection are described using published prenatal abnormal cranial development criteria. These malformations were compared to normal cranial development arising from the migration of neural crest cells. Visual and photographic investigations were performed.
Results
The malformed crania were occipital encephalocele, holoprosencephaly, anencephaly, and two without a recognizable diagnosis. The anthropological crania were malformed in the same regions as formerly observed in fetal pathology. These regions were comparable to fields formed during normal cell migration from the neural crest. This has seemingly not previously been demonstrated. One undiagnosed cranium may represent a Treacher Collins syndrome (Case 3). The other undiagnosed cranium (Case 4) could be from a scaphocephalic specimen.
Discussion
Sharp borderlines between malformed and non-malformed regions in cranial syndromes may enable improvement in diagnostics.
Introduction
Deviations in human crania can be classified and defined as deviations originating from a malformation, disruption, deformation, or dysplasia [Citation1].
A malformation is a morphological defect, resulting from an inborn abnormal developmental process. A disruption is a morphological defect, resulting from a break-down or an interference with a normal process as observed due to a traumatic injury. A deformation in a cranium can result in an abnormal cranial form or shape while dysplasia is a term used for inborn abnormal organization of cells. In other words, dysplasia is a process, and consequence of dyshistogenesis.
This article deals with inborn malformations observed in perinatal human crania.
Normal crania registered postnatally
Cranial size and growth
Studies on normal anthropological crania are predominantly performed by visual inspection of structures and by measuring distances and angles between structures [Citation2–5]. Cephalometric measurements on cranial radiographs from human individuals, focusing on cranial morphology expressed in angles and length of cranial structures are well known, specifically in orthodontics [Citation6–9]. Yearly cephalometric measurements on a profile radiograph from a non-pathological child are valuable for insight into the individual cranial growth pattern, which plays an important role in orthodontic treatment planning. Extensive differences are observed in the individual pattern of cranial growth in children with inborn malformations such as cleft lip and palate [Citation10, Citation11].
Normal cranial bones registered prenatally
Experimental studies on rats have demonstrated that the cranium arises from cell migrations from different sites of the neural crest [Citation12, Citation13]. The neural crest is the rim between neuro- ectoderm and surface-ectoderm in the cranial part of the neural tube marked NT in . The cells migrating from the neural crest give rise to neural tissue (e.g. peripheral nerves) and tissue of ecto-mesenchymal origin (e.g. bone and muscles). The different sites are marked a, b, c, d in . The uppermost part, or the occipital part, of the neural tube is marked O in .
Figure 1. A schematic overview of cranial development from the neural crest. Left: The dark yellow structure (NT) illustrates the neural tube. The notochord (n) is drawn as a red line passing through the vertebral corpora. The green structures are the facial bones developed from the neural crest. Four segments of neural crest cells are schematically demonstrated. These cells arise from the rim of the neural crest, marked by green dots (a, b, c, d), and from here, they follow the pathways (arrows) toward the anterior aspects of the face. The yellow structures illustrate the hemispheres, and below the hemispheres is the cerebellum. Center: This figure is a schematic drawing of the left illustration seen from behind. The notochord is a red line (n) and the dark yellow (NT) is the neural tube. Note that the four green dots (a, b, c, and d) appear on the right side and also on the left side of the neural tube. This is important for the understanding of unilateral malformations only involving the right or left neural crest. The arrows from the green dots indicate a pathway for the migrating cells to the jaws. Right: A seemingly normally developed perinatal cranium, seen from top to bottom. The cranium might be slightly deformed during storage and appear slightly asymmetrical. As this is a perinatal cranium, the teeth have not erupted. In the posterior view, Wormian bones appear, and the occipital squama appears divided into a lower and an upper bulging part. The upper part has a desmal origin, developed like the parietal bones. The lower part has a cartilaginous origin, comparable to the cartilaginous development of arches in the vertebrae.
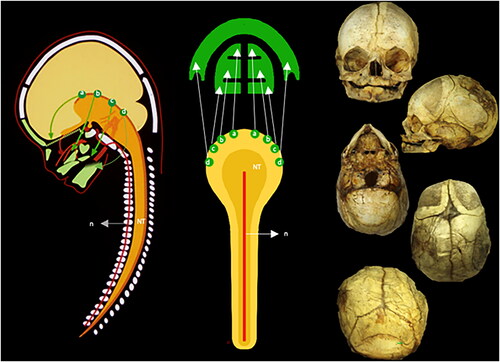
If this important information on rats is transferred to humans, as proposed by Kjær [Citation14], then it is apparent, that one part of the cranium, such as, the mandible arises from one region on the neural crest and other cranial parts from other regions [Citation14] (). Estimated from the location of the uppermost parts of the neural tube and the path of the cranial peripheral nerves Kjær suggested [Citation15–17], that the following cranial regions, named fields, are formed: occipital field which can be seen in marked O; nasopalatine field seen in marked A; maxillary field seen in marked B; palatine field seen in marked C; mandibular field seen in marked D; and Theca field seen in marked E. The problem is that the development of the cranium from different neural crest regions, and borderlines between regions, has never been proved in humans, but only experimentally in rats. It was possible in rats (not in humans) to prove cell migration by lead markings of cells, followed by radiographic analyses [Citation12, Citation13]. If this developmental pattern exists in the human cranium, then the different cranial fields could include different cranial bones or different parts of cranial bones but the exact knowledge about this does not exist.
Figure 2. Illustration of the neural crest cells schematically transferred from experimental animals to a radiograph from a child 10 years of age. Left: This figure can be compared to the left drawing in . The colors demonstrate different fields. The yellow is the frontonasal field (A). The red is the maxillary field (B). The orange is the palatine field (C). The blue color (D) is the mandibular field. Not formed by neural crest cells are the theca field in purple (E) and the occipital field in green (O). The occipital field arises, like the vertebral bodies, from cartilage, influenced by the notochord. Right: This figure indicates the anterior view of the cranium. The colors and letters are comparable to the colors and letters in the left figure. Inserted figure between the left and right: This drawing indicates the well-known developmental fields marked by body lines. For a long time, the developmental fields in the head were not known (absence of lines) The neural crest cell migration in different directions explained the cranial development (Le Douarin [Citation12, Citation13]).
![Figure 2. Illustration of the neural crest cells schematically transferred from experimental animals to a radiograph from a child 10 years of age. Left: This figure can be compared to the left drawing in Figure 1. The colors demonstrate different fields. The yellow is the frontonasal field (A). The red is the maxillary field (B). The orange is the palatine field (C). The blue color (D) is the mandibular field. Not formed by neural crest cells are the theca field in purple (E) and the occipital field in green (O). The occipital field arises, like the vertebral bodies, from cartilage, influenced by the notochord. Right: This figure indicates the anterior view of the cranium. The colors and letters are comparable to the colors and letters in the left figure. Inserted figure between the left and right: This drawing indicates the well-known developmental fields marked by body lines. For a long time, the developmental fields in the head were not known (absence of lines) The neural crest cell migration in different directions explained the cranial development (Le Douarin [Citation12, Citation13]).](/cms/asset/15bc734a-5bbe-43cd-8957-4392d31d77dd/ipdp_a_2338434_f0002_c.jpg)
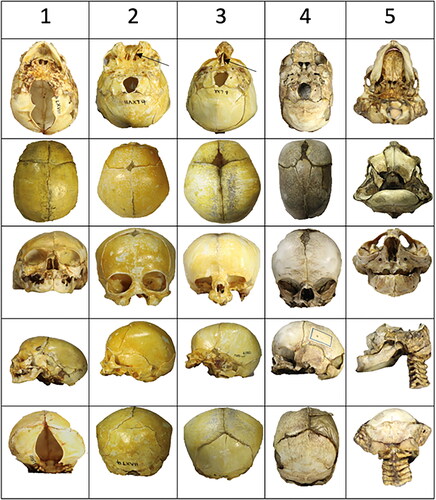
Figure 3. An overview of the five perinatal crania is analyzed from row 1 to row 5. Two crania have mandibular bones, and three do not have mandibular bones. Each cranium is demonstrated from top to bottom in the cranial base view, the thecal view, the anterior view, the lateral view, and the posterior view. Cranium 1, Row 1: A large malformation (absence of occipital bone), marked green in , is observed in the occipital view. In the thecal view, the absence (or early closure) of fontanel is apparent. In the frontal view, a low appearance of the diminutive frontal bones appears. In the posterior view, an absence of bone appears below the lambdoid suture. Cranium 2, Row 2: Absence of bone in the middle of the face (yellow area in ) and presence of septum nasi (arrow) appear in the occipital view. In the thecal view, a diminutive frontal fontanel appears. In the frontal view, the mid-axial cleft does not affect the orbital structures. In the lateral view, a short arcus zygomaticus can be observed. In the posterior view, an extra triangular bone appears above the occipital squama. Cranium 3, Row 3: In this cranium, only the mid-axial part of the face appears (this is the yellow part that was missing in Cranium 2). The regions present are the red and orange fields in . The nasal septum is marked by an arrow. In the thecal view, the fontanel appears normal. In the frontal view, the cavum nasi appears diminutive, and the lower rim of the orbita is absent. In the lateral view, the zygomatic bone and the arcus zygomaticus are missing. Cranium 4, Row 4: A narrow and long cranium appears from the occipital view. The absence of zygomatic bone and arcus is observed (orange areas in ). In the thecal view, the posterior part of the parietal bones is fused (synostosis). In the anterior part, the interfrontal bones are separated by a broad suture. Irregularities in the suture system also appear in the posterior view. Cranium 5, Row 5: In the occipital view, the palate appears narrow posteriorly. From the thecal view, parts of the frontal bone are visible anteriorly, and parts of the occipital bone are visible in the posterior direction. There is no thecal bone (purple area in ) uniting these two parts of the skull. In the frontal view, a narrow interocular distance is observed. In the lateral view, the maxillary complex appears diminutive and retrognathic. In the posterior view, there appear to be fusions between the cervical vertebrae. Bilateral malformations in the lower part of the occipital squama are observed.
Malformed crania registered postnatally
Former studies on human malformed crania have revealed different bony abnormalities, e.g. in the cranial base in newborns with cleft lip and palate [Citation18, Citation19], and also in the mandibles with inborn abnormalities in the mandibular canal [Citation20]. One aspect, that needs to be systematically elucidated, is where the borderlines are between the different developmental fields in the human postnatal cranium.
The main goal of the study of anthropological crania is to improve cranial diagnostics in newborn children. To reach this goal it is important to observe the borderlines between malformed and non-malformed regions in perinatal crania and compare the findings to normal cranial development. This is the intention of the present study.
Material and methods
Material
Perinatal crania from the Saxtorphian Collection at the Medical Museion in Copenhagen have been investigated in this study.
Information on the Saxtorphian collection
The Saxtorph collection was founded in the 1780s by Professor, Dr Med Matthias Saxtorph (1740–1800), who was head of the Danish Birth Foundation (Fødselsstiftelsen). Matthias Saxtorph had studied obstetrics in several European countries and was the leading obstetrician in Denmark. The Saxtorph collection includes relevant collections of human bones and soft tissue preparations, instruments, and a large scientific library. The collection was to be maintained, augmented, and stored at the Danish Birth Foundation, or a suitable institution, and still serve a scientific purpose. The collection was used by students and researchers and has been of great importance for the scientific development of the science of childbirth.
The collection followed the Danish Birth Foundation when it became part of Rigshospitalet (Main Hospital in Copenhagen) in 1910, but later lost its original significance.
In 1992, the collection was handed over to the Medical History Museum, now called Medical Museion, University of Copenhagen. The collection changed character, but it is still stored and used under safe and controlled conditions and with respect for human material.
The perinatal crania
The Saxtorphian collection includes both normal and pathological crania from the perinatal period. Out of the collection, twelve normal crania and five severely malformed crania were studied. An example of a normal crania is demonstrated in . The five severely malformed crania are numbered from 1 to 5 and demonstrated in . The five crania, with the tentative diagnoses are the following:
Occipital encephalocele cranium including the mandible.
Holoprosencephaly (median cleft type) cranium.
Undiagnosed cranium. Only the interocular facial bones are visible.
Undiagnosed narrow cranium. Bilateral lack of arcus zygomaticus.
Anencephaly cranium including the mandible.
The tentative diagnoses were based on a series of prenatal and postnatal pathological human specimens with occipital encephalocele [Citation21, Citation22], holoprosencephaly [Citation23–29], and anencephaly [Citation30–33]. Three of the pathological crania and several prenatal pathological crania have previously been demonstrated in a textbook [Citation13].
Methods
The crania are investigated visually. Photographs have been taken. The following analysis was performed:
Based on fetal pathological findings the perinatal pathological skulls are diagnosed [Citation21–33].
Within the pathological regions, the absence of bones and malformation of single bones are compared with illustrations of normal facial bones in Spalteholtz Atlas [Citation34].
Pathological perinatal regions are compared to hypothetically normal neural crest fields.
Results
The five pathological crania are demonstrated from an outer or inner cranial base view, from the upper cranial view seen from the outside named the thecal view, from the anterior view, the lateral view, and the posterior view illustrated in . The following were observed from the different crania.
Cranium 1, , row 1
The absence of the occipital bone and parts of the sphenoid bone is registered in this cranium. The cranium is short in the vertical dimension. In the theca cranium, the absence of the frontal fontanel is observed. Whether the fontanel region is fused prenatally cannot be ascertained from this case. The analysis documents that the diagnosis of the specimen is occipital encephalocele. The bones, not present in the cranial base are comparable to the green region, region O in . The mandible is seemingly normal.
Cranium 2, , row 2
This cranium is malformed in the mid-axial part of the face below the nasal cavity. The medial yellow parts of the maxilla () are lacking. The lateral maxillary parts and the zygomatic bones with the arcus zygomaticus are present. Compared to the schematic drawing of the frontonasal field () the cranium documents the absence of the yellow frontonasal field. Observed is a septum nasi (arrow) also a diminutive frontal fontanel. The diagnosis is holoprosencephaly, type mid-line cleft.
Cranium 3, , row 3
This cranium demonstrates the presence of the frontonasal yellow neural crest field () including the nasal bone, the nasal septum(arrow), and the medial parts of the maxillary bone. The zygomatic bone with the zygomatic arch and the lower edge of the orbital foramen is absent (red and orange fields in ). In this specimen, the frontal fontanel appears seemingly normal. There is not an exact diagnosis of the cranium.
Cranium 4, , row 4
This cranium is characterized by a bilateral absence of the lateral cranial bones including the absence of the lower edge of the orbital foramen and the alveolar bone in the palatal region (orange field in ). It is characteristic that there is an interparietal synostosis and abnormal interfrontal suture in the theca region. There is not an exact diagnosis of the cranium.
Cranium 5, , row 5
In this specimen, the development of the mandible is seemingly normal. The maxilla is severely retrognathic. The theca field illustrated in purple and marked E in is not present. It is characteristic that only the lower part of the frontal bone is formed as illustrated in and . This cranium seems to represent an anencephalic cranium.
Comparison of crania
It is interesting to compare cranium 2 with cranium 3 (). Cranium 2 has arcus zygomaticus, but this is not the case in cranium 3.
Figure 4. A comparison of cranium 2 () diagnosed holoprosencephaly, mid-line cleft type without facial midaxial structures and cranium 3 eventually diagnosed with Threscher Collin syndrome with midaxial facial structures, but absence of the lateral facial structures. Both specimens have a septum nasi (arrows) which proves that the septum does not develop from the neural crest but from the anterior cartilaginous cranial base. A comparison of the two specimens demonstrates that the orbital cavities are normal in case 2 but abnormal in case 3, lacking the zygomatic bone.
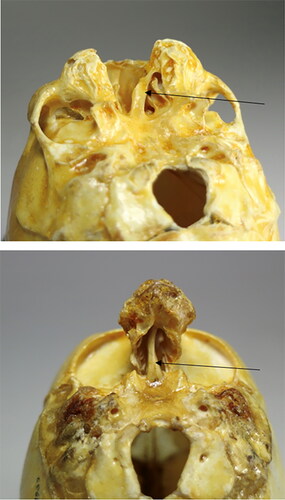
Cranium 2 has fully developed orbitae, while cranium 3 is lacking the lower edge of the orbita. The septum nasi appears in cranium 2 as well as in cranium 3, marked by arrows. This observation might confirm that the nasal septum has been developed from the anterior cartilaginous cranial base and not from the neural crest cells.
A laterally angled photograph of cranium 4 demonstrated in shows part of the absence of the palatal bone in the external view of the maxilla and parts of the zygomatic bone. The posterior part of the maxillary alveolar bone is seemingly lacking.
Figure 5. Two specific views on specimen 4 () were eventually diagnosed as scaphocephalic. The upper figure demonstrates an absence or malformation in the zygoma region and the vertical part of the palatal bone. The lower part illustrates, in a palatal view, the absence of alveolar bone in the maxillary molar region (arrows). The teeth are not visible. The timing between the tooth formation and the alveolar bone formation could be interrupted, but this cannot be concluded.

The five crania demonstrate sharp borderlines between malformed and non-malformed regions in different cranial syndromes.
Discussion
The five crania demonstrated in this study have malformations affecting different bones. This is why the crania are described individually. It is characteristic that the crania shown demonstrate a lack of development in different regions. None of the crania are similar, but collectively they show prenatal defects in different cranial regions.
The exact diagnosis of a cranium can be complicated to determine. Meanwhile, the three diagnoses of cranial encephalocele, holoprosencephaly, and anencephaly seem correct. The one undiagnosed cranium might be from a perinatal specimen with Treacher-Collins syndrome [Citation35] (Case 3, ). The other undiagnosed cranium (Case 4, ) could be from a scaphocephalic specimen. There are still many unsolved questions concerning the diagnostics of the perinatal cranium.
The neural crest cells and their developmental pathways are schematically drawn after Le Douarin et al. [Citation13]. There may exist several more neural crest fields responsible for developing facial bones. In a former investigation of human fetuses published in the Journal of the History of Collections by Meyer and Richter [Citation36] it was possible to illustrate both skeletal and visceral tissue. This was not possible in the present study where fixation and preservation of visceral tissue was not performed.
In former prenatal and postnatal studies on crania, the close interrelationship between cranial and brain development has been highlighted [Citation37–40].
The brain and the peripheral nervous system are important in cranial development. In anthropological crania, the development and orientation of the bony canals enclosing the peripheral nerves can be used in growth analysis. This has been reported in the mandible [Citation41, Citation42] and in the maxilla [Citation43].
Differences in the morphology of the frontal fontanel might be related to changes in the inter-cranial pressure.
This present study demonstrates the importance of pathological perinatal crania for visualizing the individual bones in the malformed regions, which is not currently possible to do in histological studies on prenatal tissue. An anthropological analysis of individual bones might be important for craniofacial surgery in children. The present study of perinatal crania indicates an inter-relationship between the malformed regions and the neural crest fields. It also indicates that the neural crest fields in pathological cases could include loss of entire bones and parts of bones.
The usefulness of this study is that it visualizes the different malformed developmental fields. Most importantly, the malformed area is shown both in extension and depth. In prenatal histological examinations or perinatal radiological examinations/CT scans, the extent of the malformation in depth is difficult to diagnose.
Conclusion
As a conclusion this investigation demonstrates that the anthropological crania appear malformed within local regions bordering normally developed regions. The malformed regions involved in the 5 crania are as follows: frontonasal field, maxillary field, palatal field, thecal field, and the cranial base. The development of the mandible seems to be normal. There seems to be an absence of entire bones and of parts of individual bones in different regions. This has previously been difficult to demonstrate in human individuals [Citation44]. Also, the observation of differences in the frontal fontanels and thecal sutures is important information for perinatal diagnostics.
Author contributions
Ion Meyer was responsible for the material. Amberley Marin was responsible for photography, drawings, and preparing the figures. Inger Kjær was responsible for the conception design, analysis, and interpretation of data. All authors were responsible for drafting the paper, critical revision, and final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgments
Fetal pathologists Birgit Fischer Hansen MD and Jean Keeling MD are thanked for their collaboration and outstanding support in research over many years. Thank you to Maria Kvetny for preparing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Spranger J, Benirschke K, Hall JG, Lenz W, Lowry RB, Opitz JM, Pinsky L, Schwarzacher HG, Smith DW. Errors of morphogenesis: concepts and terms. Recommendations of an international working group. J Pediatr. 1982;100(1):160–5. doi:10.1016/s0022-3476(82)80261-8.
- Sejrsen B, Kjaer I, Jakobsen J. The human incisal suture and premaxillary area studied on archeologic material. Acta Odontol Scand. 1993;51(3):143–51. doi:10.3109/00016359309041160.
- Sejrsen B, Kjær I, Jakobsen J. Human palatal growth evaluated on medieval crania using nerve canal openings as references. Am J Phys Anthropol. 1996;99(4):603–11. doi:10.1002/(SICI)1096-8644(199604)99:4<603::AID-AJPA6>3.0.CO;2-U.
- Sejrsen B, Jakobsen J, Skovgaard LT, Kjaer I. Growth in the external cranial base evaluated on human dry skulls using nerve canal openings as references. Acta Odontol Scand. 1997;55(6):356–64. doi:10.3109/00016359709059200.
- Sejrsen B, Kjaer I, Jakobsen J. Agenesis of permanent incisors in a medieval maxilla and mandible: aetiological aspects. Eur J Oral Sci. 1995;103(2 Pt 1):65–9. doi:10.1111/j.1600-0722.1995.tb00118.x.
- Axelsson S, Kjaer I, Bjørnland T, Storhaug K. Longitudinal cephalometric standards for the neurocranium in Norwegians from 6 to 21 years of age. Eur J Orthod. 2003;25(2):185–98. doi:10.1093/ejo/25.2.185.
- Brock-Jacobsen MT, Pallisgaard C, Kjaer I. The morphology of the sella turcica in monozygotic twins. Twin Res Hum Genet. 2009;12(6):598–604. doi:10.1375/twin.12.6.598.
- Kenrad A, Christensen IJ, Kjær I. Craniofacial morphology of the frontonasal segment in patients with one or two macrodontic maxillary central incisors. Eur J Orthod. 2013;35(3):329–34. doi:10.1093/ejo/cjs062.
- Krebs BJ, Kjær L. Can cephalometry reveal abnormal cerebellum development in Down syndrome? Reviews Press. 2018;(2):73–6.
- Nodal M, Kjaer I, Solow B. Craniofacial morphology in patients with multiple congenitally missing permanent teeth. Eur J Orthod. 1994;16(2):104–9. doi:10.1093/ejo/16.2.104.
- Arntsen T, Kjaer I, Sonnesen L, Mølsted K. Skull thickness in patients with clefts. Orthod Craniofac Res. 2010;13(2):75–81. doi:10.1111/j.1601-6343.2009.01477.x.
- Le Douarin NM. The neural crest in the neck and other parts of the body. Birth Defects Orig Artic Ser. 1975;11(7):19–50.
- Le Douarin NM, Ziller C, Couly GF. Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev Biol. 1993;159(1):24–49. doi:10.1006/dbio.1993.1219.
- Kjær I. Etiology-based dental and craniofacial diagnostics. Wiley Blackwell; 2017. p. 1–244.
- Kjaer I. Correlated appearance of ossification and nerve tissue in human fetal jaws. J Craniofac Genet Dev Biol. 1990;10(3):329–36.
- Kjaer I. Neuro-osteology. Crit Rev Oral Biol Med. 1998;9(2):224–44. doi:10.1177/10454411980090020501.
- Kjær I. How can cranial bones and teeth in children with craniofacial anomalies indicate disturbances in the brain and cranial nerves. Ann Pediatr Child Health. 2020;8:1188.
- Mølsted K, Kjaer I, Dahl E. Spheno-occipital synchondrosis in three-month-old children with clefts of the lip and palate: a radiographic study. Cleft Palate Craniofac J. 1993;30(6):569–73. doi:10.1597/1545-1569_1993_030_0569_sositm_2.3.co_2.
- Mølsted K, Kjaer I, Dahl E. Cranial base in newborns with complete cleft lip and palate: radiographic study. Cleft Palate Craniofac J. 1995;32(3):199–205. doi:10.1597/1545-1569_1995_032_0199_cbinwc_2.3.co_2.
- Jakobsen J, Jørgensen JB, Kjaer I. Tooth and bone development in a Danish medieval mandible with unilateral absence of the mandibular canal. Am J Phys Anthropol. 1991;85(1):15–23. doi:10.1002/ajpa.1330850104.
- Kjaer I, Hansen BF, Keeling JW. Axial skeleton and pituitary gland in human fetuses with spina bifida and cranial encephalocele. Pediatr Pathol Lab Med. 1996;16(6):909–26. doi:10.1080/15513819609168714.
- Kjaer I, Fischer Hansen B, Reintoft I, Keeling JW. Pituitary gland and axial skeleton malformations in human fetuses with spina bifida. Eur J Pediatr Surg. 1999;9(6):354–8. doi:10.1055/s-2008-1072282.
- Kjaer I, Keeling JW, Graem N. The midline craniofacial skeleton in holoprosencephalic fetuses. J Med Genet. 1991;28(12):846–55. doi:10.1136/jmg.28.12.846.
- Kjær I, Hansen BF. Human fetal pituitary gland in holoprosencephaly and anencephaly. J Craniofac Genet Dev Biol. 1995;15:222–9.
- Kjær I, Keeling J, Russell B, Daugaard-Jensen J, Hansen BF. Palate structure in human holoprosencephaly correlates with the facial malformation and demonstrates a new palatal developmental field. Am J Med Genet. 1997;73(4):387–92. doi:10.1002/(sici)1096-8628(19971231)73:4<387::aid-ajmg3>3.0.co;2-l.
- Kjaer I, Becktor KB, Lisson J, Gormsen C, Russell BG. Face, palate and craniofacial morphology in patients with a solitary median maxillary central incisor. Eur J Orthod. 2001;23(1):63–73. doi:10.1093/ejo/23.1.63.
- Becktor KB, Sverrild L, Pallisgaard C, Burhøj J, Kjaer I. Eruption of the central incisor, the intermaxillary suture, and maxillary growth in patients with a single median maxillary central incisor, SMMCI. Acta Odontol Scand. 2001;59(6):361–6. doi:10.1080/000163501317153202.
- Kjaer I, Keeling JW, Fischer Hansen B, Becktor KB. Midline skeletodental morphology in holoprosencephaly. Cleft Palate Craniofac J. 2002;39(3):357–63. doi:10.1597/1545-1569_2002_039_0357_msmih_2.0.co_2.
- Tabatabaie F, Sonnesen L, Kjaer I. The neurocranial and craniofacial morphology in children with solitary median maxillary central incisor (SMMCI). Orthod Craniofac Res. 2008;11(2):96–104. doi:10.1111/j.1601-6343.2007.00419.x.
- Kjaer I, Keeling JW, Graem N. Midline maxillofacial skeleton in human anencephalic fetuses. Cleft Palate Craniofac J. 1994;31(4):250–6. doi:10.1597/1545-1569_1994_031_0250_mmsiha_2.3.co_2.
- Kjaer I, Keeling JW, Graem N. Cranial base and vertebral column in human anencephalic fetuses. J Craniofac Genet Dev Biol. 1994;14(4):235–44.
- Keeling JW, Kjaer I. Diagnostic distinction between anencephaly and amnion rupture sequence based on skeletal analysis. J Med Genet. 1994;31(11):823–9. doi:10.1136/jmg.31.11.823.
- Lomholt JF, Fischer-Hansen B, Keeling JW, Reintoft I, Kjaer I. Subclassification of anencephalic human fetuses according to morphology of the posterior cranial fossa. Pediatr Dev Pathol. 2004;7(6):601–6. doi:10.1007/s10024-004-9098-z.
- Spalteholz W. Anatomie des Menschen. Erster band. Leipzig: Verlag von S. Hirzel; 1921.
- Gorlin RJ, Cohen MM, Levin LS. Syndromes of the head and neck. 3rd ed. New York: Oxford University Press; 1990.
- Meyer I, Richter J. The fate of a nineteenth-century Ischiopagus from Denmark. J Hist Collect. 2008;20(2):253–8. doi:10.1093/jhc/fhn009.
- Kjaer I. Human and animal studies in craniofacial embryology enrich human postnatal craniofacial insight differently. DOBCR. 2021;4(1):1–18. doi:10.31487/j.DOBCR.2021.01.02.
- Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53(3):135–43. doi:10.3109/00016359509005963.
- Kjaer I, Wagner A, Thomsen LL, Holm K. Brain malformation in single median maxillary central incisor. Neuropediatrics. 2010;40(6):280–3. doi:10.1055/s-0030-1248245.
- Kjær I. Neuro-osteology: a discipline of importance for evaluation of human craniofacial development. EC Neurol. 2018;10.6:465–9.
- Chávez-Lomeli ME, Mansilla Lory J, Pompa JA, Kjaer I. The human mandibular canal arises from three separate canals innervating different tooth groups. J Dent Res. 1996;75(8):1540–4. doi:10.1177/00220345960750080401.
- Pálsson SR, Kjaer I. Morphology of the mandibular canal and the angulation between the mandibular and mental canals in dry skulls. Eur J Orthod. 2008;31(1):59–63. doi:10.1093/ejo/cjn076.
- Caspersen LM, Christensen IJ, Kjær I. Inclination of the infraorbital canal studied on dry skulls expresses the maxillary growth pattern: a new contribution to the understanding of change in inclination of ectopic canines during puberty. Acta Odontol Scand. 2009;67(6):341–5. doi:10.1080/00016350903001858.
- Lauesen SR, Daugaard-Jensen J, Lauridsen E, Kjær I. Localised scleroderma en coup de sabre affecting the skin, dentition and bone tissue within craniofacial neural crest fields: clinical and radiographic study of six patients. Eur Arch Paediatr Dent. 2019;20(4):339–50. doi:10.1007/s40368-019-00427-7.
